?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Improvement of electro-Fenton (EF) process with granulated activated carbon (GAC) for the treatment of brewery effluent obtained from Ota, South-West Nigeria was investigated. The GAC was obtained by crushing, carbonising, sieving, and activating cow bones. Duplicate samples of the raw effluent and 10 treated brewery effluent samples were analysed for conductivity, turbidity, total dissolved solids (TDS), chemical oxygen demand (COD), copper, manganese, cadmium, lead, and zinc. The average readings were taken as final values. Results showed that the combined use of EF and GAC treatment of the effluent yielded better result than use of EF alone. The removal efficiency was 1181, 50, 565, 4375, 160, and 840% for turbidity, COD, copper, cadmium, lead, and zinc respectively. EF and GAC treatment were, however, found to be inefficient for the treatment of conductivity, TDS, and manganese. It was also found that pH had a direct impact on the treatment media. Turbidity and cadmium, which had the highest removal rates were achieved at pH 2, while COD, copper, and zinc had optimum treatment at pH 6. It was concluded that the combined advantage of electrolytic separation from EF and adsorption from GAC yielded better treatment result for the brewery effluent samples.
Public Interest Statement
It is widely known that millions of people, especially in developing countries, lack access to clean drinking water. This drives many to source water from dug wells and surface water bodies, which are prone to contaminations from the environment. One such contamination comes from industrial activities which is often laden with heavy metals. Also, the quality of discharged industrial wastewater in Nigeria is not well regulated. Consequently, heavily polluted wastewater is often discharged indiscriminately into the environment. This research, therefore, provides information on how industrialists can treat their effluent effectively with the combined use of electro-Fenton and carbonized cow-bone before discharge into the environment. The citizens can also learn how to apply the same method described herein to treat their raw water before use.
1. Introduction
Industrialization leads to economic growth of any nation. However, industrialization also leads to environmental pollution and degradation when the waste products are not well managed. This problem is particularly pronounced in developing countries, where adequate treatment technologies are unavailable or expensive (Omole, Isiorho, & Ndambuki, Citation2016). Adapting any treatment method in any area, and at any given condition depends on several factors such as economic, required efficiency, available technology, concentration of the pollutants, and national standards, among other things (Emenike, Omole, Ngene, & Tenebe, Citation2016; Omole, Ndambuki, Nwafor-Oritzu, & Obata, Citation2014). Many reviews focus on various treatment and pre-treatment methods of water and wastewater for sustainable development (Garcia-Segura, Bellotindos, Huang, Brillas, & Lu, Citation2016; Huang et al., Citation2017; Mirzaei, Chen, Haghighat, & Yerushalmi, Citation2017; Nidheesh & Gandhimathi, Citation2012; Omole & Ogugua, Citation2017; Omole et al., Citation2014). This includes advanced oxidation processes and use of activated carbon (Babuponnusami & Muthukumar, Citation2014; Emenike et al., Citation2016; Emenike, Omole, Ngene, & Tenebe, Citation2017). The electro-Fenton (EF) method is based on the principle of ionization, oxidation, and separation of wastewater constituents at atomic level using electric currents (electrolysis) while activated carbon employs the principle of surface adsorption (Atallah Aljubourya, Palaniandy, Aziz, & Feroz, Citation2015; Deng & Zhao, Citation2015; Emenike et al., Citation2016; Emenike, Omole, Ngene, & Tenebe, Citation2017; Glaze, Citation1987; Glaze, Kang, & Chapin, Citation1987; Knopp, Prasse, Ternes, & Cornel, Citation2016). Reviews on the improvement of Fenton treatment method has been done by various researchers (Babuponnusami & Muthukumar, Citation2014; Ghanbari & Moradi, Citation2015; Nidheesh & Gandhimathi, Citation2012; Wang, Zheng, Zhang, & Wang, Citation2016). The high solid sludge creation from Fenton process, is associated with the widespread use of iron salts as catalyst (Garcia-Segura et al., Citation2016; Yuan, Lai, & Tang, Citation2016). The problem of extensive use of oxidant (hydrogen peroxide) for wastewater treatment in Fenton method subsequently led to the use of EF for various organic pollutant removal (Davarnejad & Bakhshandeh, Citation2017; Iglesias, Meijide, Bocos, Sanromán, & Pazos, Citation2015; Li, Jin, Zhao, Angelidaki, & Zhang, Citation2017). The EF process can be grouped into four classes, depending on the Fenton’s reagent formation (Babuponnusami & Muthukumar, Citation2014). In class 1, a sacrificial anode and an oxygen giving cathode are used to electro-generate the hydrogen peroxide and the ferrous ion respectively (Ting, Lu, & Huang, Citation2008). In class 2, hydrogen peroxide is added while the ferrous ion is produced from the sacrificial anode (Kurt, Apaydin, & Gonullu, Citation2007) as shown in Equation Equation1(1)
(1) . In class 3, ferrous ion is inputted into the system while hydrogen peroxide is generated from oxygen giving cathode (Badellino, Rodrigues, & Bertazzoli, Citation2006; Brillas & Casado, Citation2002). In class 4, hydroxyl radical (*OH) is produced by the application of the Fenton’s reagent in an electrolytic cell. The cathode, however, regenerates ferrous ions through the reduction of ferric ions (Zhang, Fei, Zhang, & Tang, Citation2007; Zhang, Zhang, & Zhou, Citation2006).
(1)
(1)
Electro-Fenton is particularly known as an efficient method for reducing chemical contaminants from effluents (Kahoush, Citation2017; Mohapatra, Brar, Tyagi, Picard, & Surampalli, Citation2014; Pintado-herrera, Biel-maeso, & Rueda, Citation2017). Activated carbon, on the other hand, makes use of different materials with the potential for carbon for removing metal ions from wastewater (Emenike et al., Citation2016). The activated carbon is also known for its treatment efficiency (Knopp et al., Citation2016; Zhao, Wang, Chen, Tian, & Zhao, Citation2017). This study, therefore, explores the use of the combined technologies of EF and granulated activated carbon (GAC) from cow bones in varied pH environment for the treatment of industrial wastewater from brewery, which is a novel approach in treating both the physical and chemical water quality parameters. This combined technology has limited coverage in literature.
2. Materials and methods
2.1. Reagent and equipment used
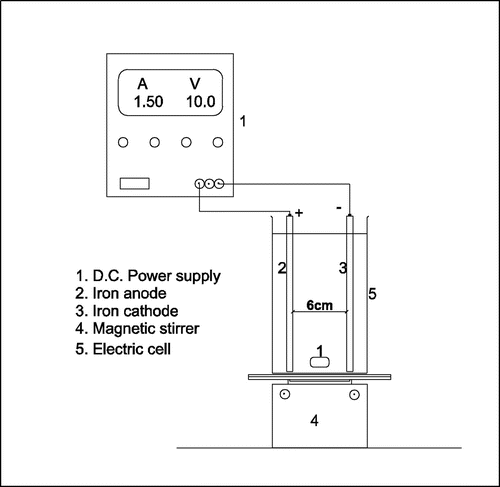
Iron (II) Sulphate Heptahydrate (FeSO4·7H2O), Sodium hydroxide (NaOH) and Tetra-oxo Sulphate (VI) acid (H2SO4), which were used during experiments, were purchased from Merck KGaA (Germany). Hydrogen Peroxide solution (H2O2) was supplied by Wine-light Analytical System Limited. Iron electrodes with thickness, length, and width dimension 2, 60, and 75 mm respectively were used as cathode and anode. The electrodes were connected to a DC power supply (KYSAN, DC Digital, 0–30 V, 0–3 A) as illustrated in Figure .
2.2. Preliminary sampling method
Duplicate effluent samples were taken from a brewery industry, located at GPS location 0523357N and 0744330E; with elevation 29.075 m along Lagos-Abeokuta expressway, Sango Otta near a tributary of the Atuwara River in Ogun state, Nigeria. The GPS location was determined using the Germin GPS Map 76 Global Positioning System (GPS). The brewery produces various malts and malt-liquor drinks daily for public consumption. Effluent generation is very high as water is an integral part of production. The effluent, which had undergone preliminary treatment prior to discharge into the river was collected from the industry’s main effluent discharge point into a sterilized 25-L plastic container. Physical parameters of the effluent such as pH, temperature and turbidity were measured in situ using Horiba U-10 multiparameter instrument. The stored effluent samples were taken to Covenant University Chemistry Research Laboratory for further analyses.
2.3. Electro-Fenton process
The EF oxidation of the wastewater was done in a 0.5-L capacity beaker. The detailed EF schematic diagram is shown in Figure . The Iron electrodes, serving as cathode and anode, were arranged in parallel. The space between the cathode and anode was 6 cm, while the effective electrode surface area was 45 cm2. The electrodes were connected to a direct current (DC) power supply and the electrochemical treatment was conducted at 10 volts, since high volts above 5 volts increase degradability of organic compounds (Ren, Zhou, Liu, Ma, & Yang, Citation2016) and 1.5 Ampere (power rating of 15 Watt). All experiments were conducted at a constant room temperature of 27 °C. The study was performed for varying pH values ranging from 2 to 6. For each experiment, the desired pH was achieved using 1 N of H2SO4 (Sulfuric acid) and subsequent incremental pH adjustments was achieved using sodium hydroxide (NaOH). The study was performed at a constant oxidant (H2O2) dose of 3000 mg/L. The EF experiments were conducted within a constant time frame of 30 min.
2.4. Granulated activated carbon process
Cow bones were obtained locally from a slaughter house in Agege, Lagos State, Nigeria. Dirt and flesh were thoroughly removed from the bone samples. Thereafter, the bone samples were washed with distilled water at 100°C, sun dried, and subsequently oven dried at 150°C for 4 h, for the removal of oily matter and moisture. A mass of 2500 g of the oven dried sample was weighed out and carbonised at 800°C (in a muffle furnace) for 3 h (pyrolysis), following which it was cooled and dried in a desiccator. The carbonised bones were subsequently crushed with a mortar and pestle. The crushed samples were passed through a sieve of 212 microns for uniformity of size. Further chemical activation of the carbonised samples was achieved by adding 250 ml of HCl (hydrochloric acid) with a concentration of 2 M (molarity) to 200 g of crushed carbonised sample until an even mixture (a paste) was formed. The paste was then transferred into a dry crucible and put into a muffle furnace at 400°C with an hourly increase of 100°C until the final temperature of 800°C. The GAC sample was then cooled to room temperature, washed with distilled water and dried for 3 h in an oven at 150°C, to remove ash particles. Finally, the dried GAC was passed through a sieve of 212 micron, once again, for size uniformity as surface area has direct effect on rate of adsorption (Emenike et al., Citation2016).
2.5. Preparation of electro-Fenton and GAC wastewater treatment system
The Ferrous Sulphate (FeSO4 ∙ 7H2O) catalyst of 0.002 M was added to the wastewater system in solid form to activate the oxidant (H2O2) in an electrolytic cell (Zhang et al., Citation2007). The EF oxidation of wastewater was conducted in an undivided reactor. The oxidant (hydrogen peroxide) was added to the wastewater treatment system (class 4, EF process). The Iron electrodes (serving as cathode and anode) were arranged in parallel and connected to a DC power supply (electrolysis). For consistency of results, the experiment was performed at constant room temperature of 27°C.
To further check the effect of the properties of the water quality parameters, the EF treated wastewater was treated with 2 g of GAC, placed on a magnetic stirrer with a revolution of 1000 revolution per minute (rpm) for 5 min after which its allowed to settle for 30 min.
3. Result and discussion
3.1. Effect on initial pH
Result of the physico-chemical characteristics of the raw effluent from the beverage industry is shown in Table . Also, results of the physico-chemical parameters of the brewery effluent post-treatment using EF alone and EF with GAC at different pH ranges of 2 to 6 are presented in Tables and . It was shown that for a treatment period of 30 min and initial pH values ranging from 2–5, the final pH and temperature values of the effluent increased. The increase in pH indicates increased production of hydroxyl ion (radical). At initial pH of 6, however, there was a marginal decrease in the final pH value to 5.94 (Table ). This slight drop in pH does not signify a reduction in the formation of the hydroxyl ion but rather attributed to the formation of colloidal ferric species such as ferric-hydroxo complexes.
Table 1. The physico-chemical parameters of the raw Brewery effluent
Table 2. Results of physico-chemical parameters after electro-Fenton treatment
Table 3. Results of physico-chemical parameters after electro-Fenton with GAC treatment
The constant increase in the effluent temperature (Tables and ) is due to the electrical energy exerted on the system (EF process). The pH of the solution was varied from 2 to 6 with the oxidant (H2O2) concentration kept constant at 3000 mg/L. Table also shows the post-treatment behaviour of the physical (conductivity, turbidity and total dissolved solids (TDS)) and chemical properties (COD and heavy metals) of the effluent with increasing pH, using EF (alone). Table , on the other hand, shows the same set of parameters using the combination of EF and GAC.
3.2. Effect on conductivity
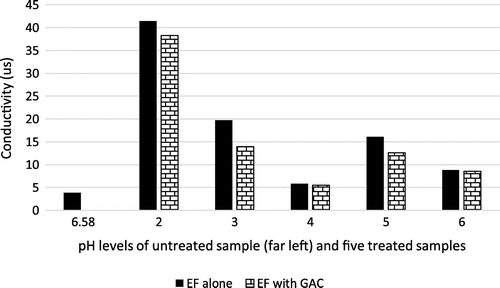
Conductivity (or specific conductance) of a solution indicates its ability to conduct electricity. Conductivity in a solution gives a measure of ionic constituent of that solution (Badejo et al., Citation2015). Figure shows that the conductivity of the solution increased drastically when the pH level was dropped to 2 from its raw state pH of 6.58; the conductivity decreased steadily as the pH level was raised until it reached pH of 4. The study suggests that use of EF or GAC for treatment of conductivity made no difference in the system. The increase in the conductivity of the raw effluent could be attributed to the ions generated from the sulfuric acid which was introduced into the solution to lower the pH. Furthermore, the combined use of EF and GAC yielded comparatively better result for all the samples than the use of EF method alone.
3.3. Effect on turbidity
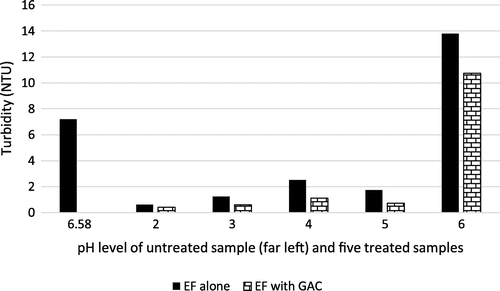
Turbidity, is a measure of clarity or cloudiness of a solution which may be brought about by suspended particles that are visible (Badejo et al., Citation2015). Turbidity is measured as nephelometric turbidity unit (NTU). Generally, Figure indicates that turbidity improved greatly when the pH of the solution was lowered between 2 and 5, with the optimum result occurring at pH 2 with a treatment efficiency of 1,181%. For EF alone, the turbidity tended to increase gradually as pH was increased. This can be attributed to the presence of ferric hydroxide precipitate and relatively redundant ferric oxyhydroxides (Fe-OOH2+) (Parsons, Citation2004). It attained its worst state at pH of 6. At this point, the amount of hydroxyl radical generation is reduced due to minimal free iron ions. After further treatment with GAC, Figure also indicates that treatment of the effluent with EF and GAC further gave better results than use of EF alone. This improvement can be explained by the added advantage of surface adsorption provided by the GAC.
3.4. Effect on total dissolved solids (TDS)
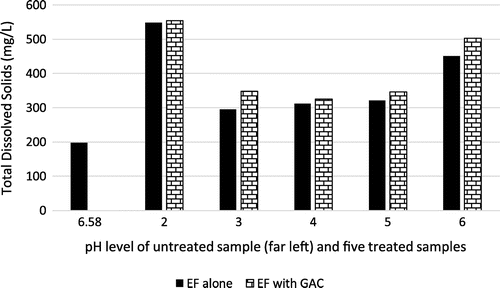
TDS is a measure of inorganic substances and small organic matter which are mostly invisible (Omole et al., Citation2017). They are contaminants, though invisible to the human eye, and they are measured in mg/L. Figure shows that TDS became worse in all treated samples than the raw effluent sample. The capacity of water (a universal solvent) to ionize substances dissolved in it, aids electrolysis. Thus, the ionization of added ferrous salts and the regeneration of the ferrous ion from ferric form increases the TDS in the solution. Also, the detected increased contaminants might have been precipitated by the introduction of the sulfuric acid and sodium hydroxide, which were used to adjust the pH levels. The observed increased turbidity might also be linked to dissolution of some particles of the GAC, when further treated. Thus, both EF and GAC are not efficient in the treatment of turbidity in the brewery wastewater.
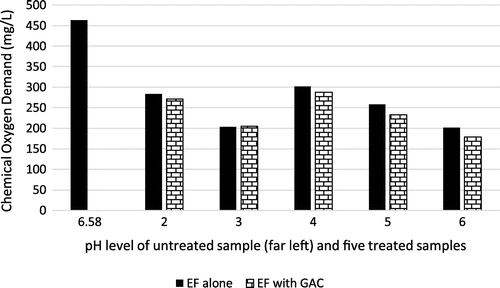
3.5. Effect on chemical oxygen demand (COD)
COD is the amount of oxygen required to break down an inorganic pollutant in water or wastewater (Badejo, Omole, Ndambuki, & Kupolati, Citation2017). Figure shows that the use of EF alone and EF with GAC led to improvement in the quality of COD in the brewery’s effluent, with the combined use of EF and GAC giving the better result in virtually all treated samples. At pH of 3, the concentration of COD was halved. This is due to high solubility of the iron species. Nevertheless, the COD removal improved just slightly across pH values of 2 to 6. This could be due to the excessive H2O2 scavenging free radicals (OH*). The optimum treatment condition, however, was achieved at pH 6 where the use of EF with GAC lowered COD by over 50%.
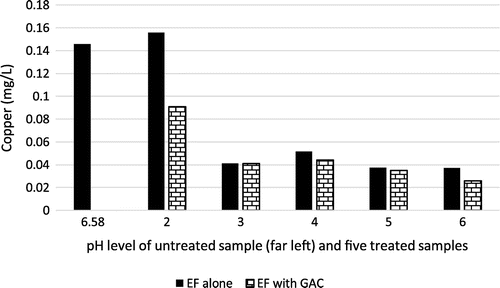
3.6. Effect on copper
Copper (Cu) is a trace metal which can be harmful to the human body if high levels of the metal are ingested. Human and environmental exposure to copper from polluted water sources is commonly reported in literature (Emenike, Omole, Ngene, Tenebe, & Maxwell, Citation2017; Solisio, Lodi, Torre, Converti, & Borghi, Citation2006). Copper causes intestinal discomfort and anaemia (Emenike, Omole, Ngene, & Tenebe, Citation2017). The effect of varying pH on copper removal in brewery wastewater was studied for both EF and combined EF and GAC treatment. From Figure , it is evident that both EF and the combined use of EF and GAC produced significant positive treatment results for copper, with the optimum treatment pH being between 3 and 6. This result is in agreement with the study conducted by Lee, Lee, Sedlak, and Lee (Citation2013) who affirm that a reaction of Cu(II) and H2O2 display better removal of impurities at pH range between 5.5–6.5. The best result, however, was achieved at pH 6 using combined EF and GAC treatment thereby giving a removal efficiency of 565%.
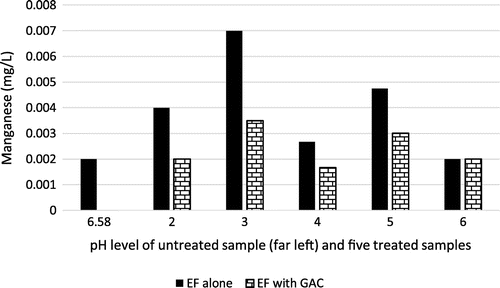
3.7. Effect on manganese
Manganese (Mn) is another trace metal that is found in air, soil or water and may lead to pneumonia or neurological problems, especially in children when they are exposed to excessive amounts (ASTDR, Citation2015). Figure indicates that use of EF and GAC was not too efficient except with the combined use of EF and GAC at pH 4 where a marginal reduction in manganese was recorded. This might be explained by the possibility the experimental pH range was not sufficient for the optimum removal of manganese. Sharifi, Hosseini, Mirzaei, and Salmani Oskuloo (Citation2015) stated that the optimum pH for removal of manganese is 9–10. The reaction of manganese in the presence of hydroxyl ions (obtainable from the hydrogen peroxide) and iron catalyst (obtainable from the sacrificial electrodes) yield manganese (II) hydroxide, which is a settleable solid that can be sieved from the solution, thus effectively reducing the Manganese content in the effluent (Sharifi et al., Citation2015).
3.8. Effect on cadmium
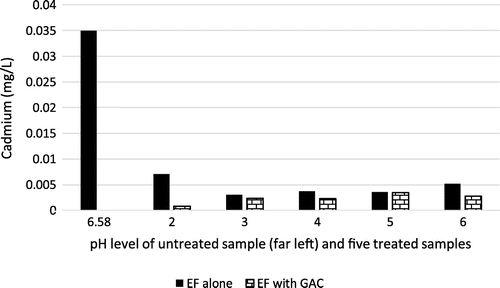
Cadmium (Cd) is a heavy metal that is very dangerous to health, even at exposure to trace amounts (Omole, Ndambuki, & Balogun, Citation2015). Adverse health effects from cadmium include kidney and liver failure. Hence, its complete removal from the aqueous environment is recommended to prevent contamination of freshwater bodies by polluted wastewater effluents. Figure demonstrates that cadmium was effectively treated using EF alone and the combination of EF with GAC. However, the optimum result was achieved with removal efficiency of 4375% using EF with GAC at pH value of 2. Cadmium was reduced from 0.035 to 0.0008 mg/L. The limit of cadmium in ingestible waster is 0.003 mg/L (Standards Organization of Nigeria, Citation2007).
Figure 8. Cadmium level of raw effluent and samples 1–5 using EF (alone) and EF + GAC at different pH level.
It is therefore advantageous to apply the EF and combine EF and GAC for Cadmium treatment in brewery’s wastewater. At atomic level, the chemical reaction can be explained by Equation Equation2(2)
(2) .
(2)
(2)
The cadmium contaminant reacts with hydroxide ions to form cadmium hydroxide (a solid material), which can thereafter be separated from the liquid.
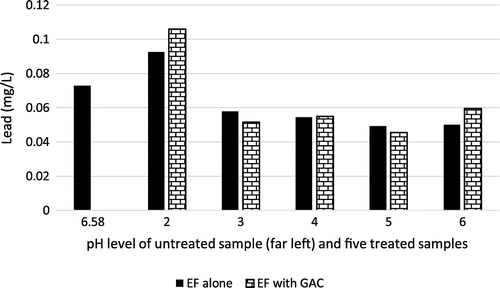
3.9. Effect on lead
Lead (Pb) is a heavy metal that may also impact negatively on central nervous system in humans, even at trace quantities, thus leading to damaged internal organs (ASTDR, Citation2017). Figure shows minimal removal of Pb from the brewery wastewater, mostly at pH level 3–6. Therefore, the concentration of Pb was better treated in a less acidic solution for both EF and combined EF and GAC treatment. The chemical equation that illustrates the removal process is shown in Equation Equation3(3)
(3) . Again, the solid is precipitated while the portion in suspended state is adsorbed from the solution by the GAC. The optimum removal efficiency of 160% occurred at pH value of 5 using EF with GAC.
(3)
(3)
3.10. Effect on zinc
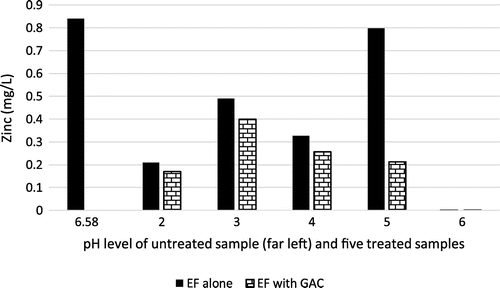
Zinc (Zn) is another trace metal that is beneficial to humans at trace levels; however when humans are exposed to levels higher than 10–15 times the permissible level for protracted periods of time, it may lead to nausea, stomach cramps, and damaged pancreas (ATSDR, Citation2015). Lower pH values seem to favour treatment of zinc when using EF and GAC (Figure ). The combination of EF and GAC generally produced better treatment results than the use of EF alone. It became evident in the sequel, that concentration of Zinc is better reduced as the pH of solution tended to neutral for both treatment (EF and GAC). A near complete removal of zinc was recorded at pH value of 6 with removal efficiency of 840%.
4. Conclusion
The study showed that combined use of EF and GAC yielded better results that use of EF alone. Obviously, the advantages of electrolysis and adsorption, which are two different wastewater treatment methods associated with EF and GAC respectively, were brought to bear on the experimental set-up. Success was recorded in the treatment of turbidity, COD, copper, cadmium, Lead, and zinc. However, conductivity, TDS, and Manganese were not significantly improved with the use of either EF or GAC in treatment. The study also showed that pH of the media for treatment of brewery effluent impacted significantly on treatment efficiency, with optimum pH for efficient treatment of turbidity and cadmium being 2, Pb being 5, and optimum pH level for reduction of COD, Cu, and Zn being 6. It is therefore recommended that the combined use of EF and GAC could be employed in place of EF alone to achieve better results in the treatment of turbidity, COD, and most heavy metals.
Funding
The authors received no direct funding for this research
Cover image
Source: Authors.
Additional information
Notes on contributors
Adebanji S. Ogbiye
Adebanji S. Ogbiye is a Senior Lecturer in the Department of Civil Engineering at Covenant University. He specialises in Water Resources and Environmental Engineering.
David O. Omole
David O. Omole is Professor of Water Resources and Environmental Engineering. He has scores of publications in reputable publications.
References
- Atallah Aljubourya, D. A. D. , Palaniandy, P. , Aziz, H. B. A. , & Feroz, S. (2015). Comparative study of advanced oxidation processes to treat petroleum wastewater. Hungarian Journal of Industry and Chemistry , 43 (2), 97–101. 10.1515/hjic-2015-0016
- ASTDR . (2015). Public health statement: Manganese . Toxic substances portal-Agency for toxic substances and disease registry. Retrieved from https://www.atsdr.cdc.gov/PHS/PHS.asp?id=100&tid=23
- ASTDR . (2017). Lead (Pb) toxicity: What are the physiologic effects of lead exposure? | ATSDR - Environmental Medicine & Environmental Health Education - CSEM . Environmental Health and Medicine - Agency for toxic substances and disease registry. Retrieved from https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=10
- ATSDR . (2015). Public health statement: Zinc . Toxic substances portal - Agency for toxic substances and disease registry (ASTDR). Retrieved from https://www.atsdr.cdc.gov/phs/phs.asp?id=300&tid=54
- Babuponnusami, A. , & Muthukumar, K. (2014). A review on Fenton and improvements to the Fenton process for wastewater treatment. Journal of Environmental Chemical Engineering , 2 (1), 557–572. 10.1016/j.jece.2013.10.011
- Badejo, A. A. , Ndambuki, J. M. , Kupolati, W. K. , Adekunle, A. A. , Taiwo, S. A. , & Omole, D. O. (2015). Appraisal of access to safe drinking water in southwest Nigeria. African Journal of Science, Technology, Innovation and Development , 7 (6), 441–445. doi:10.1080/20421338.2015.1096669
- Badejo, A. A. , Omole, D. O. , Ndambuki, J. M. , & Kupolati, W. K. (2017, February). Municipal wastewater treatment using sequential activated sludge reactor and vegetated submerged bed constructed wetland planted with Vetiveria zizanioides. Ecological Engineering , 99 , 525–529. doi:10.1016/j.ecoleng.2016.11.012
- Badellino, C. , Rodrigues, C. A. , & Bertazzoli, R. (2006). Oxidation of pesticides by in situ electrogenerated hydrogen peroxide: Study for the degradation of 2,4-dichlorophenoxyacetic acid. Journal of Hazardous Materials , 137 (2), 856–864. doi:10.1016/j.jhazmat.2006.03.035
- Brillas, E. , & Casado, J. (2002). Aniline degradation by electro-Fenton® and peroxi-coagulation processes using a flow reactor for wastewater treatment. Chemosphere , 47 (3), 241–248. doi:10.1016/S0045-6535(01)00221-1
- Davarnejad, R. , & Bakhshandeh, M. (2017). Olefin plant spent caustic wastewater treatment using electro-Fenton technique. Egyptian Journal of Petroleum . doi:10.1016/j.ejpe.2017.08.008
- Deng, Y. , & Zhao, R. (2015). Advanced oxidation processes (AOPs) in wastewater treatment. Current Pollution Reports , 1 (3), 167–176. doi:10.1007/s40726-015-0015-z
- Emenike, P. C. , Omole, D. O. , Ngene, B. U. , & Tenebe, I. T. (2016). Potentiality of agricultural adsorbent for the sequestering of metal ions from wastewater. Global Journal of Environmental Science and Management , 2 (4), 411–442. doi:10.22034/gjesm.2016.02.04.010
- Emenike, P. G. C. , Omole, D. O. , Ngene, B. U. , & Tenebe, I. T. (2017). Assessment of KOH-activated unripe Musa paradisiaca peel for adsorption of copper from aqueous solution. Cogent Engineering , 4 (1), 1–13. doi:10.1080/23311916.2017.1376488
- Emenike, P. C. , Omole, D. O. , Ngene, B. U. , Tenebe, I. T. , & Maxwell, O. (2017). Experimental investigation of copper removal from aqueous solution using Musa Paradisiaca as a novel adsorbent. WIT Transactions on Ecology and the Environment , 1, 173–179. doi:10.2495/WS170161
- Garcia-Segura, S. , Bellotindos, L. M. , Huang, Y.-H. , Brillas, E. , & Lu, M.-C. (2016). Fluidized-bed Fenton process as alternative wastewater treatment technology—A review. Journal of the Taiwan Institute of Chemical Engineers , 67 , 211–225. doi:10.1016/j.jtice.2016.07.021
- Ghanbari, F. , & Moradi, M. (2015). Journal of Environmental Chemical Engineering. A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: Electrical energy consumption and biodegradability imp. Biochemical Pharmacology , 3 (1), 499–506. doi:10.1016/j.jece.2014.12.018
- Glaze, W. H. (1987). Drinking-water treatment with ozone. Environmental Science & Technology , 21 (3), 224–230. doi:10.1021/es00157a001
- Glaze, W. H. , Kang, J.-W. , & Chapin, D. H. (1987). The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone: Science & Engineering , 9 (4):335–352. doi:10.1080/01919518708552148
- Huang, D. , Hu, C. , Zeng, G. , Cheng, M. , Xu, P. , Gong, X. , … Xue, W. (2017). Science of the total environment combination of Fenton processes and biotreatment for wastewater treatment and soil remediation. Science of The Total Environment , 574 , 1599–1610. doi:10.1016/j.scitotenv.2016.08.199
- Iglesias, O. , Meijide, J. , Bocos, E. , Sanromán, M. Á. , & Pazos, M. (2015). New approaches on heterogeneous electro-Fenton treatment of winery wastewater. Electrochimica Acta , 169 , 134–141. doi:10.1016/j.electacta.2015.04.062
- Kahoush, M. , Behary, N. , Cayla, A. , & Nierstrasz, V. (2018). Bio-Fenton and Bio-electro-Fenton as sustainable methods for degrading organic pollutants in wastewater. Process Biochemistry , 64 , 237–247.
- Knopp, G. , Prasse, C. , Ternes, T. A. , & Cornel, P. (2016). Elimination of micropollutants and transformation products from a wastewater treatment plant effluent through pilot scale ozonation followed by various activated carbon and biological filters. Water Research , 100 , 580–592. 10.1016/j.watres.2016.04.069
- Kurt, U. , Apaydin, O. , & Gonullu, M. T. (2007). Reduction of COD in wastewater from an organized tannery industrial region by Electro-Fenton process. Journal of Hazardous Materials , 143 (1–2), 33–40. doi:10.1016/j.jhazmat.2006.08.065
- Lee, H. , Lee, H.-J. , Sedlak, D. L. , & Lee, C. (2013). pH-Dependent reactivity of oxidants formed by iron and copper-catalyzed decomposition of hydrogen peroxide. Chemosphere , 92 (6), 652–658. doi:10.1016/J.CHEMOSPHERE.2013.01.073
- Li, X. , Jin, X. , Zhao, N. , Angelidaki, I. , & Zhang, Y. (2017). Novel bio-electro-Fenton technology for azo dye wastewater treatment using microbial reverse-electrodialysis electrolysis cell. Bioresource Technology , 228 , 322–329. doi:10.1016/j.biortech.2016.12.114
- Mirzaei, A. , Chen, Z. , Haghighat, F. , & Yerushalmi, L. (2017). Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes – A review. Chemosphere , 174 , 665–688. doi:10.1016/j.chemosphere.2017.02.019
- Mohapatra, D. P. , Brar, S. K. , Tyagi, R. D. , Picard, P. , & Surampalli, R. Y. (2014). Analysis and advanced oxidation treatment of a persistent pharmaceutical compound in wastewater and wastewater sludge-carbamazepine. Science of The Total Environment , 470–471, 58–75. doi:10.1016/j.scitotenv.2013.09.034
- Nidheesh, P. V. , & Gandhimathi, R. (2012). Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination , 299 , 1–15. doi:10.1016/j.desal.2012.05.011
- Omole, D. O. , Alade, O. O. , Emenike, P. C. , Tenebe, I. T. , Ogbiye, A. S. , & Ngene, B. U. (2017). quality assessment of a university campus wastewater resource. WIT Transactions on Ecology and the Environment , 1, 193–201. 10.2495/WS170181
- Omole, D. O. , Isiorho, S. A. , & Ndambuki, J. M. (2016). Waste management practices in Nigeria: Impacts and mitigation. In G. R. Wessel & J. K. Greenberg (Eds.), Geoscience for the public good and global development: Toward a sustainable future: Geological society of america special paper 520 (vol. 520, pp. 377–386). Boulder, CO: The Geological Society of America. doi:10.1130/2016.2520(33)
- Omole, D. O. , Ndambuki, J. M. , & Balogun, K. (2015). Consumption of sachet water in Nigeria: Quality, public health and economic perspectives. African Journal of Science, Technology, Innovation and Development , 7 (1), 45–51. doi:10.1080/20421338.2014.979654
- Omole, D. O. , Ndambuki, J. M. , Nwafor-Oritzu, C. A. , & Obata, C. E. (2014). Development of a water treatment plant for heavy metal adsorption. In Proceedings of the IASTED International Conference on Environment and Water Resource Management, AfricaEWRM 2014, No. AfricaEWRM (pp. 1–5). doi:10.2316/P.2014.812-002
- Omole, D. O. , & Ogugua, C. D. (2017). Use of pelletized waste electronic plastic casings in sorbing metals from water. Journal of Materials and Environmental Science , 8 (1), 60–66.
- Parsons, S. (2004). Advanced oxidation processes for water and wastewater treatment . London: IWA publishing.
- Pintado-herrera, M. G. , Biel-maeso, M. , & Rueda, J. J. (2017). Environmental risk assessment of Ef Fl uents as a whole emerging contaminant: Ef Fi ciency of alternative tertiary treatments for wastewater depuration. Water Resources , 119 , 136–149. doi:10.1016/j.watres.2017.04.021
- Ren, G. , Zhou, M. , Liu, M. , Ma, L. , & Yang, H. (2016). A novel vertical-flow electro-Fenton reactor for organic wastewater treatment. Chemical Engineering Journal , 298 , 55–67. doi: 10.1016/j.cej.2016.04.011
- Sharifi, S. L. , Hosseini, M. H. , Mirzaei, A. , & Salmani Oskuloo, A. (2015). Catalytic decomposition of hydrogen peroxide in the presence of synthesized iron- manganese oxide nanocomposites via different methods. International Journal of Nanoscience , 11 (4), 233–240.
- Solisio, C. , Lodi, A. , Torre, P. , Converti, A. , Borghi, M. D. (2006). Copper removal by dry and re-hydrated biomass of Spirulina platensis. Bioresource Technology , 97 (14), 1756–1760. doi:10.1016/J.BIORTECH.2005.07.018
- Standards Organization of Nigeria . (2007). Nigerian standard for drinking water quality . Abuja: Nigerian Industrial Standard.
- Ting, W.-P. , Lu, M.-C. , & Huang, Y.-H. (2008). The reactor design and comparison of Fenton, electro-Fenton and photoelectro-Fenton processes for mineralization of benzene sulfonic acid (BSA). Journal of Hazardous Materials , 156 (1–3):421–427. doi: 10.1016/j.jhazmat.2007.12.031
- Wang, N. , Zheng, T. , Zhang, G. , & Wang, P. (2016). Journal of Environmental Chemical Engineering. A Review on Fenton-like processes for organic wastewater treatment. Biochemical Pharmacology , 4 (1), 762–787. doi: 10.1016/j.jece.2015.12.016
- Yuan, Y. , Lai, B. , & Tang, Y.-Y. (2016). Combined Fe 0/ air and fenton process for the treatment of dinitrodiazophenol (DDNP) industry wastewater. Chemical Engineering Journal , 283 , 1514–1521. doi: 10.1016/j.cej.2015.08.104
- Zhang, H. , Fei, C. , Zhang, D. , & Tang, F. (2007). Degradation of 4-nitrophenol in aqueous medium by electro-Fenton method. Journal of Hazardous Materials , 145 (1–2), 227–232. 10.1016/j.jhazmat.2006.11.016
- Zhang, H. , Zhang, D. , & Zhou, J. L. (2006). Removal of COD from landfill leachate by electro-Fenton method. Journal of Hazardous Materials , 135 (1–3), 106–111. 10.1016/j.jhazmat.2005.11.025
- Zhao, H. , Wang, Q. , Chen, Y. , Tian, Q. , & Zhao, G. (2017). Efficient removal of dimethyl phthalate with activated iron-doped carbon aerogel through an integrated adsorption and electro-Fenton oxidation process. Carbon , 124 , 111–122. 10.1016/j.carbon.2017.08.034