Abstract
The processing and structural characterization of Si-based carbothermal derivatives of rice husk (RH) was investigated. RH, an agro-waste, was used as starting material in a single-stage processing route to produce Si-based refractory compounds via carbothermal treatment. The processing was carried out at different temperatures windows (900–1,900°C) at 10°C/min heating range in a controlled atmosphere. The functional groups of the reaction products were analyzed using Fourier transform infrared spectroscopy. The crystalline and amorphous phases were identified by X-ray diffractometer (XRD), while the morphological features were examined by scanning electron microscopy. It was observed that the major functional groups present after carbothermal treatment of RH are OH− (hydroxyl), Si–O–Si (siloxane), and Si–C groups. However, polytypes of silicon carbide (SiC) such as 3C-SiC (C = cubic), hexagonal symmetry 6H-SiC, and mixes of 3C-SiC, 2H-SiC, and 4H-SiC were various polytypes of SiC observed for different processing temperatures adopted. The SiC made up between 63 and 74 wt.% of the crystalline phases was identified by XRD from the process. Hence, the carbothermal treatment explored showed evidence of viability for production of Si-based refractory compounds which can serve as reinforcement in the development of metal matrix composites.
PUBLIC INTEREST STATEMENT
The market price of silicon carbide is on increase every year (especially in Nigeria), and this might be due to the expensive production route of producing silicon carbide; considering the cost of petroleum coke, among others. However, there are available agro-waste products in large deposits within the southwest metropolis of Nigeria, with higher percentage of silica. Environmental challenges caused by the disposal of rice husks (RHs) can be harnessed by converting it into a promising material, using a low-cost processing route (i.e. a conventional heat treatment furnace) in the synthesis of silicon refractory derivatives. The results of the study show that Si-based refractory compounds can be synthesized from RH found within the southwestern part of Nigeria for the development of composite material in automobile application.
1. Introduction
The world annual paddy production is estimated to be over 582 million tons according to the statistical data of Food and Agriculture Organization (Food and Agriculture Organization, Citation2015). From this, about 145 million tons of husk are gotten from the processing of rice bran, making it 20–25% of husk (Vlaev, Petkov, Dimitrov, & Genieva, Citation2011). Rice husk (RH), though an agro-waste has been utilized in several ways such as fillers for natural and synthetic rubbers (Soltani, Bahrami, Pech-Canul, & González, Citation2014), as effluent waste treatment (Abo-El-Enein, Eissa, Diafullah, Rizk, & Mohamed, Citation2009; Srivastava, Mall, & Mishra, Citation2007), poultry feeds (Housen, Citation1972), insulating refractories, pozzolanic materials (Boateng & Skeete, Citation1990; Larrard, Gorse, & Puch, Citation1992; Mohamed, Abd El-Aziz, Abd El-Aleem, & El-Didamony, Citation2004; Saleh, Citation2015), and as silica source in the design of metal matrix composites (Alaneme, Akintunde, Olubambi, & Adewale, Citation2013). Since 1973, when Cutler used RH as a starting material in the production of silicon carbide (SiC), it has since then attracted interest from researchers as a promising material in the synthesis of SiC. SiC is an important nonoxide ceramic material used in a wide range of engineering applications, primarily because of its high melting point, good mechanical strength, very high thermal conductivity and electrical conductivity, good microwave absorption tendency, high energy band gap, and excellent chemical and oxidation resistance (Martin, Ecke, & Müller, Citation1998; Lutsenko, Citation2008; Prabhakaran, Sreejith, Swaminathan, Packirisamy, & Ninan, Citation2009; Pierre, Citation2002). It is currently used in several areas of applications which include high-temperature materials, as particulate material in composite development, tribology materials, and grinding materials, among others (Alaneme & Aluko, Citation2012; Fu, Li, Shi, Li, & Sun, Citation2005; Li et al., Citation2007; Najafi, Fard, Rezaie, & Ehsani, Citation2012; Ravi, Omotoye, Srivatsan, Petrorali, & Sudarshan, Citation2009). Currently, the development of SiC has been modified from what it was over the past decades. Traditionally, on an industrial-scale level, the use of an electrical furnace as a chamber for the treatment of coal and quartz sand at a temperature of order 2,500°C in the production of SiC was pronounced then. Processing routes such as Acheson process, laser synthesis, microwave plasma discharge sol-gel, and chemical vapor deposition are most of the common production route in the synthesis of SiC (Kholmanov, Kharlamov, Barborini, Lenardi, & Bassi, Citation2002; Kim, Park, Cho, & Lee, Citation1994; Satapathy, Ramesh, Agrawal, & Roy, Citation2005; Yang, Wu, Chen, Song, & Pan, Citation2007; Zhong, Shaw, Manjarres, & Zawrah, Citation2010). In all these processes, a high cost of processing and reaction time are relatively common to them; therefore, there is the need for exploring an alternate and cost-effective processing route. For a while now, researchers have been using a two-stage processing route in the synthesis of SiC from RH (Della, Kühn, & Hotza, Citation2002; Krishnarao, Subrahmanyam, & Kumar, Citation2001). The two-stage processing involves a cooking at controlled temperature interphase of 400–800°C and subsequent reaction at high-temperature window (≥1,200°C)) in the production of SiC (Krishnarao & Godkhindi, Citation1992; Singh, Mohanty, & Basu, Citation2002). The strong argument behind this two-stage processing is credited to a high rate of recovery from contaminants and the liberation of metallic impurities from the starting material (Carmona, Oliveira, Silva, Mattoso, & Marconcini, Citation2013). However, the high cost of processing associated with this route, especially for developing countries like Nigeria, where power generation and distribution is currently unstable, makes it an unviable option. Hence the quest to explore a more economically viable processing route envisaged to produce SiC yield comparable with the two-stage process. Apart from the technical benefits of developing SiC cheaply, the post-harvesting and environmental challenges, as well as poor recycling technologies resulting from marginal utilization of RH locally, can be solved in the long run. Rahman and Saleh (Citation1995) reported on the formation of β-Sialon powder using carbothermal reduction of digested RHs. Lin and Tsang (Citation2003) examined the effects of colloidal silica as precursors on the carbothermal synthesis of SiC powders. It is envisaged that higher activity ratio imposed by the contact of silica with carbon informs its choice for a carbothermal route in the synthesis of SiC, thus making it an economical and efficient process (Lin & Tsang, Citation2003). SiC undergoes metamorphosis into different forms—this transformation is informed by the stacking order of Si–C arrangement. Blend such as the zinc blende characterized by 3C-SiC (C = cubic) which is β-SiC type and the hexagonal (2H, 4H or 6H) α-SiC structures are the two notable forms of SiC. The electronic structure of α-SiC was reported by Madelung (Citation1987). He reported that the energy gap for hexagonal polytypes follows the stacking order of Si–C arrangement. For 2H-SiC, for instance, it occurs as …ABAB……., with energy gap of 3.330 eV, for 4H-SiC (a hexagonal type), it follows an order…..ABCBABCB….. with energy gap of 3.265 eV, and for 6H-SiC, it occurs as …….ABCACBABCACB…, with energy gap of 3.023 eV. The adoption of a single processing route in the synthesis of SiC derivatives from RH would be economical and time-saving. The present work examines the composition and phase characterization of Si-based compounds derived from carbothermal treatment using RH as a precursor in an unorthodox route.
2. Materials and method
2.1. Materials
The RHsused in this research were sourced from Igbemo-Ekiti, Ekiti State, southwest in Nigeria. Graphite lump was purchased commercially from a vendor and was used to fabricate graphite containers, which served as crucibles for the carbothermal heat treatment.
2.2. Methods
The RHs were washed with distilled water to remove dirt and other contaminants present in them. It was first dried at an ambient temperature and later subjected to an oven drying (Model No: ES-9009, Longspring Tech Ltd, Jiangsu, China) at about 333 K for 24 h. Samples were then screened through an ASTM standard sieve to obtain different sizes such as 75, 125, 150, 212, 300, and 450 µm in order to eliminate residual rice in the husk. In the current work, grain size of 125 µm was used. The RHs were subjected to a microwave treatment of 2.45 GHz at 900W for 5 min. About 40 g of raw RH was weighed and 6 g of Fe2O3 (catalyst) to aid the reaction kinetics. Graphite particles of average particle size <75 µm were weighed and added; the mixture was then placed in a graphite crucible and closed with a graphite lid. The experiment was carried out in three stages designating samples A1, B1, and C1. A1 was treated at 1,650°C in a stoichiometry ratio of 3:1 (graphite to RH), B1 at 1,650°C in a catalytic environment of Fe2O3 with the same ratio used in A1, and C1 at 1,600°C without any addition of graphite and catalyst. The graphite crucibles with samples were then introduced into the heating zone of the furnace (Brother Furnace-XD-1700M, Zhengzhou Brother Furnace Co., Ltd, Henan, China) in a non-oxidizing atmosphere at temperatures ranging from 800–1,900°C at a heating rate of 10°C/min for 60 min soaking time. Then, the furnace was allowed to naturally cool off. The microstructural and elemental composition of the carbothermal products was examined using an ASPEX 3020 model scanning electron microscope, Thermo Fisher Scientific, Eindhoven, Netherlands at Central Lab, Covenant University, Ota, Ogun State, Nigeria (SEM) equipped with Noran-Voyager energy-dispersive spectroscope (EDX) operating with an accelerating voltage of 15 kV. The chemical groups present in the products were identified using Fourier transform infrared spectrometer (FTIR; Shimadzu, model IR AFFINITY, IS FTIR, Shimadzu Scientific Instruments, North America, LAUTECH Central Lab. Ogbomosho, Oyo State, Nigeria). About 200–250 mg of kBr powder was mixed with 10–15 mg of processed RH and pelletized within 40 MPa compaction load. Samples were carried out to confirm the different bonds of Si/O system in RHs under treatment conditions. The crystalline and amorphous phases present in the products from the carbothermal treatment of RH were examined by X-ray diffractometry (XRD). The samples were prepared for XRD analysis using a backloading preparation method, and analysis was latter done with a PANalytical Empyrean diffractometer, at Analytical and Consulting Cc, Lynnwood Glen, South Africa with PIXcel detector and fixed slits with Fe-filtered Co-Kα radiation in a scan range of 2θ = 10–90° at scan step of 10°. The phases were identified using X’Pert Highscore plus software and the relative phase amounts (weight %) was estimated using the Rietveld method (PANalytical Highscore4+).
3. Results and discussion
3.1. FTIR analysis
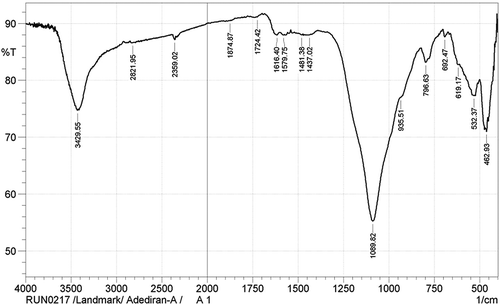
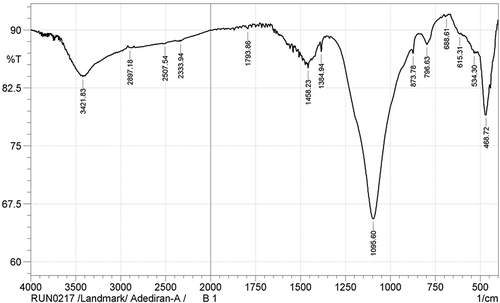
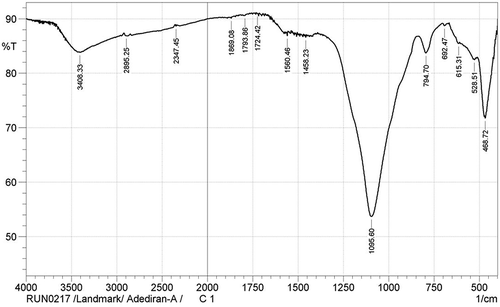
The functional groups identified in the carbothermal treatment products are as shown in the FTIR spectra presented in Figures –. Figure shows the IR spectra for sample designated A1. The OH‒ functional group was identified at the wide band 3,429.55 cm−1. The presence of the OH− functional group suggests dehydration during the carbothermal reaction, a trend which has been corroborated by several authors (Ali & Tindyala, Citation2015; Lu & Hsieh, Citation2012; Sujirote & Leangsuwan, Citation2003). The functional group Si–O–Si was present at a sharp band of peak 1,089.82 cm−1. The presence of Si–O–Si group confirms that silica was present after the thermal treatment. Similar trend was observed by Ghafoorian, Bahramian, and Seraji (Citation2015). The IR spectra (Figure ) for sample B1 at peak 468.72 cm−1 shows the presence of Si–O functional group. There are reports corroborating the detection of Si–O functional group; the reports identified an absorption range of 400–475 cm−1 for the formation of Si–O functional groups (An, Guo, Zhu, & Wangg, Citation2010; Carmona et al., Citation2013). Remarkably, the formation of Si–C was observed at peak 796.63 cm−1, which agrees with works of Ali and Tindyala (Citation2015) and Rajarao, Seyed, Rita, and Veena (Citation2014). They noted that the formation of Si–C bond was at a range of stretching bands. They observed from their individual research work that SiC was identified at a peak range of 780–800 cm−1. Rajarao et al. (Citation2014) used compact disk-CD (an electronic waste) as carbon source; from their work, they detected Si–C functional group at a peak of 790 cm−1. However, this deviates from reports by authors Najafi et al. (Citation2012) and Kim and Koh (Citation2007). From their evaluation, they reported the peak interval of 800–900 cm−1 for the presence of Si–C functional group. The representative IR spectra showing the chemical bonds for the products in sample C1 is represented in Figure . The figure shows different absorption bands, for the carbothermal treatment sequence at 1,600°C, the treatment condition for sample designated C1. From the FTIR spectra, a wide gap showing a stretching band at 3,408.33 cm−1, which corresponds to asymmetric, symmetric, and the bending modes of OH− bond, is observed. The OH− bond in Figure seems to have come from the reaction of atmospheric moisture inherent in the constituent compounds of RHs during reaction time. The steep band at 794.70 cm−1 corresponds to a stretching vibration of Si–C, which is in agreement with the stretching band for Si–C reported by Ali and Tindyala (Citation2015).
3.2. XRD analysis
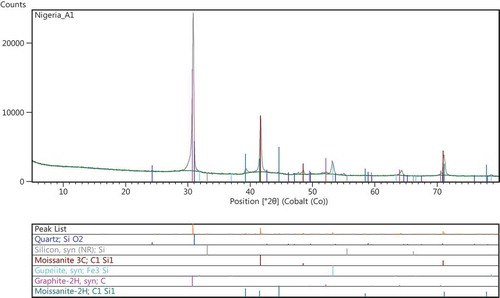
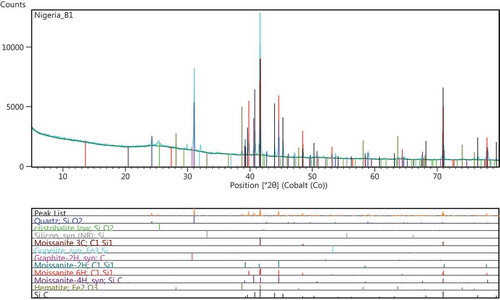
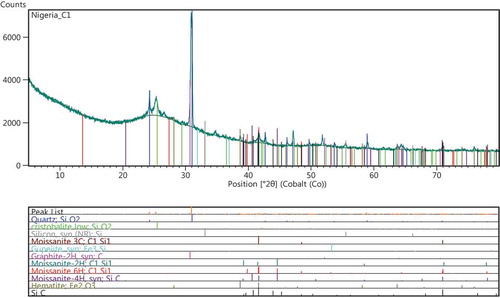
Figures – show the results of the XRD spectra. Figure shows the XRD spectrum for treatment conditions at 1,650°C. The designation was referred to as sample A1, and the phases observed at this treatment condition was predominantly β-SiC. This β-SiC type crystallizes in cubic lattice symmetry of 3C-SiC type. Dhage, Lee, Hassan, Akhtar, and Kim (Citation2009) observed the formation of β-SiC type at a diffraction peak of 2θ = 33.6°. However Guo, Zhu, Li, and Yang (Citation2013) noted that for purchased SiC nanowires, the particle diffraction peaks were for 3C-SiC phase at angles (2θ) of 35.6°, 41.06°, 60.06°, 71.84° and 75.64°, respectively, which indicates that all of the as-synthesized powders are mainly composed of β-SiC nanosized grains. The peaks of SiC particles formed in the current work falls within the diffraction peaks mentioned by Guo et al. (Citation2013). Figure shows a representative XRD pattern of various polytypes of SiC for sample B1. The sample shows diffused peaks representing at cristobalite phase at 2θ = 24.2° and 30°, respectively. It was observed that there were variations in the diffused peaks relative to the various polytypes of SiC. These variations might be partly attributed to a gradual change in the bonding of silicon to organic constituent in the RH (Krishnarao & Godkhindi, Citation1992). The diffused peak occurs at about 2θ = 39.2°, confirming the presence of SiC (Figure ), this amount to a yield of 7.97 wt. %. However, the yield of other polytypes of SiC confirmed in the reaction kinetics is about 66.43 wt. %, a characteristic treatment sequence for sample B1 treated at 1,650°C in a catalytic environment. A hexagonal SiC polytype (6H-SiC) diffused at a peak of about 2θ = 44.7°. Figure shows the XRD patterns at treatment temperature of 1,600°C in a self-catalytic environment, i.e. RH contain Fe2O3 as constituent compound (Neto & Kiminami, Citation2014). The XRD pattern for sample-designated C1 shows a yield of 3.13 wt.% of 6H-SiC, a polytype of SiC. The quantitative analysis for C1 shows a yield of 78.42 wt.% of amorphous phase; at a peak of about 2θ = 30° to 33.1°, unreacted carbon was observed to have been formed. When compared with other samples under investigation, sample C1 has more of cristobalite phase and this was observed to be more pronounced at treatment conditions than others (i.e. A1 and B1); similar behavior was described by Makornpan, Mongkolkachit, and Wasanapiarnpong (Citation2013). From the trend in the XRD patterns, crystallization of amorphous silica to cristobalite phase was dominant in sample C1. A cubic form of SiC (3C-SiC) being a polytype of SiC was observed in sample A1 with treatment temperature 1,650°C. The peaks of α-SiC being a hexagonal symmetry type of SiC was observed in sample B1, where more stacking sequence of a hexagonal silicon carbide might have favored the formation of 6H-SiC type. In sample A1 and C1, graphitic carbon peaks were observed partly due to the residual carbon in RH and the ones added as aid to enhance the Si to C reaction kinetics. However, the intensities of SiC peaks from B1 are lower than the intensities of SiC peaks from A1. A representative weight by percentage composition showing the reaction products of selected constituents is presented in Table (obtained from the quantitative analysis of the XRD diffraction peaks). As observed in the preliminary investigation, the conversion of amorphous silica to cristobalite was around a temperature interface of 1,250°C; beyond this, other polytypes of SiC crystallize. Table shows the weight percent for the reaction products; B1 has the minimum value of residual carbon, while A1 which was treated at a temperature of 1,650°C has the highest value of residual carbon. For sample A1, about 70.9 wt.% of amorphous phase was captured, while B1 has about 62.81 wt.%. However, higher proportion of polytypes of SiC were formed at B1; polytypes such as 3C-SiC, 4H-SiC and 6H-SiC have been reported as the most common polytypes by Verma and Krishna (Citation1966). They are majorly being developed for electronics application, classified as beta and alpha types. The beta type crystallizes in a cubic lattice geometry such as 3C-SiC, while the alpha type in hexagonal geometry such as 6H-SiC. Hence, these polytypes dominate all the phases obtained from sample B1. The crystal structure of polytypes in B1, going by the positions of Si and C atoms in the plane has higher number of stacking sequence as observed in this region. The total yield of SiC derivatives in sample A1 amounts to 63.04 wt.%, for sample B1 about 74.4 wt.%, and for sample C1, about 3.28 wt.%. The yields (in A1 and B1) are higher than those reported by Ali and Tindyala (Citation2015), Ahmad, Ali, Ibrahim, and Baig (Citation2014), and Janghorban and Tazesh (Citation1997). From Table , it was observed that sample A1 contains more of β-SiC. Hence, the cristobalite phase disappears as the temperature increases, which is in line with previous report (Krishnarao, Godkhindi, & Chakraborty, Citation1992). The polytypes of α-SiC phase were observed to appear though in smaller proportion. This similar observation was corroborated by the XRD spectrum in Figure .
Table 1. Composition by weight of selected reaction products
3.3. Microstructure
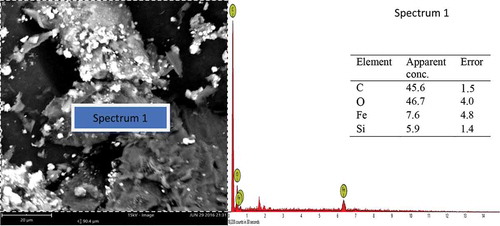
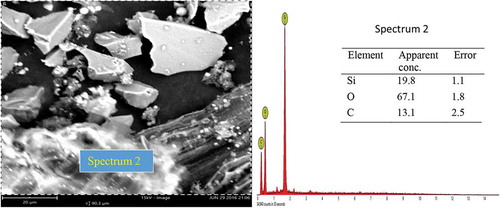
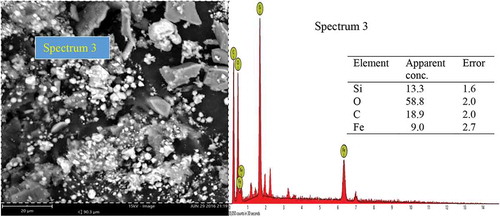
A representative SEM micrograph and EDX profile of the Si-based refractory compounds are presented in Figures –. Representative scanning electron microscope photomicrograph for sample designated A1 is presented in Figure . The formation of SiC was compensated by the disappearance of silicon and a distinct cubic lattice symmetry of beta phase SiC as noted from the XRD results. A good proportion of graphite particles was observed at this interface also, and 3C-SiC polytypes formation was observed to be on 2H-SiC; similar behaviour has been reported by Yao, Lee, and Li (Citation2003). Figure shows the representative SEM micrograph and EDX of sample designated B1; from the EDX, the presence of silicon (Si) and oxygen (O) confirms the compound SiO2. Also, the presence of silicon (Si) and carbon (C) as observed in the EDX suggests the presence of SiC and unreacted C in the structure which are confirmed by the XRD spectra. The morphology of Si-based refractory compounds for sample-designated C1 and the elemental composition captured from the EDX analysis is represented in Figure . The growth of 6H-SiC polytypes must have characterized the phases form in sample C1. The EDX profile show peaks of silicon (Si), carbon (C), oxygen (O), and iron (Fe). The presence of silicon and oxygen suggests the occurrence of SiO2, and the presence of silicon and carbon suggests the occurrence of SiC, unreacted C and Si. The peak of iron suggests the occurrence of ferric compound being a constituent of RH as reported by Adam et al. (Citation2012), precipitating at the carbothermal treatment schedule. Further observation noted on C1 was that 3C-SiC growth was on 6H-SiC substrates; this trend was reported by Syvajarvi et al. (Citation2016).
4. Conclusions
The processing and structural characterization of Si-based carbothermal derivatives of RHs via unorthodox route has been investigated. The results show that:
The major functional groups present after carbothermal treatment of RH are OH−, Si–O–Si, and Si–C groups.
3C-SiC (C = cubic) which is β-SiC type, hexagonal symmetry 6H-SiC, and mixes of 3C-SiC, 2H-SiC, and 4H-SiC were various polytypes of SiC observed for different processing temperatures adopted.
Total SiC yield between 63 and 74 wt.% of the crystalline phase was obtained from the process.
The carbothermal treatment explored showed evidence of viability for the production of Si-based refractory compounds which can serve as reinforcement in the development of metal matrix composites.
Additional information
Funding
Notes on contributors
Adeolu Adesoji Adediran
Adediran Adesoji Adeolu is a Lecturer in the Department of Mechanical Engineering, Landmark University, Omu-Aran, Kwara State Nigeria. He is a registered Engineer with the Council for the Regulation of Engineering in Nigeria (COREN) and a member of several Professional bodies. He is a Doctoral Student at the Federal University of Technology, Akure and a visiting researcher to the Department of Mechanical Engineering Science, Faculty of Engineering and the Built Environment, University of Johannesburg, South Africa. He has published in both local and international journals. His interest is in material behaviour under the influence of stress, harsh environments, contact, and vibrations; by applying the broad principles of physical metallurgy, materials characterization and materials mechanics. The group’s key research activities are centered on developing low-cost and performance efficient engineering materials for transportation, structural, vibration control, and other technological applications where proper materials selection are crucial for overall system performance.
References
- Abo-El-Enein, S. , Eissa, M. , Diafullah, A. , Rizk, M. , & Mohamed, F. (2009). Removal of some heavy metals ions from wastewater by copolymer of iron and aluminum impregnated with active silica derived from rice husk ash. Journal of Hazardous Materials , 172, 574–579. doi: 10.1016/j.jhazmat.2009.07.036
- Adama, F. , Appaturi, J. N. , & Iqbal, A. (2012). The utilization of rice husk silica as a catalyst: Review and recent progress. Catalysis Today , 190(1), 2–14. doi:10.1016/j.cattod.2012.04.056
- Ahmad, K. , Ali, M. , Ibrahim, A. , & Baig, W. M. (2014). Optimizing the yield of SiC synthesized from indigenous biomass husk using different catalysts. Journal of Material Sciences & Engineering , 3(3), 1–4.
- Alaneme, K. K. , Akintunde, I. B. , Olubambi, P. A. , & Adewale, T. M. (2013). Fabrication characteristics and mechanical behaviour of rice husk ash-alumina reinforced Al-Mg-Si alloy matrix hybrid composites. Journal of Materials Research and Technology , 2, 60–67. doi: 10.1016/j.jmrt.2013.03.012
- Alaneme, K. K. , & Aluko, A. O. (2012). Fracture toughness (K1C) and tensile properties of as-cast and age-hardened aluminium (6063)-silicon carbide particulate composites. Scientia Iranica , 19(4), 992–996. doi: 10.1016/j.scient.2012.06.001
- Ali, M. , & Tindyala, M. A. (2015). Thermoanalytical studies on acid-treated rice husk and production of some silicon based ceramics from carbonised rice husk. Journal of Asian Ceramic Societies , 3, 311–316. doi: 10.1016/j.jascer.2015.06.003
- An, D. M. , Guo, Y. P. , Zhu, Y. C. , & Wangg, Z. C. (2010). A green route to preparation of silica powders with rice husk ash and waste gas. Chemical Engineering Journal , 162, 509–514. doi: 10.1016/j.cej.2010.05.052
- Boateng, A. A. , & Skeete, D. A. (1990). Incineration of rice hull for use as a cementitious material: The Guyana experience. Cement and Concrete Research , 20(5), 795–802. doi: 10.1016/0008-8846(90)90013-N
- Carmona, V. B. , Oliveira, R. M. , Silva, W. T. L. , Mattoso, L. H. C. , & Marconcini, J. M. (2013). Nanosilica from rice husk: Extraction and characterization. Industrial Crops and Products , 43, 291–296. doi: 10.1016/j.indcrop.2012.06.050
- Della, V. P. , Kühn, I. , & Hotza, D. (2002). Rice husk ash as an alternate source for active silica production. Materials Letters , 57, 818–821. doi: 10.1016/S0167-577X(02)00879-0
- Dhage, S. , Lee, H.-C. , Hassan, M. S. , Akhtar, M. S. , & Kim, C.-Y. (2009). Sohn et al. Formation of SiC nanowhiskers by carbothermic reduction of silica with activated carbon. Materials Letters , 63, 174–176. doi: 10.1016/j.matlet.2008.09.056
- Food and Agriculture Organization . (2015). FAO statistical pocketbook world food and agriculture. (pp. 28). Rome: FAO Statistical Yearbook, United Nations.
- Fu, Q. G. , Li, H. J. , Shi, X. H. , Li, K. Z. , & Sun, G. D. (2005). Silicon carbide coating to protect carbon/carbon composites against oxidation. Scripta Materialia , 52(9), 923–927. doi: 10.1016/j.scriptamat.2004.12.029
- Ghafoorian, N. S. , Bahramian, A. R. , & Seraji, M. M. (2015). Investigation of the effect of rice husk derived Si/SiC on the morphology and thermal stability of carbon composite aerogels. Materials & Design , 86, 279–288. doi: 10.1016/j.matdes.2015.07.093
- Guo, X. , Zhu, L. , Li, W. , & Yang, H. (2013). Preparation of SiC powders by carbothermal reduction with bamboo charcoal as renewable carbon source. Journal of Advanced Ceramics , 2(2), 128–134. doi: 10.1007/s40145-013-0050-4
- Housten, D.F. (1972). Rice chemistry and technology, American association of cereal chemist. St. Paul, MN , 301–352.
- Janghorban, K. , & Tazesh, H. R. (1997). Effect of catalyst and process parameters on the production of silicon carbide from rice hulls. Ceramics International , 25, 7–12. doi: 10.1016/S0272-8842(97)00077-1
- Kholmanov, I. N. , Kharlamov, A. , Barborini, E. , Lenardi, C. , & Bassi, A. L. (2002). Bottani et al. A simple method for the synthesis of silicon carbide nanorods. Journal of Nanoscience and Nanotechnology , 2(5), 453–456. doi: 10.1166/jnn.2002.127
- Kim, D. K. , Park, S. , Cho, K. , & Lee, H. B. (1994). Synthesis of SiC by self-propagating high temperature synthesis chemical furnace. Journal of the Korean Ceramic Society , 31(11), 1283–1292.
- Kim, H.-E. , & Koh, Y.-H. (2007). Highly aligned porous silicon carbide ceramics by freezing polycarbosilane/camphene solution. Journal of the American Ceramic Society , 90, 1753–1759. doi: 10.1111/jace.2007.90.issue-6
- Krishnarao, R. V. , & Godkhindi, M. M. (1992). Distribution of silica in rice husks and its effect on the formation of silicon carbide. Ceramics International , 18, 243–249. doi:10.1016/0272-8842(92)90102-J
- Krishnarao, R. V. , Godkhindi, M. M. , & Chakraborty, M. (1992). Maximisation of SiC whisker yield during the pyrolysis of burnt rice husks. Journal of Materials Science , 27(5), 1227–1230. doi:10.1007/BF01142027
- Krishnarao, R. V. , Subrahmanyam, J. , & Kumar, T. J. (2001). Studies on the formation of black particles in rice husk silica ash. Journal of the European Ceramic Society , 21, 99–104. doi:10.1016/S0955-2219(00)00170-9
- Larrard, F. , Gorse, J. F. , & Puch, C. (1992). Comparative study of various silica fumes as additives in high-performance cementitious materials. Materials and Structures , 25, 265–272. doi:10.1007/BF02472667
- Li, B. , Zhang, C. R. , Hu, H. F. , Cao, Y. B. , Qi, G. J. , & Liu, R. J. (2007). Preparation of silicon carbide coatings from liquid carbosilanes by chemical vapor deposition. Journal of Materials Engineering and Performance , 16(6), 775–778. doi:10.1007/s11665-007-9154-8
- Lin, Y.-J. , & Tsang, C.-P. (2003). The effects of starting precursors on the carbothermal synthesis of SiC powders. Ceramics International , 29(1), 69–75. doi:10.1016/S0272-8842(02)00091-3
- Lu, P. , & Hsieh, Y.-L. (2012). Highly pure amorphous silica nano-disks from rice straw. Powder Technology , 225, 149–155. doi:10.1016/j.powtec.2012.04.002
- Lutsenko, V. G. (2008). Silicon carbide whiskers with superlattice structure: A precursor for a new type of nanoreactor. Acta Materialia , 56, 2450–2455. doi:10.1016/j.actamat.2008.01.033
- Madelung, O. (1987). Intrinsic properties of group IV elements and III–V, II–VI and I–VII compounds. Langdolt-Bornstein New Series (pp. 22). New York-London: Springer-Verlag Berlin Heidelberg.
- Makornpan, C. , Mongkolkachit, C. , & Wasanapiarnpong, T. (2013). Fabrication of SiC ceramics from rice husks. Sur Sciences Technological , 21(2), 79–86.
- Martin, H.-P. , Ecke, R. , & Müller, E. (1998). Synthesis of nanocrystalline silicon carbide powder by carbothermal reduction. Journal of the European Ceramic Society , 18(12), 1737–1742. doi:10.1016/S0955-2219(98)00094-6
- Mohamed, H. , Abd El-Aziz, M. , Abd El-Aleem, S. , & El-Didamony, H. (2004). Effect of polycarboxylate on rice husk ash pozzolanic cement. Silicates Industriels , 69(9–10), 73–84.
- Najafi, A. , Fard, G. , Rezaie, H. R. , & Ehsani, N. (2012). Synthesis and characterization of SiC nano-powder with low residual carbon processed by sol-gel method. Powder Technology , 219, 202–210. doi:10.1016/j.powtec.2011.12.045
- Neto, E. F. , & Kiminami, R. H. G. A. (2014). Synthesis of silicon nitride by conventional and microwave carbothermal reduction and nitridation of rice hulls. Advanced Powder Technology , 25, 654–658. doi:10.1016/j.apt.2013.10.009
- Pierre, M. (2002). Silicon carbide and silicon carbide-based structures: The physics of epitaxy. Surface Science Reports, 48(1–4), 1–51.
- Prabhakaran, P. V. , Sreejith, K. J. , Swaminathan, B. , Packirisamy, S. , & Ninan, K. N. (2009). Silicon carbide wires of nano to sub-micron size from phenolfurfuraldehyde resin. Journal Materials Sciences , 44, 528–533. doi:10.1007/s10853-008-3087-y
- Rahman, I. A. , & Saleh, M. I. (1995). The formation of β-Sialon in the carbothermal reduction of digested rice husks. Materials Letters , 23(1–3), 157–161. doi:10.1016/0167-577X(95)00002-X
- Rajarao, R. , Seyed, R. F. , Rita, H. F. , & Veena, S. (2014). Synthesis of silicon carbide nanoparticles by using electronic waste as a carbon source. Materials Letters , 120, 65–68. doi: 10.1016/j.matlet.2014.01.018
- Ravi, B. G. , Omotoye, O. A. , Srivatsan, T. S. , Petrorali, M. , & Sudarshan, T. S. (2009). The microstructure and hardness of silicon carbide synthesized by plasma pressure compaction. Journal of Alloys and Compounds , 299, 292–296. doi: 10.1016/S0925-8388(99)00815-4
- Saleh, A. M. (2015). Activation of granulated blast-furnace slag using lime rich sludge in presence and absence of rice husk ash. International Journal Inn Technology Exploration Engineering, 5(3), 43–51.
- Satapathy, L. N. , Ramesh, P. D. , Agrawal, D. , & Roy, R. (2005). Microwave synthesis of phase-pure, fine silicon carbide powder. Materials Research Bulletin , 40, 1871–1882. doi: 10.1016/j.materresbull.2005.04.034
- Singh, S. K. , Mohanty, B. C. , & Basu, S. (2002). Synthesis of SiC from rice husk in a plasma reactor. Bulletin of Materials Science , 25, 561–563. doi: 10.1007/BF02710551
- Soltani, N. , Bahrami, A. , Pech-Canul, M. I. , & González, L. A. (2014). Review on the physicochemical treatments of rice husk for production of advanced materials. Chemical Engineering Journal , 264, 899–935. doi: 10.1016/j.cej.2014.11.056
- Srivastava, V. C. , Mall, I. D. , & Mishra, I. M. (2007). Adsorption thermodynamics and isosteric heat of adsorption of toxic metal ions onto bagasse fly ash (BFA) and rice husk ash (RHA). Chemical Engineering Journal , 132, 267–278. doi: 10.1016/j.cej.2007.01.007
- Sujirote, K. , & Leangsuwan, P. (2003). Silicon carbide formation from pretreated rice husks. Journal of Materials Science , 38, 4739–4744. doi: 10.1023/A:1027475018767
- Syväjärvi, M., Ma, Q., Jokubavicius, V., Galeckas, A., Sun, J., Liu X., ... Svensson, B. G. (2016). Cubic silicon carbide as a potential photovoltaic material. Solar Energy Materials and Solor Cells , 145, 104–108. doi:10.1016/j.solmat.2015.08.029
- Verma, A. R. , & Krishna, P. K. (1966). Polymorphism and polytypism in crystals . New York: Wiley.
- Vlaev, L. T. , Petkov, P. , Dimitrov, A. , & Genieva, S. D. (2011). Cleanup of water polluted with crude oil or diesel fuel using rise husks ash. Journal of the Taiwan Institute of Chemical Engineers , 42(6), 954–964.
- Yang, G. Y. , Wu, R. B. , Chen, J. J. , Song, F. F. , & Pan, Y. (2007). Growth of silicon carbide whiskers in Fex.Siy flux. Materials Chemistry and Physics , 106, 236–239. doi :10.1016/j.matchemphys.2007.05.040
- Yao, Y. , Lee, S. T. , & Li, F. H. (2003). Direct synthesis of 2H–SiC nanowhiskers. Chemical Physics Letters , 381, 628–633. doi: 10.1016/j.cplett.2003.09.149
- Zhong, Y. , Shaw, L. , Manjarres, M. , & Zawrah, M. F. (2010). Synthesis of silicon carbide nanopowder using silica fume. Journal of the American Ceramic Society , 93(10), 3159–3167. doi: 10.1111/jace.2010.93.issue-10