?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This paper presents the study of an implantable biomedical device for the localized released of chemotherapeutic drugs and the controlled heating of surrounding tumor tissue to enable cancer treatment via a hyperthermia and chemotherapy combination. The coupling of magnetic induction, heat transfer, and mass diffusion concepts used to model temperature changes and drug release from the biomedical device to a surrounding environment that mimics breast tumor tissue and normal breast tissue. The predictions of temperature change in the residual tumor cells and the normal breast tissue show that when an excitation current of 25 mA supplied to the device generates heat that required to kill the residual cancer cells without damaging the nearby healthy tissue. Also, the predictions of prodigiosin concentration released from the biomedical device into selected depths in the breast phantom model show that the residual tumor has a higher concentration than the healthy tissue. The proposed system proved capable for prolonged drug delivery and temperature rise of tumor to therapeutic values for effective localize cancer treatment.
Public Interest Statement
Cancerous cell destruction using heat and chemotherapeutic drug delivered by biomedical devices at a target region is receiving attention due to the enhance efficacy and potential for localize treatment. This work presents a computational simulation on a clinical scenario with a device fabricated in our research lab that can eliminate issues such as inadequate drug concentrations reaching the primary tumor site, poor rise in temperature and skin injury and enhance the efficacy of treatment. The device comprises a resistive layer (heater) and a drugloaded thermosensitive poly (N-isopropyl acrylamide) gel, embedded within a fabricated polydimethylsiloxane (PDMS) shell. Hyperthermia condition in the implant is through inductive heating. Our predictions show that the diffused drug concentrations and the temperature changes are high enough to achieve cell death in residual tumor cells and a minimal effect on normal breast tissue. Our proposed device offers the possibility of results translate into an effective therapeutic strategy for breast cancer.
1. Introduction
Cancer is a generic term for a large group of diseases that can affect any part within or on the body. Current scientific evidence suggests that cancer can be triggered by environmental and genetic factors (Alberts et al., Citation2008). Cancer is also the second-leading cause of morbidity and mortality throughout the world (Bray, Jemal, Grey, Ferlay, & Forman, Citation2012; Ma & Yu, Citation2006; Vyas et al., Citation2012). Furthermore, the incidence of cancer is expected to continue the increase in the next few decades (van Vlerken, Vyas, & Amiji, Citation2007). According to the World Health Organization’s (WHO) International Agency for Cancer Research (IARC), the incidence of cancer worldwide is predicted to increase to 75% by the year 2030. Hence, the exact survival rate of cancer patients is heavily dependent on early diagnosis and treatment (Kumar, Mazinder Boruah, & Liang, Citation2011). The standard treatment methods, such as bulk systematic chemotherapy (Hildebrandt & Wust, Citation2007; Oni, Theriault, Hoek, & Soboyejo, Citation2011; Perry, Citation2011) surgery (Kubota, Citation2011; Schwartz et al., Citation2009; Simmonds et al., Citation2006) and radiotherapy, has exhibited severe side effects (Zhou, Citation2014). There is, therefore, a need for new cancer treatment methods that can mitigate these side effects. Within this context, a novel drug delivery system can enable more specific and effective targeting of cancer cells/tissue, while reducing or avoiding the potential effects of cancer treatment on normal cells. It could also increase the patient quality of life while motivating a multidisciplinary challenge that calls for collaboration between clinicians, biologists, materials scientists, biomedical engineers, and physicists (Kavitha & Bhalamurugan, Citation2013; Saini, Chouhan, Bagri, & Bajpai, Citation2012).
A few research groups have explored hyperthermia as a treatment modality (Marchosky et al., Citation1990; Zhao et al., Citation2013) due to its minimal side effects, and its potential to enhance the therapeutic efficacy of, conventional cancer treatments (Habash, Bansal, Krewski, & Alhafid, Citation2006; Kan-Dapaah, Rahbar, & Soboyejo, Citation2014; Moroz, Jones, & Gray, Citation2002). Within the last decade, researchers have developed multiple delivery modalities for hyperthermia in both in vitro and in vivo conditions (Heine, Sverak, Kondratick, & Bonar, Citation1971; Kase & Hahn, Citation1975; Love, Soriano, & Walsh, Citation1970). Furthermore, the use of metallic nanoparticle-based hyperthermia has been shown to have the potential for future strategies (Choi & Wang, Citation2011). These include gold nanoparticles that can be delivered and embedded into the tumor tissue region where they can be used to induce local heating when exposed to Near Infrared (NIR) laser beams. Such heating, which is associated with Plasma resonance effects, can be used to raise the temperature of tumor tissue to levels that can kill cancer cells without destroying surrounding healthy tissue (Day, Morton, & West, Citation2009; Lee, Chatterjee, Lee, & Krishnan, Citation2014). Hirsch et al. (Citation2003) carried out in vitro studies on SK-BR-3 human breast carcinoma cells incubated with the gold nanoshells/polyethylene glycol (PEG) for 1 h, and then exposed to NIR laser beams. They observed lost cell membrane integrity and cell death from the fluorescence images of treated cells. O’Neal, Hirsch, Halas, Payne, and West (Citation2004) reported impressive results in in vivo study that showed selective photothermal ablation of tumors embedded with near infrared-absorbing gold nanoshells in mice. Magnetic rods or needles have also been explored as excitable sources of heat, (Shinohara, Citation2004; Yukumi et al., Citation2008) while other radio-frequency delivery modalities have been developed (Koreckij et al., Citation2010; Raoof & Curley, Citation2011; Zhou, Citation2013). These include; high intensity focused ultrasound techniques (Orsi, Arnone, Chen, & Zhang, Citation2010; Zavaglia, Mancuso, Foschi, & Rampoldi, Citation2013) and high-frequency eddy currents (Gaitas & Kim, Citation2015; Yukumi et al., Citation2008).
Similar to other treatment modalities, the clinical objective of hyperthermia is to induce cell death of tumor-bearing tissue. Thus, localized hyperthermia can play an important role in enhancing the efficacy of anticancer drugs at the specific site of a tumor. For example, Rodriguez-Luccioni et al. (Citation2011) applied an alternating magnetic fields (AMFs) to magnetite nanoparticles in MCF-7 breast cancer cell tissue. Their work showed that magnetic hyperthermia was more effective at reducing cancer cell viability compared to hot water-induced hyperthermia. Hilger et al. (Citation2002) have also studied an immunodeficient SCID mouse model with intra-tumoral injections of iron oxide particles that were subjected to applications of AMFs. Histological examination of the mice revealed clear evidence of the early stages of coagulation necrosis in the treated tumor cells. Combinations of localized chemotherapy and hyperthermia have also been studied by a number of investigators (Berjano, Citation2006; Gasselhuber et al., Citation2010; Hayashi et al., Citation2010; Huang & Liauh, Citation2011; Landon, Park, Needham, & Dewhirst, Citation2011). These include in vitro and in vivo studies by several researchers (Landon et al., Citation2011; Mura, Nicolas, & Couvreur, Citation2013; Owusu, Abern, & Inman, Citation2013). Gautier et al. (Citation2012) formulated a poly-ethylenes-glycol-conjugated superparamagnetic iron oxide (PEG-SPIO) loaded with doxorubicin (DOX). These were heated by the application of alternating magnetic fields under in vitro conditions. Shah, Majeed, Shafique, Rashid, and Awan (Citation2013) also formulated doxorubicin-loaded thermo-responsive P(NIPA-co-Am) coated MnFe2O4 nanoparticles. These were used to study cell viability of HeLa carcinoma cells exposed to alternating magnetic fields. They discovered that at 45°C, the magnetic hyperthermia and drug delivery reduced the cell viability to 16% in 6 h. Morita, Zywietz, Kakinuma, Tanaka, and Katoh (Citation2008) have also investigated the efficacy of the drug-loaded thermo-sensitive liposomes combined with localized hyperthermia, on rat tumor models. Hayashi et al. (Citation2010) synthesized smart nanoparticles of a clustered Fe3O4/polymer that was loaded with doxorubicin. These were heated with alternating magnetic fields that resulted in improved studied therapeutic efficacy due to the combined effects of hyperthermia and chemotherapy on myeloma tumor. Hayashi et al. (Citation2010) showed that the cancer cells in the entire tumor were destroyed. They were also to achieve a complete “cure” in one treatment, without the recurrence of malignancy.
Several mathematical and computational models have been developed for the tumor prediction of heating and controlled drug delivery from biomedical devices (Huang & Liauh, Citation2011; Timko et al., Citation2014; Weinberg, Patel, Exner, Saidel, & Gao, Citation2008). These have been used to model the thermal doses (Berjano, Citation2006), temperature distributions (Huang & Liauh, Citation2011), and the concentration of drugs released to the tumors or residual tumor cells/tissue (Qian, Stowe, Liu, Saidel, & Gao, Citation2003). Qian et al. (Citation2003) have developed a mathematical model in the prediction of doxorubicin transport from polymer Milli-rods subjected to radiofrequency-induced thermo-ablation. Their results showed the influence of tissue ablation and devascularization on the transport of doxorubicin. Gasselhuber et al. (Citation2010) used a paired heat transfer and pharmaco-kinetic mathematical model to study drug delivery in a multi-compartment model consisting of low-temperature sensitive liposomes/tumor plasma. They also studied drug delivery in systemic plasma/tissue models. Their simulations showed that the thermal ablation of doxorubicin-loaded liposomes enhanced localized drug delivery into tumor tissue, compared to drug delivery via conventional chemotherapy. Prior work in our research group (Danyuo et al., Citation2014; Oni et al., Citation2011) has explored the development of an implantable anti-cancer treatment device that can deliver anticancer drugs locally while heating tumor cells/tissue. Such a device can be used, following surgery, to kill cancer/tumor tissue by localized chemotherapy and hyperthermia. This paper presents the results of a computational study of controlled drug diffusion and hyperthermia from a novel implantable biomedical device for localized cancer therapy (Danyuo et al., Citation2014; Oni et al., Citation2011). Finite element model (of the device and surrounding tissue) is developed and used to validate our prior in vitro experimental results (Danyuo et al., Citation2014). The model is also used to estimate the concentrations of prodigiosin drug released from the smart thermo-sensitive hydrogels via micro-channels to be transported to surrounding cancer cells/tissue. The implications of the results are then discussed in the development of future devices for the localized treatment of cancer/tumor tissue.
2. Methods
2.1. Device fabrication
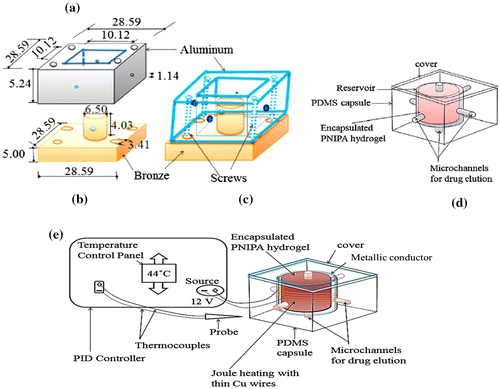
The gels were prepared by free radical polymerization, as described in Danyuo et al. (Citation2014). Briefly, molds for the processing of PDMS packages were fabricated from aluminum and bronze metal slabs that were fabricated at Princeton University (Figure (a)–(c)). The outer section was fabricated from aluminum (dimensions between 10.12 and 15.89 mm), while the internal dimensions varied between 9.66 and 13.00 mm. Bronze was machined into cylindrical rods at the middle section that contained the reservoir for the drug carrier polymer, P (NIPA). The cylindrical rods had diameters of 4.00–7. 06 mm, and heights of about 5. 24–11.20 mm. Holes with a diameter of 1.12 mm were drilled into four locations in the aluminum mold, while similar holes were drilled into the bronze cylindrical rod (Figure (a)–(b)). These were introduced to enable the molding of microchannels (obtained after the molding process) to provide a path for drug diffusion from the encapsulated hydrogel. These paths enabled drugs (from the loaded gels) to be delivered to the surrounding tissue in a controlled manner.
Figure 1. Schematics of mold and PDMS components; (a) aluminum slab; (b) brass slab with central cylindrical rod; (c) fabricated mold; (d) encapsulated biomedical device with micro-channels for drug elution; and 1(e) hyperthermia system with a PID temperature controller (used to set and monitor local temperatures). Reused with permission from Danyuo et al. (Citation2014), Materials Science and Enginering C, published by Elsevier.
PDMS packages with different channel lengths and reservoirs were fabricated by mixing Sylgard 184 kit, silicone elastomer with a silicone elastomer curing agent (a crosslinker) (Sylgard Dow Corning, Krayden Inc., Midland, Michigan, USA). These were mixed in a ratio of 10:1 (%v/v). The mixture was stirred vigorously, de-gassed with a GALVAC vacuum oven (LTE Scientific Ltd., Greenfield State) set at -24 mm Hg equivalent, with no heat, for an hour.
A complete mold was fixed by the aid of nuts and bolts, while 1.12 mm diameter thick surgical needles were passed through the four faces to produce the microchannels. In order to induce temperature responsiveness in the gels, 5–10 turns of thin copper wire (0.1 mm diameter) were incorporated into some devices to induce Joule heating degassed PDMS was poured gently into the fabricated molds. The samples were then cured at 60°C for 3 h before exposing them to room-temperature (28°C) for 12 to 24 h.
2.2. Encapsulation of P (NIPA) into PDMS capsules
Although P(NIPA) -based hydrogels have been shown to have improved biocompatibility, compared to P(NIPA) solids, (Vihola, Laukkanen, Valtola, Tenhu, & Hirvonen, Citation2005) a special effort was made to encapsulate the non-biodegradable P(NIPA) -based hydrogels into PDMS packages with reservoirs. The PDMS capsules consisted of Sylgard 184 kit, silicone elastomer and a silicone elastomer curing agent of 10:1 (%v/v) (Sylgard Dow Corning, Krayden Inc., Midland, Michigan, USA). Drug-loaded P(NIPA)-based hydrogels were then inserted into the reservoir of the PDMS capsules. These were then, sealed using a clamping device that was used to apply a slight pressure to the axes that were perpendicular to the edges. Sealed packages were then incubated at 40°C for 24 h, to ensure that the two layers were well bonded to each other.
2.3. Description of the proposed device
The implantable device was fabricated from a poly (dimethylsiloxane) (PDMS) -based elastomer that was used to encapsulate the drug-loaded P(NIPA) gels. The PDMS capsule had a 1 mm channel diameter, 1.5 mm channel lengths and a cylindrical reservoir volumeof 11.8 μm3 containing a drug loaded hydrogel (Figure (a)–(c)). The polymerized P(NIPA) hydrogels were soaked with cancer drugs (prodigiosin (PG) before inserting them into the PDMS capsules (Figure (d)).
2.4. Principle of operation
The microchannels, fabricated within the device (Figure (e)) enabled the P(NIPA) gels to load cancer drugs in solution at room temperature (28°C). The device was subjected to Joule heating at (37, 41, 43, and 45°C). The heating was controlled using a Proportional Integrated Differential (PID) controller that was used to simulate potential exposures to normal body temperature (37°C) and hyperthermia temperature ranges (41–45°C). The dissolved cancer drugs were eluted from the P(NIPA) gels were then delivered through the microchannels into the surrounding regions/tissue at physiological temperature (37°C), or hyperthermia temperatures in 41, 43, and 45°C.
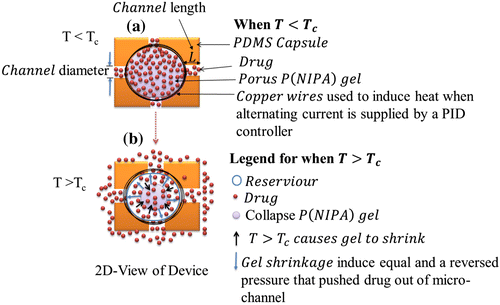
The release mechanism of drugs involved the diffusion of drug molecules from the temperature sensitive hydrogels through the microchannels. A typical P(NIPA) -based homopolymer experiences a volumechange during phase transition at the lower critical solution temperature (LCST) (Figure ) which depends on the amount of acrylamide (AM) (a hydrophilic co-monomer) in the P(NIPA) -based gels (Danyuo et al., Citation2014). This leads to the LCST of the hydrophilic copolymer increasing from 36.3°C at 5 mol % of AM to 41.7°C at 15 mol % of AM, which the pure P(NIPA) has an LCST of 33°C. In principle, when the temperature, T, in the device is less than the LCST, (as shown in Figure (a)), less pressure would be induced in the gel, which leads to a little/no drug release. However, when the gel temperature is greater than the LCST, the encapsulated gel shrinks (Figure (b)), which causes ~90% of the encapsulated drug to be released in a controlled manner through the microchannels (Danyuo et al., Citation2014). Hence, drug molecules are released from the device when T ≥ LCST,
. Hence, controlling the LCST using the control of the LCST via copolymerization with AM, as presented in recent work by Danyuo et al. (Citation2014, Citation2016) guides the choice of gel before encapsulation.
Figure 2. Phase Diagram for P(NIPA)-based Hydrogel in Water. Reused with permission from Yiporo Danyuo (2015), AUST.
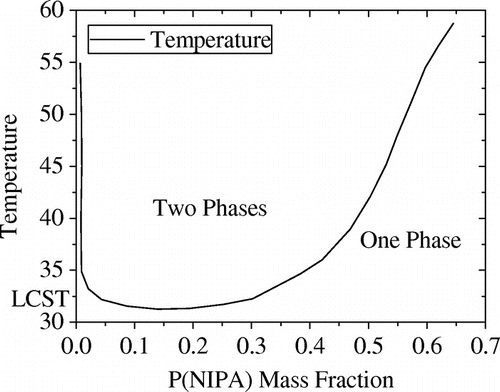
Figure 3. Operation of the Device Below and Above the LSCT: (a) When the Application Temperature is Less Than the T < LCST, Little or No Drug is Released and (b) Drug Molecules Released from the Hydrogel when T ≥ LCST.
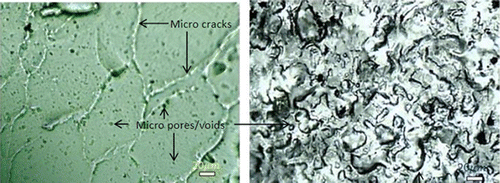
The drug kinetic studies by Danyuo (Citation2015) shows that the porous structures of the PNIPA gels (with a mesh size of about 0.5–70 μm) allows prodigiosin drug diffusion (Figure ). The PNIPA microstructure does not hinder the flow of prodigiosin molecules because of their small hydrodynamic radii, as compared to the polymer mesh size , thus, the drug release process is dominated by diffusion. The effective diffusion coefficients of drug released from the device were determined experimentally, based on the times required for the fluid to flow to occur across devices with different channel lengths (Danyuo et al., Citation2014). For such diffusion-controlled flow, a plot of L
2 vs. t gives a straight line with a gradient that is equal to the effective diffusion coefficient, has been reported by Danyuo et al. (Citation2014). This gives:
(1a)
(1a)
(1b)
(1b)
Figure 4. LCD Microscopy Analysis of PNIPA Based Hydrogels: (a) Homopolymer (100 mol% of NIPA and (b) 95 mol% of P (NIPA)-5 mol% of AM. Reused with permission from Danyuo et al. (Citation2016), Materials Science and Enginering C, published by Elsevier.
where L is the channel length, D is the effective coefficient of diffusion and t is the time taken for the fluid to flow across the channel length.
In recent work of our group (Danyuo et al., Citation2014), the linear dependence (r 2 = 0.97) of L 2 on t, yielded a slope corresponding to an effective diffusivity of 2.0 × 10−8 m2/s. It was noticed that devices with shorter channel lengths could facilitate the release of drug molecules from the device into the treatment area. Moreover, the shorter channel lengths also require less pressure to pump drugs from the device into the tumor tissue, when compared to devices with longer channels.
In conclusion, drug delivery through the channels can best be described by a diffusion process. It is also clear from the current study that the final release rate of drugs into the tumor tissue could be managed by the diffusion across the channels. Controlling the channel dimensions is particularly important for the control of initial burst effects during the early stages of drug release from the P(NIPA) -based gels. This also helps to, enable extended drug delivery, instead of the direct delivery of drugs from the gel matrix to surrounding tissue.
2.5. How the device may be used in a clinical scenario
Hence, this study suggests that temperature-responsive P(NIPA) -based hydrogels, encapsulated within PDMS-capsules, can enhance the controlled and extended delivery of cancer drugs to targeted tumors or cancer cells. The encapsulated can be inserted via surgery prior to the localized delivery of cancer drugs via diffusion through the microchannels. Furthermore, the diffusion time can be controlled by varying the channel length. In this way, the time period and the rate of diffusion (of the proposed device) can be tuned to be within relevant ranges required for cancer treatment. A schematic of how the device could be used is presented in Figure . Following the removal of a solid tumor, the device could be implanted directly in the resected region to enhance the killing of residual cells, since there is no guarantee that all the cancer cells are removed via surgery. On the other hand, the device could also be implanted at a tumor location to shrink the tumor via the localized release of cancer drugs. Such shrinkage could also be enhanced via Joule heating using the embedded copper wires that can be resistively or inductively heated (Danyuo et al., Citation2014; Theriault et al., Citation2012). The resulting heat enables the hydrogel to collapse at temperatures close to or above its transition temperature. Furthermore, the heat produced could enhance adjuvant effects of cancer treatment.
Figure 5. Schematic of How the Device Could be Used: (a) When the Device Temperature is Less Than the Lower Critical Solution Temperature (LCST) (T < LCST), Little or No Drug Will Be Released; (b) Drug Molecules Released from the Hydrogel when T
LCST; (c) Heat Trigger Mechanism Causes Loaded Cancer Drugs to be Release from the Thermo-sensitive Hydrogel; (d) Implanted Device at tumor location, and (e) Schematic of a Female Breast Containing Solid tumor.
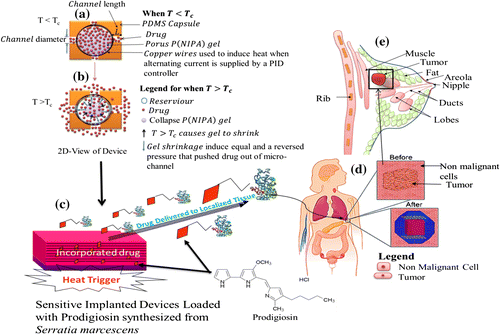
It is important to note here that the device could be removed after drug delivery and hyperthermia. The device may also be left in the body since PDMS is biocompatible (U. S. FDA approved) and poses no toxicity threat in long-term applications in humans. However, some surface change of the device may be needed to ensure good integration with the surrounding breast tissue.
2.6. Modeling
In this study, a computational model of an implantable biomedical device was developed. The model includes drug and thermal diffusion from a recently fabricated device (Danyuo et al., Citation2014). The objective of the treatment was to maintain a uniform temperature distribution and therapeutic level of diffused anti-cancer drug in the tumor tissue. To simulate clinical scenarios, we assume that the biomedical device was inserted into a breast tissue phantom, as shown schematically in Figure (a). The breast model is assumed to be hemispherical in shape with short and long axes diameters of 90 and 95 mm. The device was implanted into the breast at a depth of 23.6 mm, with its center on the z-axis (see Figure (a)), after surgical excision of a 1 cm tumor (Stage I). Figure (b) shows how the proposed biomedical device could perform during the hyperthermia process.
Figure 6. (a) Two-dimensional Model of Breast Phantom with a Long Axis Diameter of 30 mm and a Short Axis Diameter of 25 mm, (b) Schematic of Clinical Scenarios for hyperthermia treatment.
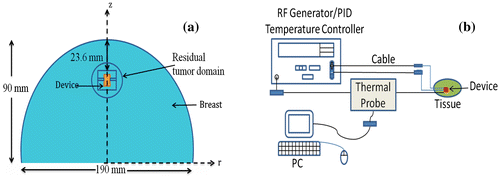
However, the main components used in the hyperthermia system are a current controlled generator, and flexible coaxial cables connecting the thin copper coils to the generator (see Figure (b)). As the RF generator reads the specified settings of the controller, an electrical excitation flows into the coils of the device via the connecting cable from the generator. This then produces heat via inductive heating, which is transferred to nearby tumor tissue to achieve hyperthermia. A thermocouple/fiber optic thermometer (in the system) is used to monitor the tissue temperature.
2.7. Hyperthermia modeling
Induction heating was accomplished using thin copper wires that were embedded initially in the PDMS package, as shown in Section 2.1. Five turns of thin copper wires (0.1 mm diameter) then used to generate alternating magnetic fields via exciting currents (Figure (e)). The change in magnetic flux produced eddy currents, which are induced in the cylindrical metallic conductor. The induced eddy currents interact with the resistance of the conductor through Joule’s law, causing Joule heating. In this model, the net effect of the induction heating equations is given by Dedulle (Citation2006), Toi and Takagaki (Citation2008)
(2)
(2)
where A
φ
Is the magnetic vector potential, is the angular frequency of oscillating magnetic flux, ɛ is permittivity,
is permeability, and
is the electrical conductivity. The function
for copper is given by
(3)
(3)
where is the resistivity at the reference temperature, α is the thermal coefficient of the resistivity and T
ref is the reference temperature.
The boundary conditions for Equation (2) are: axial symmetry, r = 0; outer edges of the PDMS under magnetic insulation, A
φ
= 0; induction coils and the conductive plate are under the continuity condition: .
The temperature distribution in solids, due to an external heat source, can be determined by Fourier heat conduction equation. This is given by Welty, Charles, Wilson, and Rorrer (Citation2000)
(4)
(4)
where is density,
is the specific heat capacity,
is the thermal conductivity, and
the heat generation term, per period of a sinusoidal function, which is responsible for the change in the temperature inside the biomedical device.
is given by Rossmanna and Haemmerich (Citation2014):
(5)
(5)
where E
p
is the peak value of electric field and is the electrical conductivity.
For biological tissue, the Pennes model (Kan-Dapaah et al., Citation2014; Pryor, Citation2009) modifies Equation (3) by including the effects of heat generation due to constant blood perfusion (BP), and the metabolic heat,
. Q
b
is defined as:
(6)
(6)
where
ρ
b
and T are the perfusion rate, specific heat capacity, density, temperature of blood and temperature of biological tissue, respectively.
The boundary conditions for the inductive heat transfer process (Equation 3) are axial symmetry (r = 0), ; temperature, T = 37°C, at the chest wall; heat transfer by convection between the surface of the breast, external environment,
where the heat transfer coefficient
, T
ext = 20°C; and continuity condition,
on the internal boundaries. The initial temperature in all the domains of the model was set to the normal body temperature of 37°C.
In addition, four different exciting currents, with an interval of 5 mA, were considered to flow through the coils of wires in the device. These were used in the numerical simulations to map out the temperature distributions in the models. The electrical currents (at 480 kHz) were set below the 100 mA (at 50 Hz) to avoid conduction that can cause ventricular fibrillation (Ni, Mulier, Miao, Michel, & Marchal, Citation2005).
Due to pronounced changes in blood perfusion, the thermal conductivity of living tissues can increase with temperature rise (Rossmanna & Haemmerich, Citation2014). The temperature-dependence of blood perfusion for normal tissue and tumor tissue can be expressed as (Drizdal, Togni, Visek, & Vrba, Citation2010; Lang, Erdmann, & Seebass, Citation1999; Sawicki & Miaskowski, Citation2014):(7a)
(7a)
(7b)
(7b)
The temperature-dependent thermal conductivity of biological tissues is modeled as linear functions. They are expressed as (Duck, Citation1990; Zhu, Shen, Zhang, & Xu, Citation2013):(8)
(8)
where is the thermal conductivity at 293.15 K. The properties of the materials that were used in the simulations were obtained from data reported in the literature (Bezerra et al., Citation2013; Blanco et al., Citation2012; Ekstrand et al., Citation2005; Goodfellow, Citation1993) and summarized in Table .
Table 1. Materials properties
2.8. Thermal damage
Although it is generally accepted that tissue damage is an outcome of several complex mechanisms, Thermal damage in the tissue model was predicted using the Arrhenius law (Bhowmik, Repaka, Mishra, & Mitra, Citation2015). However, the progression of thermal damage can be reasonably approximated by a single process that is described by a first order kinetics-thermal equation. This is given by (Bhowmik et al., Citation2015).
(9)
(9)
where , is the degree of biological tissue damage,
is the initial concentration of healthy biological cells,
is the concentration of healthy biological cells remaining after thermal stimulation, R (8.315
) is the universal gas constant, A is a frequency factor for the kinetic expression (1/s), and
is the activation energy of the thermal damage process
, and T °C is the instantaneous absolute temperature of the cells during thermal stress, which is a function of time, t (s). The parameters A and ΔE are dependent on the type of tissue and have been characterized for normal breast tissues by Henriques and Moritz (A = 3.1 × 1098 s−1 and ΔE = 6.28 × 108
) (Gould, Wang, & Pfefer, Citation2014; Henriques & Moritz, Citation1947) and breast tumor tissues (A = 1.8 × 1051 s−1 and ΔE = 3.27 × 105
) (Bhowmik et al., Citation2015).
The tissue injury integral increase as the time of exposure is increased. The critical value, , indicates that a sufficient irreversible thermal damage has been achieved. This corresponds to a viable cell concentration of 37%, which indicates a 63% cell necrosis volume. In its original formulation, the Arrhenius equation was associated with the percentage of a volumeof cells surviving a uniform exposure to temperature for a length of time. However, when the volumeconstitutes a single cell, the Arrhenius equation reflects the percent probability of cell survivability (Garcia, Davalos, & Miklavcic, Citation2014). The probability of tissue cell death, P (%), is then expressed as (Garcia et al., Citation2014):
(10)
(10)
2.9. Modeling of drug release
The governing equation for drug release in the implantable biomedical device is given by (Kothandaraman, Citation2006; Mikhailov & Ozisik, Citation1984)
(11a)
(11a)
where C is the concentration of prodigiosin, D (m2/s) is the diffusion coefficient of prodigiosin released. The diffusion coefficient of the prodigiosin drug in temperature sensitive PNIPA is given by:
(11b)
(11b)
where D o is the diffusion constant, R is the universal gas constant, T is temperature, and E a is the activation energy of the hydrogel (Danyuo et al., Citation2014).
At the initial time the three (PNIPA) -base hydrogels were assigned the following prodigiosin concentration
462 × 10−9, 231 × 10−9 and 77 × 10−9 mol/m3, respectively. These were used for the simulations. The boundary conditions during the diffusion process in the device are: axial symmetry condition along the rotational axis (r = 0),
= 0; no mass loss or diffusion of drug into the PDMS domain, assumed an insulation (impermeable) condition, n ⋅ N = 0, and continuity (
. There were enforced on all of the interior boundaries. The diffusion coefficients of P(NIPA) (100 mol %) at 37, 43 and 45°C were obtained from our prior experimental work (Danyuo et al., Citation2014) (Table ).
Table 2. Diffusivity,
 , of PNIPA-loaded Prodigionsin, Drug Release Exponent (n), and Geometric Constant of PNIPA-Based Hydrogels (k) at 37°C (Danyuo et al., Citation2014)
, of PNIPA-loaded Prodigionsin, Drug Release Exponent (n), and Geometric Constant of PNIPA-Based Hydrogels (k) at 37°C (Danyuo et al., Citation2014)
2.10. Numerical model implementation
The numerical model was implemented using the COMSOL Multiphysics (R) software package (version 4.3a, Burlington, Massachusetts, USA). In order to save computer resources, two-dimensional axisymmetric finite element (FE) model of the device/tissue structures with cylindrical coordinates were developed. Coupled Multiphysics of “induction heating” and Transport of diluted species’ physics models were used to solve Equations (2)–(4) and (11) with the given initial and boundary conditions (see Sections 2.1 and 2.2)). This was done over a computational period of 30 min. A frequency-transient scheme was used to solve the equations. The frequency and time steps used in the simulation processes are 480 kHz and 60 s, respectively. Unstructured triangular elements were used to discretize the axisymmetric finite element models. The mesh size for all calculations was defined as a physics-controlled mesh, with the element size as “finer”.
However, with the effort to determine the drug delivery and the heating capability of the biomedical under potential for clinical scenarios, a few points in the residual tumor cell domain and the normal breast tissue domain were selected to extract information on temperature, thermal damage, and concentration of prodigiosin released. The numerical solutions were obtained using the direct solver method, PARDISO of COMSOL based on LU decomposition (COMSOL, Inc, Citation2007). The simulations were performed on a computer with a 2.50 GHz Intel (R) Core (TM) i5-2450 CPU and 6 GB of RAM (HP Pavilion dm4 Notebook PC, Hewlett Packard, Princeton, New Jersey, USA).
3. Results and discussion
3.1. Predictions of heat diffusion in tissues
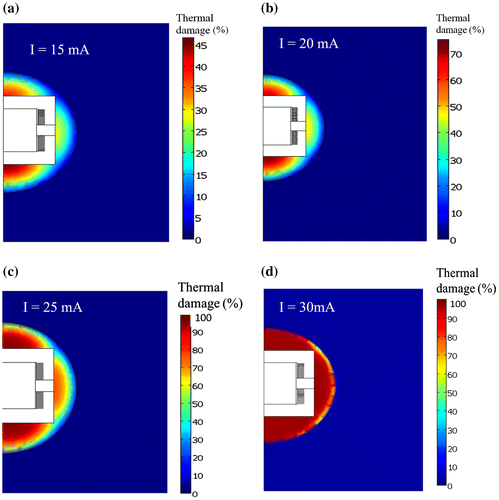
The predicting temperatures in the breast tumor tissue and healthy breast tissue (due to induction heating of the composite polymer implant) are presented in Figure (a)–(d). Figure (a) shows the temperature distribution in and around the device when subjected to an excitation current of 15 mA. A thermal gradient of 17.06°C/mm is calculated between the composite Polymer implant and the residual tumor cells in the radial direction of the FEM model. The residual tumor cells, which are close to the heated device, experienced higher temperatures than the healthy tissue cells. Figure (b) shows the temporal evolution of temperature at two points on the right-hand side of the model: one in the tumor domain ( from the device) and the other at the tumor-breast interface (
from the device). The results of both points show a similar profile of temperature increase with increasing time.
Figure 7. Temperature profile results of FEM model. (a) Temperature distribution in tissue model. (b, c) Temperature as a function of time at selected points in tumor domain and breast domain when an excitation current of 15 and 20 mA are supplied to the device respectively. (d) Temperature as a function of radial distance in tissue for different electrical current supply to the implanted device.
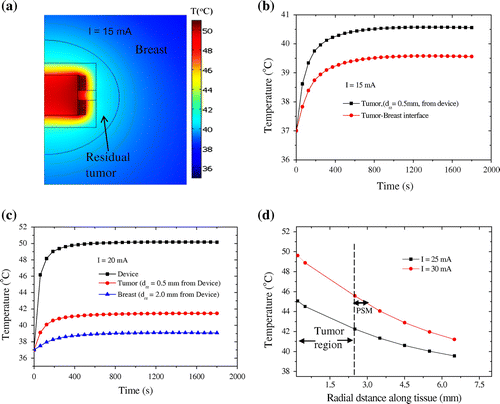
Closer to the device, the temperature of the tumor cells and normal breast tissue cells are increased when higher excitation currents of 20 to 30 mA are supplied to the device. When 20 mA is supplied to the device, the heat generated spread through the conduction process increased the temperature of the residual tumor cells to ~ 41.5°C, while the temperature at the selected point (0.5, 3.2 mm) in the normal breast tissue increased to 39.1°C after computation duration of 1,800 s as shown in Figure (c). The peak temperature changes at the selected point (0.5, 3.2 mm) on the normal breast tissue are 39.1°C compared 41.5°C in tumor tissue. Hence, the temperature of the normal tissue is not sufficient to cause tissue damage, while that of the tumor cells/tissue is sufficient to activate cell death pathways (Khan & Brown, Citation2002).
Figure (d) shows that an excitation current of 25 mA leads to the destruction of tumor cells surrounding the heated device, normal tissue cells at axial and radial distances of 3.6 mm from the heat source (device). This shows that a current of 25 mA is needed to generate heat that is needed to kill the residual tumor cells (surrounding tumor regions) without “damaging” the nearby normal breast tissue. When a 30 mA flows through the device, the temperature of a peripheral safety margin (PSM) about 0.5 mm thickness around normal breast tissue is raised to 45°C to kill any genetic mutant normal breast cells evolving to be cancerous to avoid local recurrence (Figure (d)). The use of temperature sensitivity MRI/thermal probe(s) techniques are valuable in monitoring hyperthermia temperatures (42–45°C) and thermal damage on tumor and normal breast tissues around the implanted biomedical device for safety of normal breast tissue during clinical scenario (Figure (b)). Lately, temperature-sensitive MR imaging sequences have been developed that acquire images on time scales appropriate for the real-time volumetric monitoring, documenting thermal changes in vitro, in animals or in humans (Hushek, Morrison, Kernahan, Fried, & Jolesz, Citation1994; Kettenbach et al., Citation1998) Furthermore, Figure (d) shows that the temperature changes in the tumor tissue are in agreement with those reported in the literature for programmed tumor cell death thermal during therapy (Bettaieb, Wrzal, & Averill-bates, Citation2013; Guan & Xu, Citation2016; Keisari, Citation2012; Theriault et al., Citation2012).
3.2. Predictions of tissue damage coverage
The thermal damages are presented in Figure . The predictions show that the percentage of tissue damage in the residual tumor increases with increasing the electrical current (Figure (a)–(d)). Also, the matter of damaged tumor cells was greater in the regions next to the where the temperatures were greater. The result presented in Figure (c) shows that 68.5% of residual tumor cells are damaged at tumor temperatures between 42.5 and 44.5°C. Figure (d) shows that about 99.9% of residual tumor cells and of the tumor-breast boundary are damaged when the 30 mA flows through the device to generate inductive heat. Also, at the tumor-breast boundary, 45.90% of the normal breast cells/tissue is damaged. Reports have proved that normal tissues have more blood flow than cancerous tissue so they dissipate heat better (DeNardo & DeNardo, Citation2008; Hervault & Thanh, Citation2014). Therefore, above the 30 mA, more of the neighboring healthy breast cells/tissue will be damaged. This suggests 30 mA as the optimum current to maximize thermal dose to cancer cells while minimizing damage to surrounding healthy tissue.
3.3. Predictions of drug release profile of implantable biomedical device
The simulations of prodigiosin release profiles (at 37, 43, and 45°C) from encapsulated P(NIPA) -based hydrogels in the biomedical devices are presented in Figure . Figure (a) and (b) show the released prodigiosin concentration as a function of time. These are presented for the inlet and the channel outlet of the device containing the P(NIPA) homo polymer (100 mol %). Figure (a) shows the release profiles of prodigiosin into the micro channel inlets of three implanted biomedical devices loaded with different drug concentration. For the first 360 s of the computation period, the concentrations of prodigiosin released into the channel inlets from the three encapsulated P (NIIPA) -based hydrogels in the devices are shown in Figure (a).
Figure 9. (a, b) Prodigiosin release to the inlet and outlet the micro-channels from encapsulated P(NIPA)-base hydrogels loaded with three different drug concentrations. (c, d) Prodigiosin concentration in the residual tumor domain and breast tissue domain from the P(NIPA) with diffusion coefficients at 43and 45°C respectively.
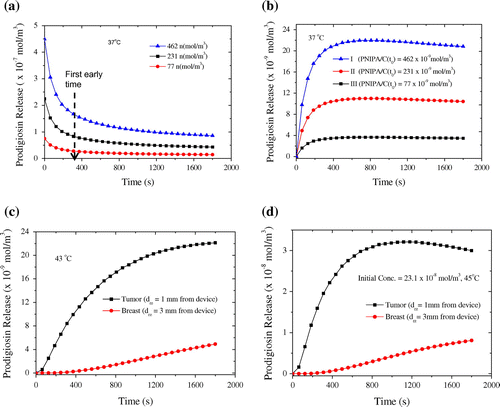
The drug concentrations that are released from the micro-channels of the three devices are presented in Figure (b). The release profile of II (PNIPA/C) in Figure (b) is in the range of clinically relevant concentrations (Zasadil et al., Citation2014). The prodigiosin concentration released into a tumor domain from PNIPA/C
(with the diffusion constant at 37, 43 and 45°C), falls into the dosage range (Zasadil et al., Citation2014). Figure (c) and (d) also predict the concentrations of prodigiosin that diffused into selected points within the tumor region and normal tissue domain. The results show that the tumor tissue had higher concentrations of prodigiosin than the normal breast tissue. The concentration of prodigiosin released into the tumor also increases as the diffusivity coefficient of PNIPA (100 mol %) increases due to temperature rise.
4. Implications
The implications of the current work are very significant. The predictions of tissue thermal damage obtained suggest that excitation currents of ~ 25–30 mA will destroy 99.9% of the residual tumor cells (Figure (c) and (d)). Furthermore, the concentration of the prodigiosin (anti-cancer drug) in the tumor tissue is about four times that in the normal breast tissue. Hence, the controlled delivery of the cancer drug is likely to enhance the local availability of cancer drug in the cancer cells/tumor tissue. Finally, the combined effects of the increased concentration of the cancer drugs (in the cancer cells/tissue) and the increase in the thermal doses in the cancer cells/tissue are likely to improve the clinical outcomes associated with cancer treatment via localized drug delivery and hyperthermia.
5. Conclusion
The paper presents the results of a computational study of temperature distributions and the cancer drug (prodigiosin) release profiles because of applied alternative magnetic fields through a multimodal biomedical implant for breast cancer therapy. The simulations reveal that tumor tissues have a higher concentration of diffused prodigiosin than normal breast tissue. Also, the temperature changes in the tumor tissue are greater than those in neighboring healthy tissue. The predictions of drug concentrations and temperature 41–43°C suggest the implant idea is more likely to induce cell death in the surrounding cancer cells/tissue than in normal cells/tissues.
6. Potential future direction
However, our future studies will aim to check the efficacy of the proposed devise in animals. These preclinical safety and efficacy data are needed for submission to drug regulatory authorities before the permission for further studies in humans are granted.
Funding
This work was supported by the National Science Foundation [grant number: DMR 0231418], the Princeton Grand Challenges Program, the School of Engineering and Applied Science (SEAS) at Princeton University, the World Bank STEP B program, the World Bank Africa Centers of Excellence Program, the African Capacity Building Foundation (ACBF), the Nelson Mandela Institution (NMI), the African University of Science and Technology (AUST) and the African Development Bank (AfDB) for their financial support.
Acknowledgments
The authors are also grateful to the Sheda Science and Technology Complex, Biotechnology Advanced Laboratories, Abuja, Nigeria.
Additional information
Notes on contributors
W.O. Soboyejo
W.O. Soboyejo received his PhD in materials science and metallurgy from Cambridge University in 1988. He then worked as a Research Scientist at McDonnell Douglas Research Labs in St. Louis, MO, from 1988 to 1992. He was a Principal Research Engineer at the Edison Welding Institute in Columbus, OH, before joining the department of materials science and engineering at The Ohio State University as an Assistant Professor in 1992. Soboyejo was tenured and promoted to the rank of Associate Professor in 1996. He then spent a year as a Visiting Martin Luther King Associate Professor at MIT (1997–1998) before moving to Princeton University as a Professor of Mechanical and Aerospace Engineering in 1999. Between 2012 and 2014, he served as the President and Provost of the African University of Science and Technology (AUST) in Abuja, Nigeria. He then returned to Princeton between 2014 and 2016 before moving to the Worcester Polytechnic Institute (WPI) as the Bernard M. Gordon Dean of Engineering and Engineering Leadership.
References
- Alberts, B. , Johnson, A. , Lewis, J. , Raff, M. , Roberts, K. , & Walter, P. (2008). Molecular biology of the cell . New York, NY : Garland Science.
- Berjano, E. J. (2006). Theoretical modeling for radiofrequency ablation: State-of-the-art and challenges for the future. Biomedical Engineering Online , 5 (24), 1–17.
- Bettaieb, A. , Wrzal, P. K. , & Averill-bates, D. A. (2013). Hyperthermia: Cancer treatment and beyond. Cancer Treatment-Conventional and Innovative Approaches , 257–283.
- Bezerra, L. A. , Oliveira, M. M. , Rolim, T. L. , Conci, A. , Santos, F. G. S. , Lyra, P. R. M. , & Lima, R. C. F. (2013). Estimation of breast tumor thermal properties using infrared images. Signal Processing , 93 , 2851–2863.10.1016/j.sigpro.2012.06.002
- Bhowmik, A. , Repaka, R. , Mishra, S. C. , & Mitra, K. (2015). Thermal assessment of ablation limit of subsurface tumor during focused ultrasound and laser heating. Journal of Thermal Science and Engineering Applications , 1–46.
- Blanco, L. , Shiakolas, P. S. , Aswath, P. B. , Alberts, C. B. , Grace, C. , Godfrey, K. , & Patin, D. (2012). A thermoresponsive hydrogel based controlled drug delivery device. International Mechanical Engineering Congress and Exposition , 2 , 371–378.
- Bray, F. , Jemal, A. , Grey, N. , Ferlay, J. , & Forman, D. (2012). Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. The lancet Oncology , 13 (8), 790–801.10.1016/S1470-2045(12)70211-5
- Choi, J. , & Wang, N. S. (2011). Nanoparticles in biomedical applications and their safety concerns. In R. Fazel-Rezai (Ed.), Biomedical engineering from theory to applications (p. 486). Rijeka: InTech.
- COMSOL, Inc . (2007). COMSOL multiphysics user’s guide . Burlington, MA .
- Danyuo, Y. (2015). Implantable biomedical devices for localized breast cancer drug delivery . African University of Science and Technology.
- Danyuo, Y. , Dozie-Nwachukwu, S. , Obayemi, J. D. , Ani, C. J. , Odusanya, O. S. , Oni, Y. , & Anuku, N. (2016). Swelling of poly(N-isopropylacrylamide) P(NIPA)-based hydrogels with bacterial-synthesized prodigiosin for localized cancer drug delivery . Materials Science & Engineering C , 59 , 19–29.10.1016/j.msec.2015.09.090
- Danyuo, Y. , Obayemi, J. D. , Dozie-Nwachukwu, S. , Ani, C. J. , Odusanya, O. S. , Oni, Y. , & Anuku, N. (2014). Prodigiosin release from an implantable biomedical device: Kinetics of localized cancer drug release. Materials Science & Engineering C , 42 , 734–745.10.1016/j.msec.2014.06.008
- Day, E. S. , Morton, J. G. , & West, J. L. (2009). Nanoparticles for thermal cancer therapy. Journal of Biomechanical Engineering , 131 , 1–5.
- Dedulle, J. M. (2006). Pedagogic using of COMSOL multiphysics for learning numerical method and numerical modeling. Proceedings of the COMSOL Users Conference , 1–7.
- DeNardo, G. L. , & DeNardo, S. J. (2008). Update: Turning the heat on cancer. Cancer Biotherapy and Radiopharmaceuticals , 23 (6), 671–680.10.1089/cbr.2008.0591
- Drizdal, T. , Togni, P. , Visek, L. , & Vrba, J. (2010). Comparison of constant and temperature dependent blood perfusion in temperature prediction for superficial hyperthermia. Radioengineering , 19 (2), 281–289.
- Duck, F. A. (1990). Physical properties of tissue . London: Academic Press.
- Ekstrand, V. , Wiksell, H. , Schultz, I. , Sandstedt, B. , Rotstein, S. , & Eriksson, A. (2005). Influence of electrical and thermal properties on RF ablation of breast cancer: Is the tumour preferentially heated ? Biomedical Engineering Online , 16 , 1–16.
- Gaitas, A. , & Kim, G. (2015). Inductive heating kills cells that contribute to plaque : A proof-of-concept. PeerJ , 3 , e929.10.7717/peerj.929
- Garcia, P. A. , Davalos, R. V. , & Miklavcic, D. (2014). A numerical investigation of the electric and thermal cell kill distributions in electroporation-based therapies in tissue. PLoS One , 9 (8), e103083.10.1371/journal.pone.0103083
- Gasselhuber, A. , Dreher, M. R. , Negussie, A. , Bradford, J. , Rattay, F. , & Haemmerich, D. (2010). Mathematical spatio-temporal model of drug delivery from low temperature sensitive liposomes during radiofrequency tumour ablation. International Journal of Hyperthermia , 26 (5), 499–513.10.3109/02656731003623590
- Gautier, J. , Munnier, E. , Paillard, A. , Hervé, K. , Douziech-Eyrolles, L. , Soucé, M. , … Chourpa, I. (2012). A pharmaceutical study of doxorubicin-loaded PEGylated nanoparticles for magnetic drug targeting. International Journal of Pharmaceutics , 423 (1), 16–25.10.1016/j.ijpharm.2011.06.010
- Goodfellow . (1993). Metals, alloys, compounds, ceramics, polymers, composites. Catalogue 1993/94 (2nd ed., p. 1). Cambridge: Cambridge Ltd..
- Gould, T. , Wang, Q. , & Pfefer, T. J. (2014). Optical-thermal light-tissue interactions during photoacoustic breast imaging. Biomedical Optics Express , 5 (3), 832–847.10.1364/BOE.5.000832
- Guan, L. , & Xu, G. (2016). Damage effect of high-intensity focused ultrasound on breast cancer tissues and their vascularities. World Journal of Surgical Oncology , 14 (619), 1–17.
- Habash, R. W. , Bansal, R. , Krewski, D. , & Alhafid, H. T. (2006). Thermal therapy, part 2: Hyperthermia techniques. Critical Reviews™ in Biomedical Engineering , 34 (6), 459.10.1615/CritRevBiomedEng.v34.i6
- Hayashi, K. , Ono, K. , Suzuki, H. , Sawada, M. , Moriya, M. , Sakamoto, W. , & Yogo, T. (2010). High-frequency, magnetic-field-responsive drug release from magnetic nanoparticle/organic hybrid based on hyperthermic effect. ACS Applied Materials & Interfaces , 53 , 8244.
- He, Y. , Shirazaki, M. , Liu, H. , Himeno, R. , & Sun, Z. (2006). A numerical coupling model to analyze the blood flow, temperature, and oxygen transport in human breast tumor under laser irradiation. Computers in Biology and Medicine , 36 , 1336–1350.10.1016/j.compbiomed.2005.08.004
- Heine, U. , Sverak, L. , Kondratick, J. , & Bonar, R. (1971). The behavior of HeLa-S3 cells under the influence of supranormal temperatures. Journal of Ultrastructure Research , 34 ( 3–4 ), 375–396.10.1016/S0022-5320(71)80079-5
- Henriques, J. F. , & Moritz, A. (1947). Studies of thermal injury: I. The conduction of heat to and through skin and the temperatures attained therein. A theoretical and an experimental investigation. The American Journal of Pathology , 23 (4), 1947.
- Hervault, A. , & Thanh, N. T. K. (2014). Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale , 6 (20), 11553–11573.10.1039/C4NR03482A
- Hildebrandt, B. , & Wust, P. (2007). Peritoneal carcinomatosis: A multidisciplinary approach . ( W. P. Ceelen , Ed.). (p. 185). New York, NY : Springer.
- Hilger, I. , Hiergeist, R. , Hergt, R. , Winnefeld, K. , Schubert, H. , & Kaiser, W. A. (2002). Thermal ablation of tumors using magnetic nanoparticles: An in vivo feasibility study. Investigative Radiology , 37 (10), 580–586.10.1097/00004424-200210000-00008
- Hirsch, L. R. , Stafford, R. J. , Bankson, J. A. , Sershen, S. R. , Rivera, B. , Price, R. E. , … Hazle , J. L. (2003). Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proceedings of the National Academy of Sciences , 100 (23), 13549–13554.10.1073/pnas.2232479100
- Huang, H. , & Liauh, C.-T. (2011). Review: Therapeutical applications of heat in cancer therapy. Journal of Medical and Biological Engineering , 32 (1), 1–11.
- Hushek, S. G. , Morrison, P. R. , Kernahan, G. E. , Fried, M. P. , & Jolesz, F. A. (1994). Thermal contours from magnetic resonance images of laser irradiated gels. Quantification and Localization Using Diffuse Photons in a Highly Scattering Medium , 2082 , 43–50.10.1117/12.243394
- Kan-Dapaah, K. , Rahbar, N. , & Soboyejo, W. (2014). Implantable magnetic nanocomposites for the localized treatment of breast cancer . Journal of Applied Physics , 116 (23), 233505.10.1063/1.4903736
- Kase, K. , & Hahn, G. M. (1975). Differential heat response of normal and transformed human cells in tissue culture . London: Nature Publishing Group.
- Kavitha, K. , & Bhalamurugan, G. L. (2013). A review on PEG-ylationin anti-cancer drug delivery systems. International Journal of Research in Pharmaceutical and Biomedical Sciences , 4 (1), 296–304.
- Keisari, Y. (Ed.). (2012). Tumor ablation: Effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions (Vol. 5). New York, NY : Springer Science & Business Media.
- Kettenbach, J. , Silverman, S. , Hata, N. , Kuroda, K. , Saiviroonporn, P. , Zientara, G. , … Jolesz, F. A. (1998). Monitoring and visualization techniques for MR-guided laser ablations in an open MR system. Journal of Magnetic Resonance Imaging , 8 (4), 933–943.10.1002/(ISSN)1522-2586
- Khan, V. R. , & Brown, I. R. (2002). The effect of hyperthermia on the induction of cell death in brain, testis, and thymus of the adult and developing rat. Cell Stress and Chaperones , 7 (1), 73–90.10.1379/1466-1268(2002)007<0073:TEOHOT>2.0.CO;2
- Koreckij, T. D. , Hill, C. , Azure, L. , Nguyen, H. , Kunz, L. L. , Azure, A. , … Vessella, R. L. (2010). Low dose, alternating electric current inhibits growth of prostate cancer. The Prostate , 70 (5), 529–539.
- Kothandaraman, C. P. (2006). Fundamentals of heat and mass transfer (3rd ed.). New Delhi: New Age International.
- Kubota, K. (2011). Recent advances and limitations of surgical treatment for pancreatic cancer. World Journal of Clinical Oncology , 2 (5), 225–228.10.5306/wjco.v2.i5.225
- Kumar, A. , Mazinder Boruah, B. , & Liang, X.-J. (2011). Gold nanoparticles: Promising nanomaterials for the diagnosis of cancer and HIV/AIDS. Journal of Nanomaterials , 2011 , 22–38.
- Landon, C. D. , Park, J. , Needham, D. , & Dewhirst, M. W. (2011). Nanoscale drug delivery and hyperthermia: The materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. The Open Nanomedicine Journal , 3 , 38–64.
- Lang, J. , Erdmann, B. , & Seebass, M. (1999). Impact of nonlinear heat transfer on temperature control in regional hyperthermia. IEEE Transactions on Biomedical Engineering , 46 (9), 1129–1138.10.1109/10.784145
- Lee, J. , Chatterjee, D. K. , Lee, M. H. , & Krishnan, S. (2014). Gold nanoparticles in breast cancer treatment: Promise and potential pitfalls. Cancer Letters , 347 (1), 46–53.10.1016/j.canlet.2014.02.006
- Love, R. , Soriano, R. Z. , & Walsh, R. J. (1970). Effect of hyperthermia on normal and neoplastic cells in vitro1. Cancer Research , 30 (5), 1525–1533.
- Ma, X. , & Yu, H. (2006). Global burden of cancer , 79 (3–4), 85–94.
- Marchosky, J. A. , Babbs, C. F. , Moran, C. J. , Fearnot, N. E. , DeFord, J. A. , & Welsh, D. M. (1990). Conductive, interstitial hyperthermia: A new modality for treatment of intracranial tumors. Consensus on Hyperthermia for the 1990s , 129–143.
- Mikhailov, M. D. , & Ozisik, M. N. (1984). Unified analysis and solutions of heat and mass diffusion (1st ed.). New York, NY : Dover Publications.
- Morita, K. , Zywietz, F. , Kakinuma, K. , Tanaka, R. , & Katoh, M. (2008). Efficacy of doxorubicin thermosensitive liposomes (40 °C) and local hyperthermia on rat rhabdomyosarcoma. Oncology Reports , 20 (33), 365–372.
- Moroz, P. , Jones, S. , & Gray, B. (2002). Magnetically mediated hyperthermia: Current status and future directions. International Journal of Hyperthermia , 18 (4), 267–284.10.1080/02656730110108785
- Mura, S. , Nicolas, J. , & Couvreur, P. (2013). Stimuli-responsive nanocarriers for drug delivery. Nature Materials , 12 (11), 991–1003.10.1038/nmat3776
- Ni, Y. , Mulier, S. , Miao, Y. , Michel, L. , & Marchal, G. (2005). A review of the general aspects of radiofrequency ablation. Abdominal Imaging , 30 , 381–400.10.1007/s00261-004-0253-9
- O’Neal, D. P. , Hirsch, L. R. , Halas, N. J. , Payne, J. D. , & West, J. L. (2004). Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Letters , 209 (2), 171–176.10.1016/j.canlet.2004.02.004
- Oni, Y. , Theriault, C. , Hoek, A. V. , & Soboyejo, W. O. (2011). Effects of temperature on diffusion from PNIPA-based gels in a BioMEMS device for localized chemotherapy and hyperthermia. Materials Science and Engineering: C , 31 (2), 67–76.10.1016/j.msec.2010.07.016
- Orsi, F. , Arnone, P. , Chen, W. , & Zhang, L. (2010). High intensity focused ultrasound ablation: A new therapeutic option for solid tumors. Journal of Cancer Research and Therapeutics , 6 (4), 414–420.
- Owusu, R. A. , Abern, M. R. , & Inman, B. A. (2013). Hyperthermia as adjunct to intravesical chemotherapy for bladder cancer. BioMed Research Internationa , 2013 , 262313.
- Perry, M. . (2011). Approach to the patient with cancer. In L. Goldma & A. I. Schafer (Eds.), Goldman’s cecil medicine (24th ed., p. 3031).
- Pryor, R. W. (2009). Multiphysics modeling using COMSOL: A first principles approach . Sudbury, MA: Jones and Bartlett Publishers.
- Qian, F. , Stowe, N. , Liu, E. H. , Saidel, G. M. , & Gao, J. (2003). Quantification of in vivo doxorubicin transport from PLGA millirods in thermoablated rat livers. Journal of Controlled Release , 91 , 157–166.10.1016/S0168-3659(03)00237-2
- Raoof, M. , & Curley, S. A. (2011). Non-invasive radiofrequency-induced targeted hyperthermia for the treatment of hepatocellular carcinoma. International Journal of Hepatology , 2011 , 676957.
- Rodriguez-Luccioni, H. L. , Latorre-Esteves, M. , Mendez-Vega, J. , Soto, O. , Rodriguez, A. R. , Rinaldi, C. , & Torres-Lugo, M. (2011). Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. International Journal of Nanomedicine , 6 , 373.
- Rossmanna , C. , & Haemmerich, D. (2014). Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Critical Reviews in Biomedical Engineering , 42 (6), 467–492.10.1615/CritRevBiomedEng.v42.i6
- Saini, R. K. , Chouhan, R. , Bagri, L. P. , & Bajpai, A. K. (2012). Strategies of targeting tumors and cancers. Journal of Cancer Research Updates , 1 , 129–152.
- Sawicki, B. , & Miaskowski, A. (2014). Nonlinear higher-order transient solver for magnetic fluid hyperthermia. Journal of Computational and Applied Mathematics , 270 , 143–151.10.1016/j.cam.2014.02.008
- Schwartz, J. A. , Shetty, A. M. , Price, R. E. , Stafford, R. J. , Wang, J. C. , Uthamanthil, R. K. , … Payne, J. D. (2009). Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Research , 4 , 1659–1667.10.1158/0008-5472.CAN-08-2535
- Shah, S. A. , Majeed, A. , Shafique, M. A. , Rashid, K. , & Awan, S. U. (2013). Cell viability study of thermo-responsive core–shell superparamagnetic nanoparticles for multimodal cancer therapy. Applied Nanoscience , 4 (2), 227–232.
- Shinohara, K. (2004). Thermal ablation of prostate diseases: Advantages and limitations. International Journal of Hyperthermia , 20 (7), 679–697.10.1080/02656730412331286876
- Simmonds, P. , Primrose, J. , Colquitt, J. , Garden, O. , Poston, G. , & Rees, M. (2006). Surgical resection of hepatic metastases from colorectal cancer : A systematic review of published studies. British Journal of Cancer , 94 , 982–999.10.1038/sj.bjc.6603033
- Theriault, C. , Paetzell, E. , Chandrasekar, R. , Barkey, C. , Oni, Y. , & Soboyejo, W. O. (2012). An in vitro study of the effects of temperature on breast cancer cells: Experiments and models. Materials Science & Engineering C , 32 (8), 2242–2249.10.1016/j.msec.2012.06.010
- Timko, B. P. , Arruebo, M. , Shankarappa, S. A. , McAlvin, J. B. , Okonkwo, O. S. , Mizrahi, B. , … Kohane, D. S. (2014). Near-infrared-actuated devices for remotely controlled drug delivery. Proceedings of the National Academy of Sciences , 111 (4), 1349–1354.10.1073/pnas.1322651111
- Toi, Y. , & Takagaki, M. (2008). Computational modeling of induction hardening process of machine parts. In Proceedings of the World Congress on Engineering and Computer Science (pp. 1–5).
- Vihola, H. , Laukkanen, A. , Valtola, L. , Tenhu, H. , & Hirvonen, J. (2005). Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam) . Biomaterials , 26 (16), 3055–3064.10.1016/j.biomaterials.2004.09.008
- van Vlerken, L. E. , Vyas, T. K. , & Amiji, M. M. (2007). Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharmaceutical Research , 24 (8), 1405–1414.10.1007/s11095-007-9284-6
- Vyas, A. , Das, S. K. , Singh, D. , Sonker, A. , Gidwani, B. , Jain, V. , & Singh, M. (2012). Recent nanoparticulate approaches of drug delivery for skin cancer. Trends in Applied Sciences Research , 7 (8), 620–635.10.3923/tasr.2012.620.635
- Weinberg, B. D. , Patel, R. B. , Exner, A. A. , Saidel, G. M. , & Gao, J. (2008). Modeling doxorubicin transport to improve intratumoral drug delivery to RF ablated tumors. Journal of Control Release , 124 (1–2), 11–19.
- Welty, J. R. , Charles, W. E. , Wilson, R. E. , & Rorrer, G. L. (2000). Fundamentals of momentum, heat, and mass transfer (5th ed.). Hoboken, NJ: John Wiley & Sons.
- Yukumi, S. , Watanabe, Y. , Horiuchi, A. , Doi, T. , Sato, K. , Yoshida, M. , … Kawachi, K. (2008). Feasibility of induction heating using a sintered MgFe2O4 needle for minimally invasive breast cancer therapy. Anticancer Research , 28 (1A), 69–74.
- Zasadil, L. M. , Andersen, K. A. , Yeum, D. , Rocque, G. B. , Wilke, L. G. , Tevaarwerk, A. J. , … Weaver, B. A. (2014). Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Science Translational Medicine , 6 (229), 229ra43.10.1126/scitranslmed.3007965
- Zavaglia, C. , Mancuso, A. , Foschi, A. , & Rampoldi, A. (2013). High-intensity focused ultrasound (HIFU) for the treatment of hepatocellular carcinoma: Is it time to abandon standard ablative percutaneous treatments? Hepatobiliary Surgery and Nutrition , 2 (4), 184–187.
- Zhao, L. Y. , Liu, J. Y. , Ouyang, W. W. , Li, D. Y. , Li, L. , Li, L. Y. , & Tang, J. T. (2013). Magnetic-mediated hyperthermia for cancer treatment: Research progress and clinical trials. Chinese Physics B , 22 (10), 108104.10.1088/1674-1056/22/10/108104
- Zhou, Y. (2013). Noninvasive treatment of breast cancer using high-intensity focused ultrasound. Journal of Medical Imaging and Health Informatics , 3 (2), 141–156.10.1166/jmihi.2013.1156
- Zhou, Y. (2014). High intensity focused ultrasound in clinical tumor ablation. World Journal of Clinical Oncology , 2 (1), 8–27.
- Zhu, Q. , Shen, Y. , Zhang, A. , & Xu, L. X. (2013). Numerical study of the influence of water evaporation on radiofrequency ablation. Biomedical Engineering Online , 12 , 1–16.