?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present work investigated the preparation of valine of polyaspartic acid (Val-PASP) and leucine of poly aspartic acid (Leu-PASP) by using polysuccinimide. PASP compounds were used as corrosion and scale inhibitors. The prepared derivatives in this study were characterized by using Fourier transform infrared spectroscopy and nuclear magnetic resonance 1HNMR. The electrochemical behavior of the synthesized compounds was studied on carbon steel surface using potentiodynamic polarization and electrochemical impedance spectroscopy techniques. The morphology of the C-steel specimens was surveyed using scanning electron microscopy and energy dispersive analysis of X-rays investigations. Scale agglomeration inhibition was evaluated using different concentrations of the inhibitors using standard scale test method. The highest corrosion inhibition efficiency for Val-PASP and Leu-PASP reaches 86.94 and 88.62% respectively at 250 ppm concentration, while their scale inhibition efficiency reaches 92.3 and 94.1% respectively at 125 ppm concentration.
Public interest Statement
Seawater is the fundamental natural resource present used for cooling purposes in industrial utilities. Carbon Steel is the most important metal used in every part of the desalination plant equipment and it is used extensively in cooling water systems pipe lines.
Corrosion and scale are two problems resulted from using seawater in desalination process. The threat of Corrosion is the destructive attack of a metal causing catastrophic failure, metallic deterioration and cost governments economy a lot of money. Corrosion science and engineering are the design and application of methods to prevent these defects. Scale is the formation of precipitates whenever water is produced leads to reduce flow line diameter and decrease the production capacity.
This research aims to synthesis of polyaspartic acid derivatives and investigate their corrosion inhibition performances in seawater and reducing calcium sulfate scaling. This work reaches the target of reducing corrosion defects and scale precipitation.
1. Introduction
The needs for producing clean and desalinated water increase with increasing industry propagation so seawater is the suitable and prevalent source to cover this growing demand (Cipollina, Micale, & Rizzuti, Citation2009). Seawater treatment has grown substantially so increasing in desalination capacity is very important but corrosion disasters and scaling precipitation from this process is a threat to the carbon steel piping and desalination environments materials.
Low cost and availability of carbon-steel metal make it the prevalent metal used in desalination plants piping and construction materials (Badiea & Mohana, Citation2009; Mostafaei, Peighambari, & Nasirpouri, Citation2013; Nasirpouri, Mostafaei, Fathyunes, & Jafari, Citation2014) corrosion occurs in widely differing environments and the most corrosion phenomena are in fact related to aqueous corrosion such as in seawater utilities.
Seawater is naturally aggressive solution as it contains different ions such as sulfates and chlorides which increase electrical conductivity leading to corrosion current flow so it cause catastrophic deterioration for metallic surface (Water Research Centre, Citation1981) and presence of calcium and sulfates ions cause agglomeration of insoluble particles on internal pipes walls which hinder water streaming. Using seawater in desalination process cause significant problems such as corrosion of the material and scale formation so the prevalent way to overcome these problem is by using corrosion and scale inhibitors.
Corrosion process expresses the relationship between the aggressive solution and the metallic surface so it lead desalination process to shut down for fixing corrosion damages and it is caused by electrochemical oxidation-reduction reaction (You, Tseng, Guo, & Yang, Citation1999). Corrosion procedures include metal oxidation by oxide formation and dissolution in the aggressive surrounding environment and hence refer to electrochemical redox reactions.
Corrosion breaks through the metal causing deteriorations so producing corrosion inhibitors is essential to overcome these defects by adding in the corrosive environment with certain concentrations to reduce the corrosion rate of the metal.
Eco-friendly, safe and effective chemicals are the going trend for using in desalination industries and water treatment recirculating systems (Choi, You, & Kim, Citation2002). Corrosion and scale inhibition for desalination processes increase the amount of water recovery per unit size given (Al-Ansari & Al-Masri, Citation2010).
Corrosion inhibitors play the role in inhibition processes by adsorption on the metallic surface with complication of its ions and molecules at C-steel surface and depend on metallic surface, chemical structure of compound and aggressive solution conditions (Al-Rawajfeh & Al-Shamaileh, Citation2007).
Scaling is the conjugated challenge for desalination process as it case reducing in production quantity by accumulation on pipes surface then reducing porosity and tube diameter then cause operational problems (Atamanenko, Kryvoruchko, Yurlova, & Tsapiuk, Citation2002) so scale inhibitors are used for mineral sedimentation control (Amjad, Citation1988) so corrosion and scale inhibition performance depend on the inhibitors types and efficiencies resulted from the chemicals used.
Green, low-toxicity and economical chemicals regarding environmental protection from hazardous defects are the better choice for choosing inhibitors (Sastri, Citation2011) such as polymeric compounds to overcome spontaneous corrosion process and undesired accumulation of insoluble particles (ZhenHua et al., Citation2008). The pollution produced from hazardous inhibitors is one of the most serious environmental problems, so scientists tend to produce bi functional inhibitors to eliminate these defects with environmental and economic considerations.
Polymeric compounds are good inhibitors and give effective corrosion inhibition efficiency as reported (Alsabagh, Migahed, & Awad, Citation2006; Migahed, Abdul Raheim, & Atta, Citation2010). Polyamino acids were reported as corrosion and scale inhibitors, particularly polyaspartic acid (Chen, Xu, Han, Su, & Wu, Citation2015; Gao, Fan, Ward, & Liu, Citation2015; Xu, Wang, Zhao, & Cui, Citation2011; Xu, Zhang, Zhao, & Cui, Citation2013; Xu, Zhao, Wang, Xu, & Cui, Citation2012; Zhang, Li, Lv, Cui, & Xu, Citation2014; Zhang, Zhou, Lv, Xu, & Cui, Citation2013).
Polyaspartic acid is a biodegradable water soluble polymer (Kumar et al., Citation2012) and eco-friendly (Guofu et al., Citation2014) with characteristic of non-phosphorus green inhibitor and used in water treatment operations (Guolin, Shan, Shulin, Lijie, & Lingrong, Citation2010). Polyaminoacids such as PASP has several characteristics such as side chain carboxylic acid, chelation effect, functional polymers, environmentally friendly and biodegradation (Liu, Liu, Zhang, & Yan, Citation2011) and will be suitable for using in seawater desalination process.
The aim of the present work is to prepare novel, non-phosphorus and highly effective polyaspartic acid derivatives and to characterize them with Fourier transform infrared spectroscopy (FTIR) and 1H NMR spectrum and then to investigate the corrosion inhibition performance of valine of polyaspartic acid (Val-PASP) and leucine of polyaspartic acid (Leu-PASP) with electrochemical measurements such as potentiodynamic polarization (Tafel plots) and electrochemical impedance spectroscopy (EIS) and to study the calcium-sulfate scale inhibition performance for the same derivatives with standard test with increasing in the dosage continuously till reaching the perfect inhibition results and finally studying specimen morphological surface using scanning electron microscopy (SEM) and energy dispersive analysis of X-rays (EDX).
2. Experimental procedures
2.1. Materials preparation and tested solution composition
2.1.1. Working electrode preparation and chemical composition
The working electrode (WE) type in the present electrochemical tests was from X-65 carbon steel. The Electrode was prepared by metallic turning of unused cut off wall of a pipeline section till reaching appropriate cylindrical rode cross section. The obtained rode was cleaned carefully from lubricates and oily particles by placing in acetone and by using an ultrasonic water bath cleaner for 15 min and followed by air drying.
The Rode Specimen end was welded with a copper wire for electrical connection and both were place in a glass tube and fixed with epoxy resin filling. The exposed surface area of the rod is 1 cm2 in contact with the corrosive test solution. The exposed surface polishing and treatments were performed by using abrasive papers starting from 800 till 1,200 grit of type silicon carbide and followed by 7 min in an ultrasonic acetone bath for removing any residuals, rinsed with distilled water and air drying to be ready for tests using. The chemical composition of the working electrode is illustrated and tabulated in Table .
Table 1. Chemical composition of the C-steel working electrode
2.1.2. Immersion seawater solution chemical composition
Seawater was used in this investigation as a corrosive solution. Chloride ions presence was the largest constituents.
Seawater globally has constant ratio of the concentrations of the main constituents. The Chemical composition of the studied seawater is illustrated and tabulated in Table and showing concentrations of the major 11 ions and molecules accounting 99.95% of total solutes at 25°C, Density (ρ) of 1.023 gcm−3 and 35.0% Salinity.
Table 2. Chemical composition of the investigated seawater test solution
2.2. Synthesis of inhibitors
2.2.1. Synthesis of (Val-PASP) and (Leu-PASP) products
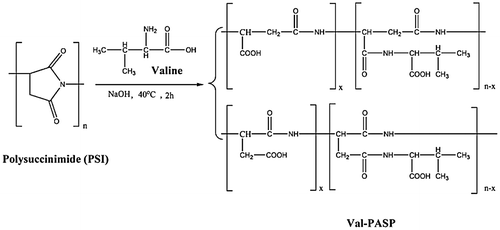
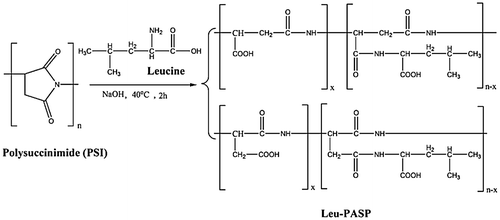
Polysuccinimide (PSI) is used primarily as an intermediate polymeric compound in the production of Polyaspartic acid derivatives. Firstly, synthesis of (Val-PASP), A suspension of Polysuccinimide and deionized water as starting materials was formed in a 500-mL beaker at 25°C. A determinate amount of 10.0% NaOH solution of valine was added in the above suspension then the temperature raised and adjusted to 40°C for 2 h with continuous magnetic stirring. The pH of the obtained solution was adjusted to 7 values by using HCl solution. For precipitation, the obtained yellowish solution mixed with C2H5OH then sediments formed then filtration and the resulted crystals was washed and dried. Yellowish solid crystals of valine of polyaspartic acid (Val-PASP) were produced and the relevant reaction steps were shown in Figure . Secondly, synthesis of Leucine of polyaspartic acid (Leu-PASP) was performed with the same procedures as indicated previously and Figure represents the reaction steps.
2.3. Technical instruments and tests
2.3.1. Chemical structures identification and characterization equipment
2.3.1.1. FTIR spectroscopic analysis
The synthesized PASP derivatives were examined and characterized by Fourier transform infrared by using ATI Mattson-Genesis Series FTIRTM spectrophotometer. FTIR spectra were in the range of 4,000–500 cm−1. The chemical structures of Products were confirmed by FTIR analysis provided the presence of characteristic functional groups evidence.
2.3.1.2. 1H NMR spectroscopic analysis
Nuclear magnetic resonance (1HNMR) measurements were conducted on Varian NMR-300-Mercury 300 MHz Spectrometer. Synthesized products analysis was performed using dimethyl sulphoxide (DMSO) solvent. 1HNMR interpretations are used to confirm PASP derivatives chemical structures.
2.3.2. Electrochemical corrosion tests
2.3.2.1. Potentiodynamic polarization technique
It is a commonly technique used to monitor the corrosion rate of a metal immersed in a corrosive solution with and without inhibitors and for determining inhibition efficiency. Electrochemical corrosion tests were conducted on Volta lab80 (Tacussel-radiometer PGZ402) and the shown tafel plots on computer screen were quantitatively analyzed using Volta master 4 software for obtaining corrosion parameters. Electrochemical measurements were performed at ambient temperature in a conventional three-electrode Pyrex glass cell. Three electrodes participate in the test identified as working electrode (WE) which previously prepared from carbon steel, a platinum electrode as the auxiliary electrode and potentials were measured against a saturated calomel electrode (SCE) as the reference electrode. The tafel plots were obtained at 25°C at 1 mV s−1 scan rate starting from −800 mV till −350 mV against SCE. Scans conducted in both cathodic and anodic potentials to investigate the behavior of obtained polarization potential.
2.3.2.2. EIS technique
Electrochemical impedance curves of the carbon steel metal in the absence and presence of different doses of the synthesized inhibitors in the aggressive electrolyte were obtained by using the same operating Volta master 4 software and performed by Volta lab 80 (Tacussel-radiometer PGZ402) potentiostat. The impedance spectra were recorded after the C-steel working electrode was stabilized at the open circuit potential (OCP) for an hour in the test solution. EIS measurements were performed at applied AC excitation amplitude of 10 mV potential perturbation peak to peak over a frequency range of 100 kHz reach to 10 mHz using 10 steps per decade. EIS was provided in Nyquist and Bode curves.
2.3.3. Scale inhibition test procedure
In the present study, Polyaspartic acid derivatives were evaluated as scale-inhibitors for water treatment against calcium sulfate CaSO4 scale. Scale test was performed according to NACE standard TM 0374-2001. Scale laboratory test conditions and procurers as follow, a Particular amount (7.3 g) of Sodium sulfate (Na2SO4) dissolved in 1 L of double distilled water forming Sulfate solution. Certain quantity (9.11 g) of calcium chloride (CaCl2) dissolved in 1 L of double distilled water forming calcium solution.
In 250-mL conical flask, 50 mL of sulfate solution mixed with 50 mL of calcium solution forming 100 mL mixture with ratio of (1:1). The obtained mixture solution in the conical flask was put into water thermostat at a constant temperature of 90°C for 24 h then cooled at ambient temperature and followed by filtration.
The calcium ions Ca2+ concentration of the filtrate was measured by Complexometric titration (NACE Standard TM0374-2007, Citation2007) using 0.01 M of (EDTA) and Murexide indicator. The experiments were carried out under different concentrations of PASP derivatives starting from 25 ppm reaching to 125 ppm for the synthesized valine of polyaspartic acid (Val-PASP) compound and the same test was repeated for the second synthesized inhibitor with the same experimental conditions and concentrations of Leucine of polyaspartic acid (Leu-PASP) and then the inhibition efficiency of each inhibitor was calculated and studied.
2.3.4. Surface morphology and X-ray analysis
SEM and EDX were performed on C-steel specimen with the same grade used for the previous studies which cut off wall of the same unused pipeline section.
2.3.4.1. Scanning electron microscopic morphologies
The change in surface morphology of C-steel specimen was characterized by means of scanning electron microscope Quanta 200. The metallic surface modification was investigated after drying in case of immersion in absence and presence of certain concentration of the novel synthesized compounds in the tested seawater solution. The surface morphology of the inhibited carbon steel sample was compared visually with the polished and with the uninhibited sample and the inhibition efficiency of the newly inhibitors was studied.
2.3.4.2. X-ray analysis technique
The EDX spectra of the polished metal, after immersion in the blank solution and after immersion in the inhibited solution using certain dose from the inhibitors was studied by using the X-rays of EDAX analyzer system attached to an analytical scanning electron microscope Quanta 200. The surface composition was characterized by means of electromagnetic radiation of the specimens by electron, X-ray beam excitation.
3. Results and discussion
3.1. Characterization of novel PASP derivatives
3.1.1. Characterization of (Val-PASP) and (Leu-PASP)
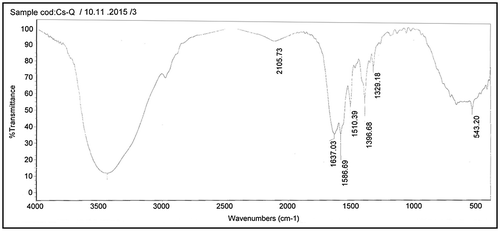
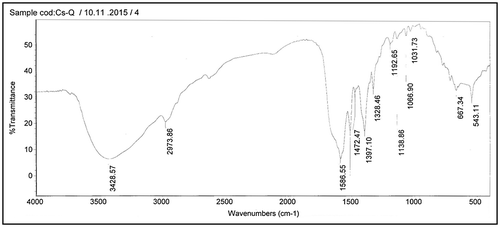
The structure of valine of polyaspartic acid (Val-PASP) and leucine of poly aspartic acid (Leu-PASP) were analyzed and characterized using infrared spectra as shown in Figures and respectively in the region of (4,000–500 cm−1) and 1H NMR spectra as represented in Figures and respectively. Firstly, it is shown from FTIR spectrum analysis of the synthetic products that there is no absorption peaks in the region 1,700–1,800 cm−1 in both figures besides absence of 1,714 cm−1 which is finger print of a cyclic five membered ring imide structure proving that effect of two neighbor carbonyl group in polysuccinimide (PSI) as intermediate material isn’t existed in the novel compounds and ring opening occurred.
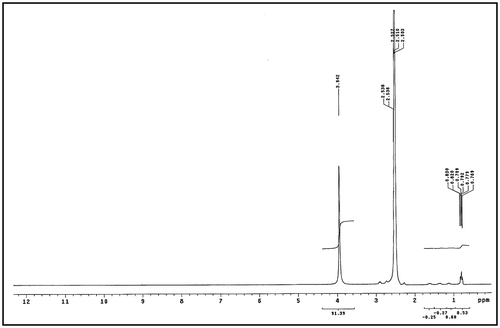
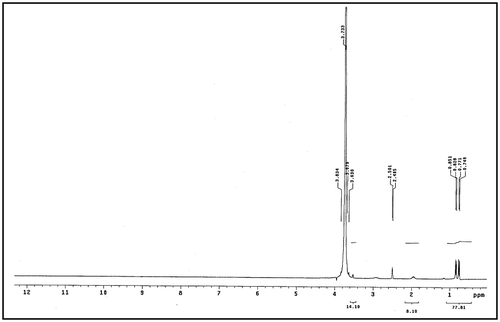
Secondly, the absorption broad bands at 3,434.77 in Figure and 3,428.57 in Figure correspond to 2° amide N–H bond stretching. The observed characteristic peaks at 2,973.86 in Figure and similar peak is present in Figure are belonging to asymmetric stretch of CH2 group.
It is seen that the characteristic absorption bands appear within a wavenumber 2,105 cm−1 in Figure and similar in Figure (1,031.73 + 1,066.90) which is 2,098.63 cm−1 is related to combination CH3 bend and the vibration of NH2 groups which indicate combination of two fundamental modes. The bands at 1,637.03 in Figure and similar to in Figure are belonging to C=O stretching. Peaks at 1,586.69 and 1,586.55 cm−1 in Figures and respectively are ascribed to the stretching vibrations of COO−.
The absorption peaks at 1,510.39 and 1,510.86 cm−1 in Figures and respectively are belonging to the vibration of bending N_H for Amides. The observed characteristic peaks at 1,396.68 and 1,397.10 cm−1 in Figures and respectively are belonging to symmetric bending of CH3. It is seen from Figures and absorption peak at 1,329.18 and 1,328.46 cm−1 respectively which assigned to CH.
The remarkable absorption peak in Figure at 1,472.47 cm−1 is assigned to bending Vibration of CH2. And also it is shown from the same figure that the bending (rocking) motion associated with CH2 group in an open chain occurs at about 667.34 cm−1 and called a long-chain band. Presence of peaks at 543.20 cm−1 in Figure and similar in Figure at 543.11 cm−1 refers to rocking . It is shown from FTIR spectrum in both Figures and that Val-PASP and Leu-PASP were synthesized.
Structure confirmation for the novel products were completed by using 1H NMR. The obtained spectra were performed by using dimethyl sulphoxide (DMSO) and Figures and illustrate the 1H NMR spectrum for Val-PASP and Leu-PASP respectively where 0.83 and 0.85 ppm in Figures and respectively is belonging to –CH (CH3)2 and 3.94 and 3.83 ppm in Figure and respectively is related to –CH2– side chain. So by using FTIR and 1HNMR characterization the Val-PASP and Leu-PASP were synthesized.
3.2. Tafel polarization studies
Tafel plots are suitable for detecting the compounds inhibition efficiencies on the metallic surface. Polarization curves were recorded by using Volta lab 80 potentiostat (Tacussel-radiometer PGZ402) with operating software (Volta master 4). Potentiodynamic polarization measurements were carried out at ambient temperature. The effects of various concentrations of the synthesized compounds on the polarization curves for carbon steel in the aggressive seawater solution were studied.
Open circuit potential (OCP) was carried out for reaching steady state potential (Eocp) after 60 min immersion of the C-steel working electrode in the tested blank solution and repeated each time in case of inhibited solution. Tafel plots were obtained with a scan rate of 1 mV/s in both anodic and cathodic potentials.
Potentiodynamic polarization measurements were performed for each concentration to get the representative plot and then collected in one figure. Tafel polarization curves of carbon steel in blanked seawater and with the addition of 50, 100, 150, 200 and 250 ppm of Val-PASP are presented in Figure and the typical Tafel plots obtained in case of addition of Leu-PASP with the same doses are shown in Figure .
Figure 7. The Potentiodynamic polarization curves (E – Log I) relationship for C-steel in tested sea water with and without addition of different concentrations of Val-PASP compound.
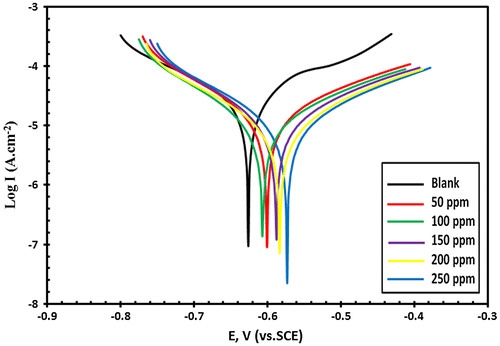
Figure 8. The Potentiodynamic polarization curves (E – Log I) relationship for C-steel in tested sea water with and without addition of different concentrations of Leu-PASP compound.
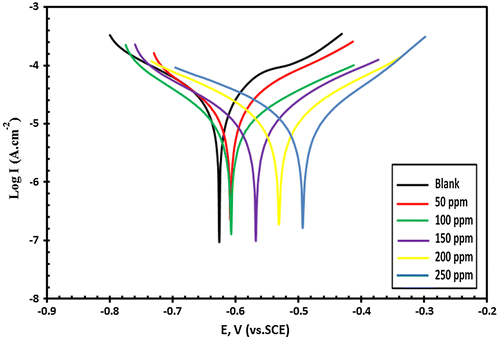
Extrapolation of the anodic and cathodic curves gives the corrosion current density (Icorr) and the addition of Val-PASP and also Leu-PASP inhibitors cause a decrease in the current density (Icorr). Tafel plots show a positive shift in the corrosion potential (Ecorr) with respect to the blank solution without inhibitor. It is shown, from the polarization curves and data obtained, that the compounds behavior affect the anodic and cathodic reaction and there is a remarkable decrease in both anodic and cathodic current density with respect to the blank solution without inhibitor indicating that they act as mixed-inhibitors. The inhibition efficiency for each inhibitor was calculated by using the following equation:(1)
(1)
where Icorr (inhibited) is the corrosion current at a certain inhibitor concentration, and Icorr (uninhibited) is the corrosion current in the absence of inhibitor in the tested solution.
Table represents the corresponding electrochemical parameters of tafel plots in case of blanked solution and in case of addition of certain concentrations of the newly inhibitors including corrosion potentials (Ecorr), corrosion current densities (Icorr), cathodic Tafel slopes (βc), anodic Tafel slopes (βa) and the calculated inhibition efficiencies percentage (IE%). The inhibition efficiency performances of both compounds obtained from Tafel polarization were studied.
Table 3. The Potentiodynamic polarization data at room temperature for C-steel working electrode in the tested solution starting from 0 to 250 ppm addition of (Val-PASP) and also for (Leu-PASP) inhibitor
It is obvious from Table that the IE percentage is low in the presence of 50 ppm, but inhibition efficiency increases remarkably at higher concentrations till reaching 250 ppm and reaches a value of 86.94 and 88.62% for Val-PASP and Leu-PASP respectively. The Corrosion inhibition influenced towards increasing by inhibitors addition and the current intensities influenced by these compounds towards decreasing which is the main target of the inhibitors.
The efficiency depends on the inhibitor concentration so the obtained diagrams results demonstrate that the addition of 50 ppm concentration from the novel compounds give weak inhibition efficiency indicating that this is insufficient for the formation of a stable protective film on the carbon steel electrode surface but at higher concentration, the film which formed was highly effective and stable giving high inhibition efficiency at 250 ppm dosage addition.
Potentiodynamic tafel polarization results show that the addition of 250 ppm dose of the synthesized PASP derivatives have the ability to reduce the destructive corrosion defect for C-steel surface in seawater and protect metallic surface from deterioration and reach significant and remarkable inhibition efficiencies.
Polymers have the ability of film-formation with a good protective barrier character giving the best inhibition performance (Tiu & Advincula, Citation2015) so addition of Polyaspartic acid (PASP) derivatives with also a biodegradability character have the ability to adhere on the metallic surface by the molecular adsorption and this means that polymeric inhibitors reduce the metallic dissolution and hence decreases the corrosion process.
Formation of a passive and barrier film on the C-steel surface play the rule in corrosion inhibition and decreasing oxidation as this film isolate the metallic surface from the aggressive ions such as chlorides which are the big part in the constituents of the seawater. Presence of strong bond between metallic surface molecule and the inhibitor compound isolate iron from the aggressive seawater surroundings (Matsuda & Uhlig, Citation1964). Adsorption caused by the inhibitors molecules on the surface leads to little shift in the corrosion potential of the C-steel negatively or positively (Sastri, Citation2011).
From tafel plots and related parameters it is also clear that there is a shift towards positive region in the values of corrosion potential (Ecorr). Potential shifting of Ecorr towards the positive region is a characteristic of adsorption of the polymeric compounds on metallic surface and as a result in anodic protection which obey the theory of mixed potential (Mansfeld, Xiao, Han, & Lee, Citation1997). Tafel plots show a parallel shifting of both anodic and cathodic plots and the decrease in the anodic and cathodic current densities show that the inhibitors are in the mixed category.
Potentiodynamic polarization measurements for investigation corrosion inhibition for carbon steel in aggressive seawater solution in case of presence the inhibitors show decreasing in the current density and hence increase in the inhibition efficiency leading to required protection.
3.3. Impedance investigations
EIS measurements were performed at ambient temperature in the same glassy electrochemical cell with the three electrodes. Impedance measurements were conducted in the aggressive environment (seawater) in case of absence and presence of inhibitors by using the same apparatus (Volta lab 80 potentiostat) Tacussel-radiometer - PGZ 402 and processed by (Volta master 4) software.
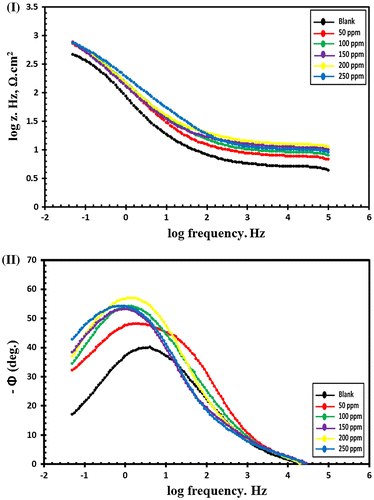
EIS is a nondestructive electrochemical technique and impedance spectra of C-steel in blanked seawater solution and inhibited seawater solution with various doses of synthesized compounds were represented in nyquist plots and bode plots. Zi and Zr are the imaginary and the real impedance and by drawing a figure between −Zi and Zr data we obtain the Nyquist plot as shown in Figures and for Val-PASP and Leu-PASP respectively. By plotting log frequency vs. log Z we obtain the bode plot as represented in Figures (I) and (I) for Val-PASP and Leu-PASP respectively and also we obtained a graph by drawing log frequency vs. phase angle as illustrated in Figures (II) and (II) for Val-PASP and Leu-PASP respectively.
Figure 9. Nyquist curves for C-steel in blanked seawater solution and in case of addition of certain doses of Val-PASP inhibitor.
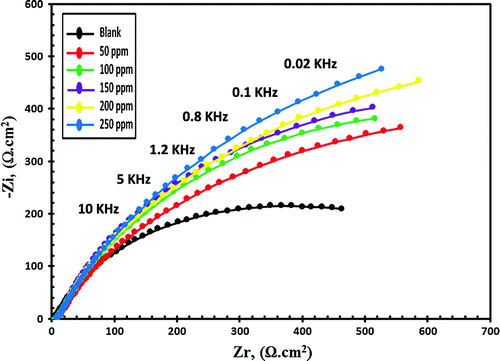
Figure 10. Nyquist curves for C-steel in blanked seawater solution and in case of addition of certain doses of Leu-PASP inhibitor.
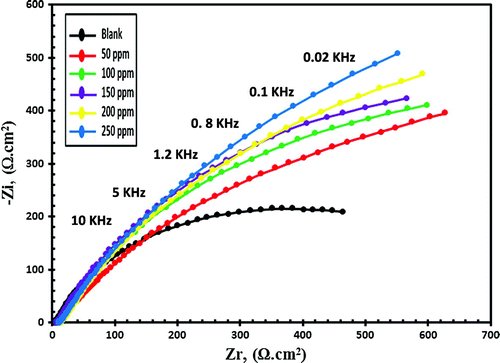
Figure 11. Bode curves for C-steel in blanked seawater solution and in case of addition of certain doses of Val-PASP inhibitor: (I) log frequency vs. log Z, (II) log frequency vs. phase angle.
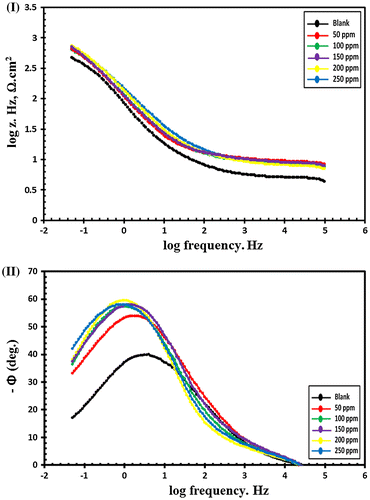
Figure 12. Bode curves for C-steel in blanked seawater solution and in case of addition of certain doses of Leu-PASP inhibitor: (I) log frequency vs. log Z, (II) log frequency vs. phase angle.
The inhibition efficiency (IE%) for each concentration of the inhibitors was calculated separately by using the charge transfer resistance from the following equation:(2)
(2)
where Rt (blank) and Rt (inhibited) are the charge transfer resistance in the solution without inhibitor and with inhibitor addition respectively.
Table shows the blanked solution without inhibitor and the different inhibitor concentrations starting from 50 ppm till reaching dose of 250 ppm and for each concentration it collects the related values of the solution resistance (Rs), the film resistance (Rf), the film capacitance (Cf), the charge transfer resistance (Rt), the double layer capacitance (Cdl) obtained from impedance spectra analysis besides the inhibition efficiency (IE%). Analysis of the collected data of impedance show an increase in addition of compound concentrations faces an increase in the charge transfer resistance (Rt) and a decrease in double layer capacitance (Cdl) leading to an increase in the inhibition efficiency (IE%) values. Further detailed analysis is required to ascertain the nature of the protective barrier layer will be considered in future work and through fully detailed schematic diagram inhibition mechanism. The inhibition efficiencies increase gradually with the corresponding increasing in concentrations of the tested compounds reaching IE. 87 and 88.69% for (Val-PASP) and (Leu-PASP) respectively at 250 ppm.
Table 4. Impedance investigation data for C-steel in blanked seawater solution and in case of addition of certain concentrations of Val-PASP and Leu-PASP products
It is clear from EIS inhibition efficiencies obtained that there is a good agreement and fitting with IE% results obtained from Potentiodynamic polarization measurements. In both electrochemical techniques the inhibition efficiencies improved with the increasing of inhibitors concentrations. Both techniques investigate the effects of certain doses addition of inhibitors on carbon steel corrosion inhibition in aggressive seawater solution and both show good results in inhibition efficiencies.
3.4. Inhibition studies of calcium sulfate scale formation by the synthesized compounds
The inhibition performance of the newly polyaspartic acid derivatives as CaSO4 scale inhibitors in simulated cooling water was evaluated by using standard method ASTM G 3-89 and Re-approved 1994. It was found that the addition of inhibitors with different concentrations has a good effect on the scale inhibition performance and the test was carried out under identical conditions. The percentage inhibition efficiency (IE%) of inhibitors for calcium sulfate scale was calculated using the following equation:(3)
(3)
where
[Ca]inhibited = Calcium ion concentration for the sample treated with the inhibitor after precipitation test.
[Ca]blank = Calcium ion concentration in the blank solution after precipitation test.
[Ca]c = Calcium ion concentration in the blank solution before precipitation test.
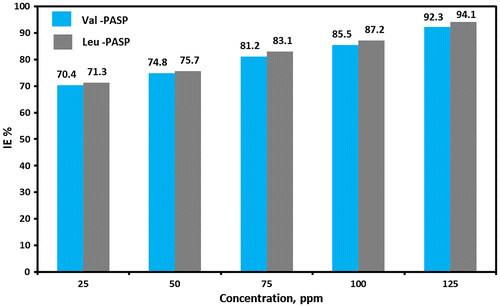
Resulted data listed in Table and show that scale inhibition efficiency increases from 70.4 to 71.3% reaching 92.3 and 94.1% respectively for Val-PASP and Leu-PASP by concentrations addition from 25 to 125 ppm respectively.
Table 5. The Percentage inhibition efficiency (IE%) data of Val-PASP and Leu-PASP compounds as CaSO4 scale inhibitors at various doses as calculated from (ASTM G3-89 Standard Re-approved 1994)
Figure shows the relation between the various concentrations of inhibitors (25, 50, 75, 100 and 125 ppm) and the calculated inhibition efficiency percentage (IE%) for each concentration. Addition of 125 ppm Concentration from the synthesized Polyaspartic acid derivatives was the reason for getting good calcium sulfate scale inhibition efficiency proving the ability in reducing CaSO4 scale accumulation which leads to reduce water hardness in industrial water treatment systems by reducing scale growth.
3.5. Surface morphology investigations using SEM
The surface investigation of the C-steel specimens was determined by SEM, scanning electron microscope Quanta 200 attached with EDAX, Ametek Energy dispersive analysis of X-rays. Surface morphologies were determined in three cases which First case is represented in Figure (I) and shows the representative SEM image of the carbon steel specimen after polishing with 1,200 carbide silicon paper.
Figure 14. SEM images of the carbon steel samples surface: (I) polished sample, (II) after immersion in the seawater solution without inhibitor and (III) after immersion in the seawater solution in the addition of 250 ppm of Leu-PASP compound.
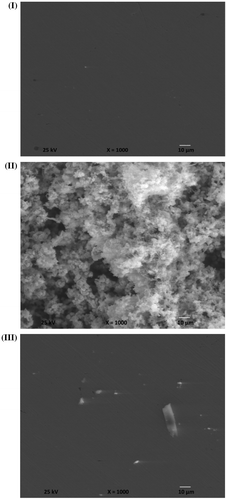
Second case is represented in Figure (II) and shows the representative SEM image of the carbon steel specimen after exposure to the aggressive tested seawater without addition of inhibitor for 90 days duration. Third case is represented in Figure (III) and shows the representative SEM images of the carbon steel specimen after exposure to the aggressive seawater with 250 ppm addition of Leu-PASP inhibitor for 90 days period leading to form an adhesive and Protective Film which isolate the metallic surface from the aggressive seawater environment.
The C-steel surface morphology displays a significant change after exposure to the newly synthesized compound for 90 days with 250 ppm concentration by comparing the third case with the second case. The large magnification in the three cases reveals the protection of carbon steel surface which happened in case of presence of the inhibitor and the target of the inhibition efficiency happened. From the scanning electron microscope investigations it is clear that the synthesized compound is suitable inhibitor in case of the aggressive seawater indicating that there is an improvement in the surface morphology of C-steel in presence of the inhibitor.
3.6. EDX analyzer observation
The inhibition efficiency for the novel inhibitor on the carbon steel surface was investigated and analyzed by using Ametek Energy dispersive analysis of X-rays, EDAX analyzer connected to scanning electron microscope Quanta 200, SEM. Energy dispersive X-rays analyses for the C-steel samples surface were determined for the previous three cases where the surface composition was studied. EDX results are presented in three figures identifying some characteristic elements peaks.
Figure (I) reveals the representative EDX analysis peaks for the carbon steel sample after polishing and shows the surface chemical composition. Figure (II) evaluates the representative EDX results for the carbon steel specimen after exposure to the seawater solution without addition of the inhibitor after 90 days. Figure (III) represents EDX data for the carbon steel slide after exposure to the seawater solution with addition of 250 ppm concentration from the synthesized Leu-PASP inhibitor after 90 days which have a stable protective layer.
Figure 15. The (EDX) analysis of the carbon steel samples surface: (I) polished sample, (II) after immersion in the seawater solution without inhibitor and (III) after immersion in the seawater solution in the addition of 250 ppm of Leu-PASP compound.
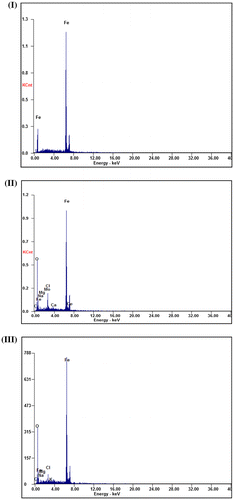
The EDX results assessed by comparing between the three cases and Decreasing in Fe peak intensity for the inhibited sample is relative to the other samples indicating formation of protective film formed by absorption on the C-steel surface which isolate the metallic surface from deterioration by the corrosive media and presence of oxygen signal show a characteristic inclusion as indication of an oxide inclusion as a result of inhibitor molecules addition forming a good adherent layer demonstrating increase in inhibition efficiency by using the novel synthesized compound. Scanning electron microscope micrographs and Energy dispersive X-rays analysis reveal that there is an improvement in the surface morphology of the inhibited carbon steel specimen compared with the uninhibited carbon steel specimen and provide more information about increasing inhibition efficiency due to the addition of inhibitor to the natural seawater solution compared to the blank sample without inhibitor.
3.7. Mechanism of the corrosion and scale inhibition
Corrosion and scale inhibition of the newly synthesized inhibitors for C-steel surface in the aggressive solution depends on products chemical composition, structure and their bonding mechanism.
Polymeric compounds have multiple adsorption sites for bonding with metallic surface so the increase in the molecular weight of the polymeric compounds increase the adsorption sites and polymeric chain displaces water molecules from the surface of the metal and also slow desorption of the polymers due to multiple bonding sites (Amin, EI-Rehim, El-Sherbini, Hazzazi, & Abbas, Citation2009; Encyclopaedia of Polymer Science & Technology, Citation1964).
Metallic surface/Polymeric inhibitor interface include chemical reactions and physical adsorption so inhibitor chelates with Fe2+ ion accelerating the formation of a protective film protecting C-steel surface from Aggressive environment (Huo, Cai, Zhao, & Lu, Citation2001).
Adsorption of the synthesized PASP derivatives on the C-steel surface then formation a protective film leading to corrosion inhibition is due to the reaction between oxygen and nitrogen atoms which have lone pair of electron in –CONH and –COOH interacting with the vacant d-orbital of iron by donor acceptor interactions (Behpour, Ghoreishi, Salavati-Niasari, & Ebrahimi, Citation2008; Yurt, Balaban, Kandemir, Bereket, & Erk, Citation2004).
Chemisorption mechanism for inhibitors includes displacing adsorbed water molecules from metallic surface and sharing of electrons between iron and the heteroatoms (Migahed, Attia, & Habib, Citation2015). The protective film block the active sites on the metallic surface resisting aggressive chloride ions and dissolved oxygen from transferring to the metal in the seawater and hence reduce corrosion (Matsuda & Uhlig, Citation1964).
Polymers have some advantages like multi-functional chemistries, and better film-forming leading to improve protective barrier properties and also complex formation with the metallic surface or chelation on corrosive agents resulting to better inhibition efficiency (Tiu & Advincula, Citation2015).
Amino and carboxyl groups are chemically active; they participate in chelation, cyclization, racemization and ability of picking-up ions (Wu, Citation2013).
Figure shows illustration of the adsorption modes at the metal surface /aggressive solution interface and how inhibitor molecules chelate calcium ions and remaining sulfate ions soluble in the solution.
Figure 16. Schematic illustration of corrosion and scale inhibition mechanism of the Synthesized PASP derivatives, R=–CH (CH3)2 in case of Val-PASP, R=_CH2–CH (CH3)2 in case of Leu-PASP.
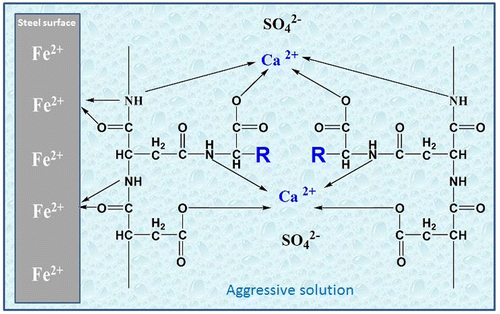
Scale formation path through several steps from Calcium and sulfate ions, precipitation, crystal growth leading to scaling and agglomeration. Inhibitors play the role in active crystal growth sites, modification, crystal dispersion, delaying precipitation and prevent buildup of crystalline lattice. Scale Inhibition depends on Crystal structure modification and preventing the formation of an intensive and hard adherent scales on the metallic steel surface (Liu, Dong, Li, Hui, & Lédion, Citation2012).
Scale inhibition depends on the ability of the compounds in reducing scale crystals growth rate in the aggressive solution (Martel & Calvin, Citation1952) the inhibition performance of PASP derivatives against CaSO4 scale depends on chelation of Ca2+ ions as presented in Figure in the solution and presence of functional groups which play the rule in scale inhibition (Feng et al., Citation2014; Zhou, Sun, & Wang, Citation2011).
The synthesized PASP derivatives with the active functional groups have the ability in corrosion inhibition by adsorption on the steel surface and formation a protective film on it and also the synthesized compounds have the ability in scale inhibition by chelation with Ca2+ ions in the aggressive solution decreasing the precipitation of calcium sulfate scales CaSO4.
4. Conclusions
Ecofriendly and biodegradable polyaspartic acid derivatives were synthesized by using Polysuccinimide as starting material with certain reactions steps. Val-PASP and Leu-PASP compounds were characterized by using FTIR and1HNMR.Corrosion inhibition efficiencies for Val-PASP and Leu-PASP inhibitors were studied by using electrochemical corrosion tests including tafel polarization measurements and impedance technique. By using polarization test it was shown a decrease in corrosion current leading to an increase in inhibition efficiencies for both inhibitors by increasing doses. By using impedance test there was an improve in inhibition efficiencies for both compounds by increasing in concentration addition due to an increase in Rt and there was a decrease in Cdl values.
The synthesized derivatives were suitable for decreasing corrosion deterioration for carbon steel by adsorption on the metallic surface leading to isolation for the metal form the aggressive seawater environment. Valine of polyaspartic acid and leucine of poly aspartic acid were tested as CaSO4 Scale inhibitors by using standard method, ASTM G3-89, Re approved 1994 and the scale inhibition efficiency for each inhibitor reaches 92.3 and 94.1% respectively at 125 ppm.
SEM and EDX were used to photograph and analyze the C-steel surfaces in case of polished, blanked and inhibited with certain dose addition from the inhibitor and The results showed an improvement for the metallic surface upon using the inhibitor compared to the surface appearance of the blanked sample in absence of inhibitor and these examinations support the electrochemical measurements results and prove the inhibition efficiency.
Present work reached the target in evaluation of newly synthesized and characterized polyaspartic acid derivatives as corrosion and scale inhibitors for sea water applications in desalination process.
Funding
The authors received no direct funding for this research.
Additional information
Notes on contributors
M.A. Migahed
M.A. Migahed is a Professor of Physical Chemistry, Head of Petroleum Applications Department, and Head of scale and corrosion inhibitors lab in Egyptian Petroleum Research Institute (EPRI). Consultant for corrosion protection and scale formation retardation in all oil and gas companies in Egypt. His research interests are focused on Electrochemistry.
S.M. Rashwan
S.M. Rashwan is a Professor of Physical Chemistry in Department of Chemistry of Faculty of Science at Suez Canal University, Ismailia, Egypt. His research interest includes Corrosion and scale topics, Supervise master and doctoral theses.
M.M. Kamel
M.M. Kamel is a senior lecturer holds a PhD degree in Department of Chemistry of Faculty of Science at Suez Canal University, Ismailia, Egypt. His research interest includes corrosion and scale inhibition topics
R.E. Habib
R.E. Habib is a PhD student in physical chemistry in corrosion and scale inhibition in Department of Chemistry of Faculty of Science at Suez Canal University, Ismailia, Egypt.
References
- Al-Ansari, M. S., & Al-Masri, N. (2010). Future sustainable desalination technologies for the GCC. In Third SQUJ-CCP Joint Symposium, Muscat, December.
- Al-Rawajfeh, A. E., & Al-Shamaileh, E. M. (2007). Inhibition of corrosion in steel water pipes by ammonium pyrrolidine dithiocarbamate (APDTC). Desalination, 206, 169–178.10.1016/j.desal.2006.02.065
- Alsabagh, A. M., Migahed, M. A., & Awad, H. S. (2006). Reactivity of polyester aliphatic amine surfactants as corrosion inhibitors for carbon steel in formation water (deep well water). Corrosion Science, 48, 813–828.10.1016/j.corsci.2005.04.009
- Amin, M. A., EI-Rehim, S. S. A., El-Sherbini, E. E., Hazzazi, O. A., & Abbas, M. N. (2009). Polyacrylic acid as a corrosion inhibitor for aluminium in weakly alkaline solutions. Part I: Weight loss, polarization, impedance EFM and EDX studies. Corrosion Science, 51, 658–667.10.1016/j.corsci.2008.12.008
- Amjad, Z. (1988). Calcium sulfate dihydrate (gypsum) scale formation on heat exchanger surfaces: The influence of scale inhibitors. Journal of Colloid and Interface Science, 123, 523.10.1016/0021-9797(88)90274-3
- Atamanenko, I., Kryvoruchko, A., Yurlova, L., & Tsapiuk, E. (2002). Study of the CaSO4 deposits in the presence of scale inhibitors. Desalination, 147, 257–262.10.1016/S0011-9164(02)00547-7
- Badiea, A. M., & Mohana, K. N. (2009). Corrosion mechanism of low-carbon steel in industrial water and adsorption thermodynamics in the presence of some plant extracts. Journal of Materials Engineering and Performance, 18, 1264–1271.10.1007/s11665-009-9378-x
- Behpour, M., Ghoreishi, S. M., Salavati-Niasari, M., & Ebrahimi, B. (2008). Evaluating two new synthesized S-N Schiff bases on the corrosion of copper in 15% hydrochloric acid. Materials Chemistry and Physics, 107, 153–157.10.1016/j.matchemphys.2007.06.068
- Chen, J., Xu, L., Han, J., Su, M., & Wu, Q. (2015). Synthesis of modified polyaspartic acid and evaluation of its scale inhibition and dispersion capacity. Desalination, 358, 42–48.10.1016/j.desal.2014.11.010
- Choi, D., You, S., & Kim, J. (2002). Development of an environmentally safe corrosion, scale, and microorganism inhibitor for open recirculating cooling systems. Materials Science and Engineering: A, 335, 228.10.1016/S0921-5093(01)01928-1
- Cipollina, A., Micale, G., & Rizzuti, L. (2009). Seawater desalination—Conventional and renewable energy processes (pp. 1–2). Berlin Heidelberg: Springer-Verlag.
- Encyclopaedia of Polymer Science and Technology. (1964). Amorphous polymers (Vol. 1, 2nd ed., p. 558). Hoboken, NJ: Wiley
- Feng, J., Gao, L., Wen, R., Deng, Y., Wu, X., & Deng, S. (2014). Fluorescent polyaspartic acid with an enhanced inhibition performance against calcium phosphate. Desalination, 345, 72–76.10.1016/j.desal.2014.04.019
- Gao, Y., Fan, L., Ward, L., & Liu, Z. (2015). Synthesis of polyaspartic acid derivative and evaluation of its corrosion and scale inhibition performance in seawater utilization. Desalination, 365, 220–226.10.1016/j.desal.2015.03.006
- Guofu, M., Yajuan, W., Kanjun, S., Hui, P., Haiping, W., & Ziqiang, L. (2014). High performance nitrogen-doped carbon for supercapacitor obtained by carbonizing eco-friendly and cheap polyaspartic acid. Materials Letters, 132, 41–44.
- Guolin, J., Shan, T., Shulin, L., Lijie, X., & Lingrong, K. (2010). The optimal preparation of scale inhibitor polyaspartic acid. Advanced Materials Research, 113–114, 694–697.
- Huo, Y., Cai, Z., Zhao, Y., & Lu, Z. (2001). Effect of the corrosion inhibition behavior of polyaspartic acid and its mixture of zinc ion on carbon steel. Journal of East China University of Science and Technology, 27, 669–672.
- Kumar, N. M., Varaprasad, K., Rao, K. M., Babu, A. S., Srinivasulu, M., & Naidu, S. V. (2012). A novel biodegradable green poly(l-aspartic acid-citric acid) copolymer for antimicrobial applications. Journal of Polymers and the Environment, 20, 17–22.10.1007/s10924-011-0335-z
- Liu, D., Dong, W., Li, F., Hui, F., & Lédion, J. (2012). Comparative performance of polyepoxysuccinic acid and polyaspartic acid on scaling inhibition by static and rapid controlled precipitation methods. Desalination, 304, 1–10.10.1016/j.desal.2012.07.032
- Liu, Z., Liu, H., Zhang, L., & Yan, M. (2011). Study on performance of scale and corrosion inhibition of polyaspartic acid composite material. Advanced Materials Research, 233–235, 50–53.10.4028/www.scientific.net/AMR.233-235
- Mansfeld, F., Xiao, H., Han, L., & Lee, C. (1997). Electrochemical impedance and noise data for polymer coated steel exposed at remote marine test sites. Progress in Organic Coatings, 30, 89–100.10.1016/S0300-9440(96)00675-3
- Martel, A. E., & Calvin, M. (1952). Chemistry of metal chelate compounds. New York, NY: Prentice-Hall chemistry series.
- Matsuda, S., & Uhlig, H. H. (1964). Effect of pH, sulfates, and chlorides on behavior of sodium chromate and nitrite as passivators for steel. Journal of the Electrochemical Society, 111, 156–161.10.1149/1.2426075
- Migahed, M. A., Abdul Raheim, A. M., & Atta, A. M. (2010). Synthesis and evaluation of a new water soluble corrosion inhibitor from recycled poly(ethylene terphethalate). Materials Chemistry and Physics, 121, 208–214.10.1016/j.matchemphys.2010.01.018
- Migahed, M. A., Attia, A. A., & Habib, R. E. (2015). Study on the efficiency of some amine derivatives as corrosion and scale inhibitors in cooling water systems. Royal Society of Chemistry Advances, 5, 57254–57262.
- Mostafaei, A., Peighambari, S. M., & Nasirpouri, F. (2013). Failure analysis of monel packing in atmospheric distillation tower under the service in the presence of corrosive gases. Engineering Failure Analysis, 28, 241–251.10.1016/j.engfailanal.2012.10.028
- NACE Standard TM0374-2007. (2007). (formerly TM0374-2001).
- Nasirpouri, F., Mostafaei, A., Fathyunes, L., & Jafari, R. (2014). Assessment of localized corrosion in carbon steel tube-grade AISI 1045 used in output oil–gas separator vessel of desalination unit in oil refinery industry. Engineering Failure Analysis, 40, 75–88.10.1016/j.engfailanal.2014.02.012
- Sastri, V. S. (2011). Green corrosion inhibitors (p. 26). Hoboken, NJ: John Wiley & Sons.10.1002/9781118015438
- Tiu, B. D. B., & Advincula, R. C. (2015). Polymeric corrosion inhibitors for the oil and gas industry: Design principles and mechanism. Reactive and Functional Polymers, 95, 25–45.10.1016/j.reactfunctpolym.2015.08.006
- Water Research Centre. (1981). A guide to solving water quality problems in distribution systems (Technical report TR 167). Medmenham.
- Wu, G. (2013). Amino acids: Biochemistry and nutrition (p. 27). Boca Raton, FL: CRC Press.10.1201/b14661
- Xu, Y., Wang, L., Zhao, L., & Cui, Y. (2011). Synthesis of polyaspartic acid– aminobenzenesulfonic acid grafted copolymer and its scale inhibition performance and dispersion capacity. Water Science & Technology, 423–430.10.2166/wst.2011.526
- Xu, Y., Zhao, L., Wang, L., Xu, S., & Cui, Y. C. (2012). Synthesis of polyaspartic acid–melamine grafted copolymer and evaluation of its scale inhibition performance and dispersion capacity for ferric oxide. Desalination, 286, 285–289.10.1016/j.desal.2011.11.036
- Xu, Y., Zhang, B., Zhao, L., & Cui, Y. (2013). Synthesis of polyaspartic acid/5- aminoorotic acid graft copolymer and evaluation of its scale inhibition and corrosion inhibition performance. Desalination, 311, 156–161.10.1016/j.desal.2012.11.026
- You, S. H., Tseng, D. H., Guo, G. L., & Yang, J. J. (1999). The potential for the recovery and reuse of cooling water in Taiwan. Resources, Conservation and Recycling, 26, 53–70.10.1016/S0921-3449(98)00075-5
- Yurt, A., Balaban, A., Kandemir, S., Bereket, G., & Erk, B. (2004). Investigation on some Schiff bases as HCl corrosion inhibitors for carbon steel. Materials Chemistry and Physics, 85, 420–426.10.1016/j.matchemphys.2004.01.033
- Zhang, B., Li, J., Lv, X., Cui, Y., & Xu, Y. (2014). Synthesis of polyaspartic acid/2-amino-2-methyl-1,3-propanediol graft copolymer and evaluation of its scale inhibition and corrosion inhibition performance. Desalination and Water Treatment, 1–7.
- Zhang, B., Zhou, D., Lv, X., Xu, Y., & Cui, Y. (2013). Synthesis of polyaspartic acid/3-amino-1H-1,2,4-triazole-5-carboxylic acid hydrate graft copolymer and evaluation of its corrosion inhibition and scale inhibition performance. Desalination, 327, 32–38.10.1016/j.desal.2013.08.005
- ZhenHua, Q., Chang, C. Y., XiuRong, W., Cheng, S., YunJie, L., & Fang, M. C. (2008). Experimental study on scale inhibition performance of a green scale inhibitor polyaspartic acid. Science in China Series B: Chemistry, 51, 695–699.
- Zhou, X. H., Sun, Y. H., & Wang, Y. Z. (2011). Inhibition and dispersion of polyepoxysuccinate as a scale inhibitor. Journal of Environmental Sciences, 23, S159–S161.10.1016/S1001-0742(11)61102-9