 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, the structural modification of Siam weed (Chromolaena odorata) was performed using NaOH–H2O2- and Ca(OH)2–H2O2-based oxidative pretreatment for the enzymatic conversion of the biomass to a biocommodity, reducing sugar (RS). Pretreatment of raw sample was evaluated at temperatures of 60°C, 70°C, 80°C, 90°C for different time intervals of 3, 6, 9, 12 h in alkaline medium (NaOH or Ca(OH)2). The effects of pretreatment time and temperature were considered in obtaining the optimum conditions. The optimum conditions for NaOH–H2O2 was obtained at 70°C and 3 h with a maximum cellulose content of 44.29%(w/w), lignin content reduced to 21.09% from the initial raw value of 24.2%. Pretreatment with Ca(OH)2–H2O2 resulted in the optimum conditions obtained to be 70°C for 3 h with a cellulose content of 47.18%. Enzymatic hydrolysis on the pretreated biomass at the optimum conditions showed NaOH–H2O2-treated sample yielded 424.35 mg equivalent glucose/g biomass of RS while Ca(OH)2–H2O2-treated sample yielded 335.81 mg equivalent glucose/g biomass of RS. The untreated raw sample yielded 68.75 mg equivalent glucose/g biomass of RS. Consequently, NaOH–H2O2 pretreatment displayed a higher efficiency than Ca(OH)2–H2O2 pretreatment. Stereomicroscopic and scanning electron microscopic imaging of the treated and untreated samples revealed morphological disruptions brought about by the treatments.
PUBLIC INTEREST STATEMENT
Currently, majority of the world’s population rely on this biomass as primary source of energy. In order to effectively utilize biomass as an alternative and sustainable source of energy, fuels, to the traditional fossil fuels, adequate and promising methodologies should be developed for the treatment and conversion of the biomass to fuels and chemicals. Lignocellulosic biomass materials remain the next primary energy supply to the world after coal and gas. Generally, to be liable for effective conversion to high energy value products in a biorefinery, lignocellulosic biomass needs to undergo initial pretreatment because of the complex structure composed mainly of cellulose, hemicellulose, lignin, and other components in trace amounts. Several pretreatment methodologies exist for the deconstruction of lignocelluloses for the production of fuels and chemicals. This study investigated the applications of alkaline peroxide oxidation pretreatment to deconstruct Siam weed in preparation for further enzymatic digestion leading to the production of biocommodities.
1. Introduction
The potential of biomass energy as an alternative to fossil-based energy sources for fuels and chemicals production is very huge. Clean energy from lignocellulosic materials is extremely promising because it is available in large quantity. Lignocelluloses are cheap, and they have near-zero greenhouse emissions. This evolution as alternative to fossil fuel currently cuts across the entire world. The growing demand for energy, materials, and food; fear of depletion of fossil reserves and environmental concerns associated with the use of fossil fuels have fuelled the continuing interest of researchers in renewable resources (Awosusi et al., Citation2017a). The production of bioenergy or renewable energy from energy crops and lignocellulosic residues, agricultural and wood based, is undergoing worldwide economic analysis for efficient commercialization. Some of the energy crops have already been used on the commercial scale. Biofuels derived from these sources are important choices for exploiting alternative energy and reduction of pollution gases. The production of biofuels and some biocommodities until recently has been focused on food-based resources like grains, cereals, and other edible starch-derived crops. With the competition that this is bringing to already depleted supplies of food to growing world populations, researchers are looking inwards to developing biofuels and chemicals production based on agricultural residues, wood, municipal and industrial wastes. Biomass energy may be consumed either directly or indirectly. The largest potential feed stocks for biocommodities is plant biomass (lignocellulosic) (Kim & Dale, Citation2004). Production of biocommodities from biomass could be an attractive alternative to disposal of these residues (Wyman, Citation2001). Utilization of these waste materials is very important as there is reduction in the overdependency on fossil fuels that degrade our environment. Lignocelluloses take about 50% of the total biomass in the world with an estimated annual production of between 1 × 105 and 50 × 105 tonnes (Classen, Sijtsma, Stams, De Vries, & Weusthuis, Citation1999). The overall efficiency of processes designed to convert lignocellulosic biomass to valuable products lies on the pretreatment method used and in determining the compositions of such material. Standard methods as gravimetric, chromatography, and spectroscopic are routinely explored in the scientific literature. Lignocelluloses mainly consist of cellulose, hemicelluloses, and lignin which are bonded together by covalent bonding, various intermolecular bridges, and van der Waals’ forces forming a complex structure, making it resistant to enzymatic hydrolysis and insoluble in water (Ayeni et al., Citation2018; O’Sullivan, Citation1997). These polysaccharides (cellulose and hemicelluloses) and lignin in lignocelluloses are useful precursors in producing fuels and chemicals such as ethanol, butanol, succinic acid, furfural, energy as electricity, etc. (Awosusi et al., Citation2017a; Ghosh et al., Citation2015). Hemicelluloses as xylan-derived products such as ethanol, xylitol (a low-caloric sweetener and preservative agent against dental decay found as ingredient in chewing gums, toothpaste, and diabetic products), and xylo-oligosaccharides (a bioactive ingredient of food and health products) have already commercial applications in a wide range of industries (Deutschmann & Dekker, Citation2012). Cellulose is recalcitrant to biodegradation and needs to be hydrolyzed in an initial pretreatment step into its constituent cellobiose units and into simpler D-glucose units in order to be liable to biochemical conversion (Ayeni et al., Citation2013a, Citation2018, Citation2013b). The ash and extractive components are in trace amounts in the entire complex structure. The successful action of enzymatic hydrolysis on lignocellulosic biomass requires a suitable pretreatment that can effectively break the linked lignin and crystalline cellulose complex. Some kinds of chemical, physical, and/or biological pretreatments break the lignin sheath. Their actions on biomass also reduce the degree of cellulose crystallinity, remove or separate hemicellulose from cellulose and increase the accessible surface area of biomass, resulting in an enhancement of lignocellulosic substrate digestibility. Hydrogen peroxide and alkaline oxidative pretreatment (alkaline peroxide oxidation (APO)) is known to decrystallize cellulose (Gould, Citation1984).
In this study, the raw and pretreated materials (Chromolaena odorata: Siam weed) for the production of the reducing sugar (RS) (a reliable precursor for bioethanol and other chemicals production) were characterized through gravimetric analysis. The biomass quality parameters (proximate, ultimate, and high heating value (HHV)) analyses were carried out on the raw biomass (RB) in order to reveal the usability of chosen material for the sustainable production of energy, chemicals, and a wide range of commodities. The efficiency of pretreatment process was also evaluated based on the lignin removal (%w/w), hemicellulose solubilization, (%w/w), and cellulose content (%w/w). Furthermore, the effects of enzymatic digest ability on NaOH–H2O2-and Ca(OH)2–H2O2-pretreated samples for the bio-based commodity (RSs) production were investigated.
2. Materials and methods
2.1. Raw material preparation and APO pretreatment
Siam weed (Chromolaena odorata), is locally called “ewe Akintola” in some parts of Nigeria. Siam weed is an invasive exotic weed (Zhao, Zhang, & Liu, Citation2010), and a typical fast-growing perennial herb. Materials were prepared from the field to the laboratory before analysis by harvesting the shoots (leaves and stems) from an open fallow land around Iju town, near Ota (6°40ʹ N 3°08ʹ E), South West, Nigeria (the growth period of the plant on the land was monitored to be 6 months). Sourcing and preparations for the biomass materials were as previously described (Ayeni, Omoleye, Hymore, & Pandey, Citation2016; Ayeni, Omoleye, Mudliar, Hymore, & Pandey, Citation2014a). The leaves were removed off from the branches manually. The stems were cut to 5 ± 1 cm equal lengths and dried in an open space (35 ± 2°C) for 3 days (8 h each day). Biomass samples were further shredded in a rotor mill (Retsch1 SE-200, Germany) fitted with a 4-mm screen (Ayeni & Daramola, Citation2017). This was followed by sieving into different size fractions (Chang & Holtzapple, Citation2000). Sieved fractions were dried in a convection oven at 105°C for 3 h to a dry matter content of 88%. Screened sizes ranged from 0.25 to 1 mm. The screened sample within the size range of 1 and 0.5 mm were retained while smaller particles were discarded because they corresponded mainly to sand. The bigger size fractions were manually mixed for 10 min to obtain some homogeneous equal proportions of sizes. The dried RB was stored in plastic containers and kept in a locker in the laboratory until appropriate time to be used. All pretreatment experiments were carried out as previously described (Ayeni & Daramola, Citation2017; Ayeni et al., Citation2014a). Pretreatments were carried out in a stainless steel pressured vessel heated in an oven to the required reaction temperature. Six millilitres of 35%(w/v) H2O2 was added to 194 mL distilled water in a 250-mL Erlenmeyer flask with the appropriate weight of the alkali (NaOH or Ca(OH)2) making a total working volumeof 200 mL. This resulted in a 1%(v/v) H2O2–alkaline solution. NaOH weighing 1.8 g was added to the solution of 6 mL H2O2 in 194 distilled water in order to make pH of 11.5 for SHP (sodium hyroxide–hydrogen peroxide) pretreatments and 2.3 g Ca(OH)2 was added to make pH of 11.5 for CHP (calcium hyroxide–hydrogen peroxide) pretreatments. A slurry (mixing was done with a magnetic stirrer for uniformity) was formed with the 1%(v/v) H2O2-alkaline solution and 10 g of dried solid biomass sample. The mixture was later transferred into the stainless steel pressured vessel. All other working volumes were based on this initial estimation. Reactions were made to occur for the specified time periods (3, 6, 9, and 12 h) and temperatures (60°C, 70°C, 80°C, and 90°C) (Tables –). Extractives of 4.8%(w/w) and 0.97%(w/w) ash in trace amounts were also included in the RB total compositions. All data generated were taken in duplicate to guarantee reproducibility and repeatability of the study.
Table 1. Cellulose content remaining after pretreatments (%w/w)
Table 2. Hemicellulose content remaining after pretreatments (%w/w)
Table 3. Lignin content remaining after pretreatments (%w/w)
After pretreatment, the mixture was cooled to room temperature, and the slurry was separated into solid and liquid fractions by vacuum filtration. The solid fraction was properly washed with distilled water to a pH of 7. Small portion of the liquid fraction was used to estimate the soluble lignin content (Sluiter et al., Citation2008). Total solid left after pretreatment was estimated by weighing 2 g of the treated wet sample and drying at 105°C until constant weight was achieved. It is expected that during pretreatment there will be weight loss due to lignin removal and hemicellulose solubilization/hydrolysis, the total solid left was used for material balance in estimating cellulose left, hemicellulose solubilized, and lignin removal. The remaining wet treated materials were later used to estimate the digest ability of treated materials by the cellulase enzymes.
2.2. Biomass quality parameters estimation
The HHV content, proximate, and ultimate analyses are excellent indicators for biomass quality for processing into fuels, chemicals, and related end products. HHV was measured by DryCal oxygen bomb calorimeter equipped with DryCal 2010 software (ASTM CitationD2015-00). For the ultimate analysis, carbon and sulphur elemental compositions were determined using a LECO SC632 (equipped with LECO SC632 sulphur/carbon determinator software) with the furnace temperature maintained at 1350°C. Elemental compositions of hydrogen and nitrogen were determined by using Flash 2000 CHNS/O analyser (Thermo Scientific, USA) (Awosusi, Ayeni, Adeleke, & Daramola, Citation2017b). Oxygen (wt.%) was measured by the difference of C, H, N, S, and ash sum from 100%. The proximate analysis was carried out by the three steps of drying, devolatilization in inert gas, oxidation in oxygen making use of the thermogravimetric analyzer (El-Sayed & Mostafa, Citation2015; Mani, Murugan, Abedi, & Mahinpey, Citation2010). Volatile matter (VM), fixed carbon (FC), and moisture contents (M) were measured according to ASTM D-5142-02a (Asadieraghi, Ashri, & Daud, Citation2014) using a Perkin-Elmer STA 6000 Simultaneous Thermal Analyzer (STA) equipped with Pyris series-STA 6000 instrument viewer software. Nitrogen as purge gas and oxygen for oxidation were operated at 400 kPa.
2.3. Compositional analysis of the raw and pretreated materials
Compositional analysis on the raw and pretreated samples was carried out gravimetrically as described elsewhere (Ayeni et al., Citation2014a; Blasi, Signorelli, Russo, & Rea, Citation1999; Li, Xu, Liu, Yang, & Lu, Citation2004; Lin, Yan, Liu, & Jiang, Citation2010). Gravimetric analysis describes a set of methods for the quantitative determination of a sample or material based on the mass of a solid. Dried solids are weighed with an analytical balance. Accurate weighing of materials can provide precise analysis. Gravimetric analysis provides very little room for instrumental error. It does not require expensive equipment. Extractives were determined by means of the Soxhlet extractor. Acetone of 150 mL was used as the solvent for the extractives on 2.5 g of dried biomass. Solvent extraction was carried out at 70°C for a 4-h run period. The residence time for the rising stage was 25 min. Samples were air-dried at room temperature for few minutes. Constant weight was achieved by drying samples at 105°C in a convection oven. The extractives weight percent, %(w/w) was calculated based on the loss in weight between the dried RB and extracted sample. Mineral components were determined by ashing at 575°C. The hemicellulose content was estimated by placing 1 g of dried biomass from the extractive analysis into a 250-mL Erlenmeyer flask. One hundred and fifty millilitres of 0.5 mol/L NaOH solution was added. The mixture was boiled for 3.5 h with distilled water. Slurry was vacuum filtered after cooling to room temperature and washed (until pH value of solution approached 7). The residue was dried to a constant weight at 105°C in a convention oven after cooling. The difference between the sample weight before and after this treatment is the hemicellulose. Lignin content was estimated by weighing 300 mg of dry biomass in glass test tubes. Three millilitres of a 72% H2SO4 was added to the solid. Acid hydrolysis was made to occur by keeping the samples at room temperature for 2 h with manual mixing of samples every 30 min. Eighty-four milliliters of distilled water was added to each test tube after the 2-h acid hydrolysis step bringing the total volumeto 87 mL. The samples were autoclaved for 1 h at 121°C. After the second weak acid hydrolysis step, the mixtures were cooled to room temperature and filtered through vacuum using a filtering crucible. The acid insoluble lignin was determined by drying the residue at 105°C and accounting for ash by incinerating the hydrolyzed samples at 575°C in a muffle furnace. The acid-soluble lignin fraction was determined by measuring the absorbance of the acid hydrolyzed samples at 320 nm (Sluiter et al., Citation2008). The cellulose content was calculated by difference, assuming that extractives, hemicellulose, lignin, ash, and cellulose are the only components of the entire biomass. The material balance for the lignin removal (%w/w) and hemicellulose solubilization (%w/w) were estimated based on the following equations:
where
LCP = Lignin content remaining after pretreatment
LCU = Lignin content in the untreated biomass
TS = Total dry solids remaining after pretreatment
where
HCP = Hemicellulose content remaining after pretreatment
HCU = Hemicellulose content in the untreated biomass
LCP, LCU, TS, HCP, and HCU are in %weight.
2.4. Enzymatic hydrolysis of raw and optimized pretreated samples
The raw and optimized treated samples were hydrolysed by cellulase enzymes to determine the efficiency of substrate conversion for the APO pretreatment. The enzymatic process is as reported by Ayeni et al. (Citation2014a). Enzymatic conversion was performed at 2% dry biomass content of total saccharification volume. Five milliliters of sodium citrate buffer at 0.1 mol/L concentration and pH of 4.8 was added to the wet materials in 50-mL culture tubes. Trichoderma reesei cellulase enzymes system was prepared with an activity of 57.8 filter paper unit (FPU)/mL. The treated biomass loading was 25 FPU/g dry biomass (wet materials were used for the enzymatic hydrolysis and the material balance was calculated based on equivalent dried weight). Total mixture volumeof 20 mL was attained by adding an appropriate volumeof distilled water to the citrate buffer and wet biomass. After the hydrolysis period of 72 h, 0.5 mL aliquot was sampled and analysed for RSs. Experiments were conducted at 50oC in an incubator. To quench the hydrolysis, the samples were boiled for 15 min and then cooled in an ice bath. After hydrolysis, the samples were centrifuged at 4000 rpm for 5 min to remove residual solids. Fermentable sugars were estimated as RS with 3,5-dinitrosalicylic acid method (Miller, Citation1959), using glucose as standard. RS yields from enzymatic hydrolysis were calculated as mg equivalent glucose per g dry substrate (based on equivalent glucose in the hydrolyzed sample) (Ayeni et al., Citation2013b; Kim, Citation2004).
where Y = RS yield (mg equivalent glucose/g dry biomass)
S = sugar concentration in diluted sample (mg equivalent glucose/mL)
D = dilution factor
V = working volume(mL)
W = weight of dry treated biomass (g)
2.5. Stereomicrograph imaging
Air-dried raw and treated Siam weed biomasses were subjected to stereomicroscope imaging. The samples were placed on a background. Images were captured with a Nikon DS-Fi2 CCD camera operated by a Nikon Digital Sight System (Nikon, Japan). The Nikon SMZ745T stereomicroscope was equipped with NIS-Element D Z-Series 7 software. A Motic MLC-150C was used to adjust the brightness of the microscope to between 0% and 100%.
2.6. Scanning electron microscope imaging
The morphological changes of the biomass during the pretreatment were monitored by using a scanning electron microscope (SEM). The dried samples were mounted on aluminium stubs using conductive carbon tape followed by sputter coating with carbon and gold–palladium at 5-nm scale. Biomass samples were examined using FEI Quanta 200 SEM operated under vacuum between 3.9 × 10−4 to 2.2 × 10−3 Pa with a voltage of 30 kV.
3. Results and discussion
3.1. Quality parameters estimation
The proximate fractions representation of the RB (on dry basis) is shown in Figure .
Figure 1. Proximate fractions representation (weight%) of raw Siam weed biomass: (M) moisture, (VM) volatile matter, (FC) fixed carbon.
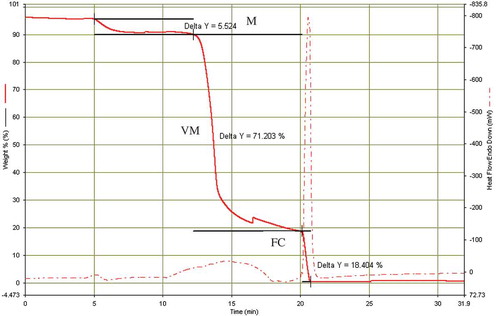
The moisture contained was recorded as 5.52%, VM was 71.20%, and the FC as 18.40%. The ash content, 4.67%, was obtained by subtracting the summation of moisture, VM, and fixed matter from 100%. The low moisture content in Siam weed shows that the biomass is suitable for fuels, chemicals, and other biocommodities production. High moisture content can lead to microbial respiratory activity thereby deteriorating the quality of the fuel. The VM involves the short and long chain, and aromatic hydrocarbons which are useful precursors for the production of platform chemicals. The high value of 71.20% is indicative of the suitability of the biomass material for fuels and energy thermochemical conversions. The low ash content (about 5%) reduces harmful deposits during thermochemical processing and further reduces extensive equipment maintenance.
The ultimate analysis and the higher heating value are given in Table . Generally, during biomass combustion and processing, SO2 and NOx gases are common emissions. Therefore, high level of sulphur content in biomass can evolve SO2 which generates sulphates, thereby leading to ash deposits. In the raw Siam weed biomass, ultimate analysis shows negligible sulphur content (0.15%) and nitrogen composition (1.48%). In addition, high carbon content in RB (43.38%) and higher heating values (about 17 MJ/kg) (Table ) point to the suitability of the biomass for fuels and chemicals production. Furthermore, using the ultimate analysis values, a representative formula in relation to carbon is CH0.97N0.03S0.001O0.863. This establishes the empirical formula for the Siam weed biomass sample.
Table 4. The ultimate analysis and higher heating value of the raw biomass
3.2. Effect of pretreatment on the solid biomass
APO is an efficient pretreatment method. It fractionates the biomass into the solid and liquid fractions. The solid fraction is majorly of polysaccaharides (more of cellulose and less of hemicellulose). After the APO pretreatments, part of the lignin in the RB was expected to have been dissolved into the liquid fraction. The solubilization of hemicellulose and lignin into the liquid fraction allows the cellulose retained in the solid fraction to be better exposed to further enzymatic attacks in order to liberate fermentable sugars. During lignocelluloses solubilization in hydrogen peroxide oxidative condition, hydroxyl radicals are formed which degrade lignin, enhances cellulose content, and produce low-molecular-weight products (Kumar & Sharma, Citation2017). Percentage of cellulose content in the solid fraction varied from 38.2%(w/w) (pretreatment of NaOH–H2O2 at 6 h and 70°C to the highest value of 48.8%(w/w) (pretreatment with NaOH–H2O2 at 9 h and 90°C) (Table , Figure ). It can be noticed in Figure that the cellulose content reduced to 38.2%(w/w) from an initial RB cellulose content of 40.1%(w/w) (higher temperature of 80°C and pretreatment time of 12 h). This showed that the cellulose degradation occurs at much higher temperature and longer pretreatment period instead of an enrichment.
Figure 2. Effect of time and temperature on cellulose content for NaOH–H2O2 (SHP)- and Ca(OH)2–H2O2 (CHP)-treated samples.
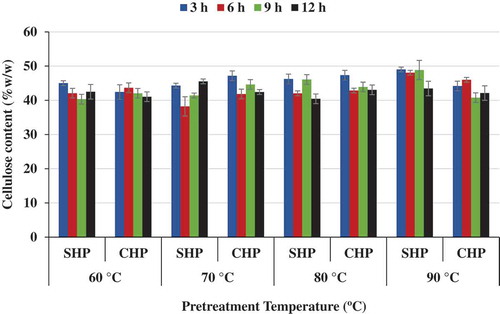
An efficient pretreatment process should have an appreciable increase in the cellulose content in the solid fraction, less of hemicellulose and less of the lignin. In other words, the more the lignin removal, the better the pretreatment process. In general, there was a better performance for the NaOH–H2O2-pretreated biomass than that for the Ca(OH)2–H2O2 pretreatments. For example, SHP at 3 h and 90°C produced a cellulose content of 49% compared to CHP under the same conditions producing 44.2% cellulose content. The hemicellulose solubilization increased at higher temperatures for the CHP process than at lower temperatures. At 80°C and 3 h, under the CHP process, the hemicellulose solubilization was 69.5% (Figure ). This supports the fact that increasing temperatures made the Ca(OH)2 more soluble. Also, lignin removal for the SHP process was 48.6%(w/w) for 3 h and 70°C pretreatment conditions (Figure ). CHP process for these conditions yielded 42.4%(w/w) lignin removal. It was also noted that increased temperatures favoured more cellulose content, which in most cases was related to increases in hemicellulose solubilization and lignin removal. In all, no matter how little lignin removal was in the experiments, favourable effects for further enzymatic hydrolysis are expected to be caused by the APO process. In the APO process, there is an appreciable increase in the degree of hydration of the cellulose polymer (Gould, Citation1984). From all the experimental conditions, it was noticed that more of the hemicellulose was solubilized than lignin removal (Figures and ).
Figure 3. Effect of time and temperature on hemicellulose solubilization for NaOH–H2O2 (SHP)- and Ca(OH)2–H2O2 (CHP)-treated samples.
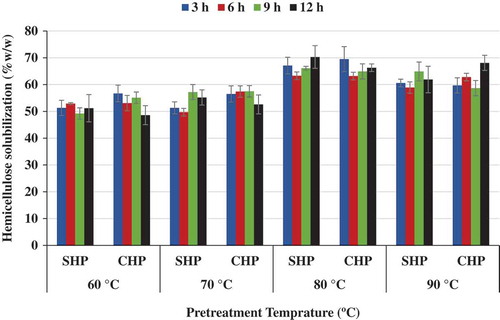
Figure 4. Effect of time and temperature on lignin removal for NaOH–H2O2 (SHP)- and Ca(OH)2–H2O2 (CHP)-treated samples.
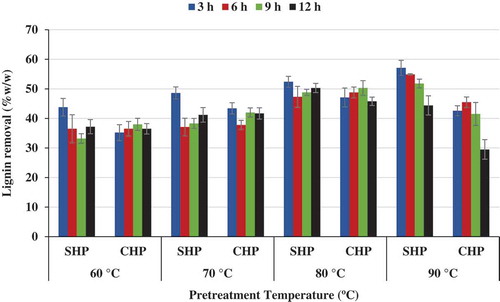
Maximum hemicellulose solubilization, 70.3%(w/w), was achieved for SHP process at 80°C and 12 h. The corresponding value for the CHP process was 66.3%(w/w). Maximum lignin removal, 57.1%, occurred for SHP at 90°C and 3 h, and the CHP also produced 42.6%(w/w) lignin removal at 90°C and 3 h. The lignin removal was at the maximum of 50.5% for CHP (at 80°C and 9h) (Figure ).
3.3. Optimization of pretreatment process and enzymatic hydrolysis
Lignin and acetyl groups in hemicellulose as well as crystallinity in cellulose and hemicellulose are significant challenges for cellulase enzymes to access lignocellulosic fibre matrix (Ayeni, Adeeyo, Oresegun, & Oladimeji, Citation2015; Chang & Holtzapple, Citation2000). The optimization of the pretreatment was determined on the best combinations yielding highest cellulose content, highest hemicellulose solubilization (hydrolysis), and highest lignin removal (for both NaOH–H2O2 and Ca(OH)2–H2O2 pretreatments). The optimization was considered for only the pretreated solid fraction and for the best pretreatment conditions combination. The optimum responses (cellulose content, hemicellulose solubilization, and lignin removal) were obtained at 70°C and 3 h (Figure –). At the optimized pretreatment conditions, the cellulose content was 44.3%(w/w), 50.3%(w/w) for hemicellulose solubilization and 48.6%(w/w) lignin removal for the SHP treated samples. In addition, at these same conditions, CHP-treated samples yielded 47.2%(w/w) cellulose content, 56.5%(w/w) hemicellulose solubilization, and 43.4%(w/w) lignin removal.
The enzymatic digest ability of biomass is affected by the pretreated methods used and the structural modification of the biomass (e.g. lignin content, acetyl group content, and crystallinity) (Holtzapple, Cognata, Shu, & Hendrickson, Citation1990). Considering RS yields after enzymatic hydrolysis of pretreated Siam weed samples (based on the optimized pretreatment conditions), the maximum RS yield showed NaOH–H2O2(SHP)-treated sample produced 424.35 mg equivalent glucose/g dry biomass of RS while Ca(OH)2–H2O2(CHP)-treated sample produced 335.81 mg equivalent glucose/g dry biomass of RS. The untreated raw sample yielded 68.75 mg equivalent glucose/g dry biomass of RS. NaOH–H2O2 pretreatment showed to be more efficient than Ca(OH)2–H2O2 pretreatment. In our previous work (Ayeni et al., Citation2014b), for APO pretreatment on unscreened sugarcane bagasse under the same hydrogen peroxide concentration but different temperature and pretreatment time, maximum RS obtained was 297.56 mg equivalent glucose/g dry biomass (pretreatment conditions of 50°C, 4 h, and 1%H2O2). Comparing with Ayeni et al. (Citation2014b), this study showed a higher RS obtained at pretreatment conditions of 70°C, time 3 h, and 1%H2O2, than at 50°C, time 4 h, and 1%H2O2. The ease of the production of RS at higher temperature of 70oC was because the lignocellulosic complex structure is ruptured at increasing temperature (Ayeni & Daramola, Citation2017; Kim, Citation2004). At higher temperature, there will be more pores for cellulolytic attacks and at the same time lignin removal will be enhanced, thereby increasing RS yields during enzymatic conversion (Holtzapple et al., Citation1990; Kim, Citation2004; Kim & Dale, Citation2004). Increasing the temperature increases the rate of reaction. In most reactions, rate of reaction doubles for every 10°C rise in temperature.
3.4. Physical and morphological deconstruction of biomass
The stereomicroscope imaging of the treated and untreated biomass showed size variability and clumping of particles (Figure and 5). The treated materials appeared thinner, smaller, and clumped together unlike the RB particles arrangements which were more scattered. These variations are attributed to surface morphology, area, and energy (as a result of relocalization of lignin to the particle surface (Ayeni & Daramola, Citation2017; Chundawat et al., Citation2011).
Figure 5. Stereomicroscopic images showing particle size variability. (a) Raw Siam weed (b) Treated Siam weed.

Scanning electron microscopic images of the untreated, recovered treated solids are shown in Figure and 6. Figure , which is the image of the untreated biomass, shows arranged structures. However, during treatments, the arranged structures have been extremely made irregular by pretreatments (Figure ), thereby opening more pores for enzymatic hydrolysis. The SEM image (Figure ) displays cell wall distortion, and the microfibres have been pulled out from their coverings (red arrows in Figure ). The images further reveal significant surface disruptions in the treated samples.
4. Conclusion
The study evaluated the feasibility of using APO pretreatment methods namely, sodium hydroxide–hydrogen peroxide enhanced pretreatment (SHP) and calcium hydroxide–hydrogen peroxide enhanced pretreatment (CHP), to deconstruct Siam weed towards its bioconversion to RS. Gravimetric analysis was used to quantify the compositions of both treated and untreated materials. The efficiency of any pretreatment methodology used on lignocellulosic biomass is directly linked to the ease of hydrolysis on treated materials by cellulase enzymes in order to produce fermentable sugars for easy microbial digestion yielding bioproducts such as ethanol. Considering RS yields after enzymatic hydrolysis of pretreated Siam weed samples (based on the optimized pretreatment responses of cellulose content, hemicellulose solubilization, and lignin removal at 70°C and 3 h), the maximum RS yield showed NaOH–H2O2(SHP)-treated sample produced 424.35 mg equivalent glucose/g biomass while Ca(OH)2–H2O2 (CHP)-treated sample produced 335.81 mg equivalent glucose/g biomass of RS. The SHP process yielded higher lignin removal than CHP-treated samples; as a result, SHP proved to be a better choice than CHP process. However, future studies will be focused on how to compare the economic benefits of the two processes. Results of the morphological examination of the raw and treated materials substantiate the observed effectiveness of the pretreatment methods to disrupt the complex structure of the lignocellulosic biomass, thereby enhancing susceptibility of the biomass to enzymatic and microbial attacks.
Acknowledgement
The management of Covenant University, Canaan land, Ota, Nigeria is appreciated for the funding of this publication.
Additional information
Funding
Notes on contributors
Augustine O. Ayeni
A.O. Ayen holds a PhD degree in Chemical Engineering. He had his postdoctoral fellowship experience at Wits University, Johannesburg, South Africa. His research areas include solid and liquid waste treatment and beneficiation to fuels and chemicals, environmental pollution, sustainable and renewable energy, statistical modelling and optimization, bioprocess engineering.
Michael O. Daramola
M.O. Daramola research interests include membrane technology and catalysis, carbon capture and utilization waste treatment and beneficiation strategies, renewable and sustainable energy. M. O . Daramola researches include membrane technology and catalysis, carbon capture and utilization of wastes, waste treatment and beneficiation strategies.
Adeola Awoyomi
Adeola Awoyomi holds a master’s degree in process systems engineering from Cranfield University, Cranfield, United Kingdom. She is currently a doctoral researcher at Cranfield University with interests in carbon capture and storage. Adeola Awoyomi holds an MSc degree from Cranfield University, United Kingdom. She is a doctoral researcher at Cranfield University with interests in carbon capture and storage.
Francis B. Elehinafe
F.B. Elehinafe is a researcher with interests in environmental monitoring, air pollution modelling and control engineering. F. B. Elehinafe is a researcher with interests in environmental monitoring, air pollution engineering.
Ajibola Ogunbiyi
A. Ogunbiyi research interests include renewable energy, simulation and modelling of chemical processes.
Patrick T. Sekoai
P. T. Sekoai research areas include biohydrogen and biofuels production from wastes, bioprocessing.
Johnson A. Folayan
J. A. Folayan research interests include drilling fluids production and enhancement relevant to oil and gas industry.
References
- Asadieraghi, M. , Ashri, W. M. , & Daud, W. (2014). Characterization of lignocellulosic biomass thermal degradation and physiochemical structure: Effect of demineralization by diverse acid solutions. Energy Conversion and Management , 82, 71–82. doi:10.1016/j.enconman.2014.03.007
- ASTM D2015-00 . Standard test method for gross caloric value of coal and coke by adiabatic bomb calorimeter. Retrieved from https://www.astm.org/Standards/D2015.htm
- Awosusi, A. A. , Ayeni, A. , Adeleke, R. , & Daramola, M. O. (2017a). Effect of water of crystallization on the dissolution efficiency of molten zinc chloride hydrate salts during the pretreatment of corncob biomass. Journal of Chemical Technology & Biotechnology , 92, 2468–2476. doi:10.1002/jctb.2017.92.issue-9
- Awosusi, A. A. , Ayeni, A. O. , Adeleke, R. , & Daramola, M. O. (2017b). Biocompositional and thermocompositional analysis of South African agro-waste corncob and husk towards production of biocommodities. Asia-Pacific Journal of Chemical Engineering , 12, 960–968. doi:10.1002/apj.2138
- Ayeni, A. O. , Adeeyo, O. A. , Oresegun, O. M. , & Oladimeji, T. E. (2015). Compositional analysis of lignocellulosic materials. Evaluation of an economically viable method suitable for woody and non-woody biomass. American Journal of Engineering Research , 4, 14–19.
- Ayeni, A. O. , Ayoola, A. , Oresegun, O. , & Efeovobhhan, V. (2014b). Effect of particle size on enzymatic hydrolysis of alkaline peroxide oxidation pretreated sugarcane bagasse. Assumption University Journal of Technology , 18, 59–68.
- Ayeni, A. O. , Banerjee, S. , Omoleye, J. A. , Hymore, F. K. , Giri, B. S. , Deskmukh, S. C. , … Mudliar, S. N. (2013a). Optimization of pretreatment conditions using full factorial design and enzymatic convertibility of shea tree sawdust. Biomass and Bioenergy , 48, 130–138. doi:10.1016/j.biombioe.2012.10.021
- Ayeni, A. O. , & Daramola, M. O. (2017). Lignocellulosic biomass beneficiation: Evaluation of oxidative and non-oxidative pretreatment methodologies for South African corn cob. Journal of Environmental Chemical Engineering , 5, 1771–1779. doi:10.1016/j.jece.2017.03.019
- Ayeni, A. O. , Daramola, M. O. , Sekoai, P. T. , Adeeyo, O. , Garba, M. J. , & Awosusi, A. A. (2018). Statistical modelling and optimization of alkaline peroxide oxidation pretreatment process on rice husk cellulosic biomass to enhance enzymatic convertibility and fermentation to ethanol. Cellulose , 25, 2487–2504. doi:10.1007/s10570-018-1714-6
- Ayeni, A. O. , Hymore, F. K. , Mudliar, S. N. , Deskmukh, S. C. , Satpute, D. B. , Omoleye, J. A. , & Pandey, R. A. (2013b). Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel , 106, 187–194. doi:10.1016/j.fuel.2012.12.078
- Ayeni, A. O. , Omoleye, J. A. , Hymore, F. K. , & Pandey, R. A. (2016). Effective alkaline peroxide oxidation pretreatment of shea-tree sawdust for the production of biofuels: Kinetics of delignification and enzymatic conversion to sugar and subsequent production of ethanol by fermentation using saccharomyces cerevisiae. Brazilian Journal of Chemical Engineering , 33, 33–45. doi:10.1590/0104-6632.20160331s20140258
- Ayeni, A. O. , Omoleye, J. A. , Mudliar, S. , Hymore, F. K. , & Pandey, R. A. (2014a). Utilization of lignocellulosic waste for ethanol production: Enzymatic digestibility and fermentation of pretreated shea tree sawdust. Korean Journal of Chemical Engineering , 31, 1180–1186. doi:10.1007/s11814-014-0026-2
- Blasi, C. D. , Signorelli, G. , Russo, C. D. , & Rea, G. (1999). Product distribution from pyrolysis of wood and agricultural residues. Industrial Engineering and Chemistry Research , 38, 2216–2224. doi:10.1021/ie980711u
- Chang, V. S. , & Holtzapple, M. T. (2000). Fundamental factors affecting biomass enzymatic reactivity. Applied Biochemistry and Biotechnology , 84-86, 5–37. doi:10.1385/ABAB:84-86:1-9:5
- Chundawat, S. P. S. , Donohoe, B. S. , Sousa, L. D. , Elder, T. , Agarwal, U. P. , Lu, F. C. , … Dale, B. E. (2011). Multi-scale visualization and characterization of lignocellulosic plant cell wall deconstruction during thermochemical pretreatment. Energy and Environmental Science , 4, 973–984. doi:10.1039/c0ee00574f
- Classen, P. A. M. , Sijtsma, L. , Stams, A. J. M. , De Vries, S. S. , & Weusthuis, R. A. (1999). Utilization of biomass for the supply of energy carriers. Applied Microbiolology and Biotechnology , 52, 741–755. doi:10.1007/s002530051586
- Deutschmann, R. , & Dekker, R. F. H. (2012). From plant biomass to bio-based chemicals: Latest developments in xylan research. Biotechnology Advances , 30, 1627–1640. doi:10.1016/j.biotechadv.2012.07.001
- El-Sayed, S. A. , & Mostafa, M. E. (2015). Kinetic parameters determination of biomass pyrolysis fuels using TGA and DTA techniques. Waste and Biomass Valorization , 6, 401–415. doi:10.1007/s12649-015-9354-7
- Ghosh, D. , Dasgupta, D. , Agrawal, D. , Kaul, S. , Adhikari, D. K. , Kurmi, A. K. , … Negi, M. S. (2015). Fuels and chemicals from lignocellulosic biomass: An integrated biorefinery approach. Energy & Fuel , 29, 3149–3157. doi:10.1021/acs.energyfuels.5b00144
- Gould, J. M. (1984). Alkaline peroxide delignification of agricultural residues to enhance enzymatic saccharification. Biotechnology and Bioengineering , 24, 46–52. doi:10.1002/bit.260260110
- Holtzapple, M. , Cognata, M. , Shu, Y. , & Hendrickson, C. (1990). Inhibition of Trichoderma reesei cellulase by sugars and solvents. Biotechnology and Bioengineering , 36, 275–287. doi:10.1002/(ISSN)1097-0290
- Kim, S. , & Dale, B. E. (2004). Global potential bioethanol production from wasted crops and crop residues. Biomass and Bioenergy , 29, 361–375. doi:10.1016/j.biombioe.2003.08.002
- Kim, S. H. (2004). Lime pretreatment and enzymatic hydrolysis of corn stover , PhD thesis. Texas A & M University, Texas.
- Kumar, A. K. , & Sharma, S. (2017). Recent updates on different methods of pretreatment of lignocellulosic feed stocks: A review. Bioresources and Bioprocessing , 4, 7. doi:10.1186/s40643-017-0140-1
- Li, S. , Xu, S. , Liu, S. , Yang, C. , & Lu, Q. (2004). Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Processing Technology , 85, 1201–2011. doi:10.1016/j.fuproc.2003.11.043
- Lin, L. , Yan, R. , Liu, Y. , & Jiang, W. (2010). In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: Cellulose, hemicelluloses, and lignin. Bioresource Technology , 101, 8217–8723. doi:10.1016/j.biortech.2010.05.084
- Mani, T. , Murugan, P. , Abedi, J. , & Mahinpey, N. (2010). Pyrolysis of wheat straw in a thermogravimetric analyser: Effect of particle size and heating rate on devolatilization and estimation of global kinetics. Chemical Engineering Research and Design , 88, 952–958. doi:10.1016/j.cherd.2010.02.008
- Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry , 31, 426–428. doi:10.1021/ac60147a030
- O’Sullivan, A. C. (1997). Cellulose: The structure slowly unravels. Cellulose , 4, 173–207. doi:10.1023/A:1018431705579
- Sluiter, A. , Hames, B. , Ruiz, R. , Scarlata, C. , Sluiter, J. , & Templeton, D. (2008, April). Determination of structural carbohydrates and lignin in biomass: Laboratory analytical procedure (LAP) . (NREL Report No.: TP-510-42618). Golden, CO: National Renewable Energy Laboratory.
- Wyman, C. E. (2001). Twenty years of trials, tribulations, and research progress in bioethanol technology. Applied Biochemical and Biotechnology , 91, 2–12.
- Zhao, X. B. , Zhang, L. H. , & Liu, D. H. (2010). Pretreatment of Siam weed stem by several chemical methods for increasing the enzymatic digestibility. Biotechnology , 5, 493–504.

