 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A renewable clean energy is sine qua non for mitigation of toxic and greenhouse gas emissions, preservation of natural ecosystem, biodiversity and its sustainability. Hence, this research work focuses on the evaluation of biofuel potential characteristics of Salicornia plant’s oil (halophytes) that grows in arid and salt water environment devoid of suitable edaphic and ecological factors that could support the growth and development of normal vegetable plants. Two species of the Salicornia plants were investigated. These are Salicornia bigelovii and Salicornia brachiata. A supercritical fluid extraction (SFE) process using carbon dioxide (CO2) modified with ethanol as extraction solvent yielded 32.14% of S. bigelovii oil and an oil yield of 26.58% S. brachiata. The fatty acid compositional analysis was determined by Agilent, HP6890 gas chromatograph with flame ionization detector (GC-FID). A 30% concentration of Candida antarctica lipase catalyst that was immobilized on a macro-porous, acrylic resin particles was used for the transesterification process. While biodiesel yield of 92.80% and 81.30% were obtained with t-butanol solvent, a yield of 59.34% and 48.76% was obtained in solvent-free system for S. bigelovii and S. brachiata, respectively. Finally, S. bigelovii biodiesel exhibited better cold flow properties but with very poor fuel critical characteristics, such as low cetane number, high iodine value and low heating value, which when applied in diesel engine could result in abnormal combustion, performance and emission behaviour. Whereas, S. brachiata showed a superlatively promising biodiesel fuel characteristics that is typical of those recommended by American and European biodiesel standards, and thus a technically viable diesel alternative in compression-ignition engines.
PUBLIC INTEREST STATEMENT
The non-renewable nature of fossil fuels (Petroleum) coupled with environmental pollution arising from harmful release of greenhouse gases have always been the impetus for exhaustive research into a clean burning fuel that is environmentally benign and from sustainable feed stock biomass. However, in order to forestall the eventual depletion of edible-oil supply in the world as a result of competition from using soyabean, coconut and palmkernel oil as biodiesel feed stocks, hence it is imperative to examine the biofuel potential of non-edible and salt water (halophytes) plants oil biomass. This study investigates Salicornia seed oil extraction with supercritical fluid method by using carbon-dioxide as extraction solvent and ethanol as co-solvent, determination of fatty acid profile of the extracted oil, determination of free fatty acids of the oil, review of enzymatic transesterification process, production of enzyme biodiesel of Salicornia oil and characterization of fuel properties of the synthesized biodiesel.
1. Introduction
The American Society for Testing and Materials (ASTM) defines biodiesel as a mixture of long-chain mono-alkyl esters of fatty acids obtained from renewable sources, such as vegetable oils and animal fats as alternative fuel in diesel engines. Various biomass feed stocks used for biodiesel production are rape-seed oil that is commonly used in European countries, soyabean oil that is widely employed in united states of America and Argentina, and palm-kernel oil that is used in central American countries and Asia. While Jatropha oil is used in India. Other plant oil biomass that is used for biodiesel production includes: sunflower oil, pea nut oil, linseed oil, saff flower oil, coconut oil, avocado and a host of others.
Various edible oils such as soybean oil, palm kernel oil and sunflower oil are being widely used in commercial quantities as biomass feed stocks in United States of America, Asia and Europe, respectively. However, because of increasing competition for food that arises from the use of edible oils as biodiesel starting materials, the world supply of edible oils may be depleted in no fewer time due to competition from biofuel demands. One possible method of addressing this impending ugly scenario is the use of non-edible oil sources from Madhuca indica, bitter Almond oil, Jatropha curcas, animal fats, waste cooking oil, castor seed oil, algae oil, Karanja oil and Pongamia pinnata oil.
Similarly, salt water plants (halophytes) that grow in arid areas may also be used as alternative to these edible oils that grows in non-salt water environment. Hence, the focus of this research work is on the assessment of the potential biofuel characteristics of Salicornia plant oil (halophytes), growing in arid and saline aquatic environment, devoid of suitable edaphic and ecological factors, such as temperature, rainfall and humidity that can support the growth and development of normal vegetable plants.
The use of biodiesel fuel in compression ignition engines offers many benefits such as non-toxicity, easily biodegradable, renewable and non-contribution to the net accumulation of greenhouse gases (Bajpai & Tyagi, Citation2006; Fellows, Citation2000). Moreover, the sulphur and aromatic content of biodiesel is lower than the conventional diesel fuel. Biodiesel has higher cetane number, flash point, iodine value, increased lubricity and lower gaseous fuel emission (Al-Zuhair, Citation2007; Al-Zuhair, Ling, & Jun, Citation2007; Anawe & Folayan, Citation2018a; Demirbas, Citation2007; Fukuda, Kondo, & Noda, Citation2001; Ranganathan, Narasimhan, & Muthukumar, Citation2008). In terms of emission characteristics, Schumacher et al. (Schumacher, Marshall, Krahl, Wetherell, & Grabowski, Citation2001) reported that a 45% reduction in total HC’s emission, 47% reduction in carbon-monoxide and 66% reduction in particulate matter emissions were brought about by the use of biodiesel compared to petroleum diesel. However, the direct use of plant oil as alternative fuel in diesel engines has been attributed to cause the following engine failure: high carbon deposits, scuffing of engine liner, injection nozzle failure, gum formation and lubricating oil thickening among others (Murugesan, Umarani, Chinnusamy, Krishnan, & Subramania, Citation2009). These problems arise because of abnormally high kinematic viscosity of plant oil, high density and poor cold flow properties. Hence, physical and or chemical modification of plant oils for biodiesel production by alkaline hydrolysis, hydrogenation, pyrolysis or cracking, micro-emulsification, blending, fractionation and transesterification becomes imperative for amelioration of these anomalies. While pyrolysis involves chemical reduction of triglyceride (TG) molecules to fatty acid alkyl esters (FAAEs) through the application of heat, micro-emulsification is used for solvents to physically reduce the viscosity of vegetable oil biomass. However, due to high cost and low yield quality of other methods of biodiesel production, transesterification is the most widely embraced method of transforming plant oil into biodiesel. The process is relatively simple with biodiesel properties that are identical or superior to petroleum diesel.
Transesterification can be divided into alcoholysis, acidolysis and inter-esterification reaction. Alcoholysis is a term that is used to describe transesterification reaction where an ester is reacted with an alcohol to produce a new ester and glycerol. Whereas, acidolysis is a chemical reaction whereby an ester reacts with carboxylic acid to generate a new ester and an acid. While, inter-esterification refers to transesterification reaction between ester and another ester (ester exchange) to produce a new ester and triacylglycerol.
Trans-esterification reaction is the main process of converting TG present in vegetable oil to alky esters by using alcohol in the presence of sodium, potassium hydroxide or lipase catalyst and under optimum condition (Anawe & Folayan, Citation2018b; Rashid, Anwar, Moser, & Samia, Citation2008; Zhou, Konar, & Boocock, Citation2003). It is a reaction between TG and an acyl acceptor. The acyl acceptor can be carboxylic acids, alcohols or another ester and the reaction may be catalyzed or non-catalyzed by using supercritical alcohol. Catalysts promote hydrolysis of TG and bring about formation of methyl ester and glycerol on a short time. The catalysts used may be homogenous or heterogeneous. In homogenous catalysis, the produced fatty acid methyl ester (FAME) has to be purified in order to remove the catalyst. This process requires large amount of water and it is very energy consuming. Heterogeneous catalysts can be separated from the biodiesel by filtration and thus encourages catalyst re-use.
Sodium hydroxide (NaOH), potassium hydroxide (KOH), sodium methoxide (CH3ONa) are the most commonly used homogenous catalysts for alkaline transesterification. While zeolites, magnesium oxide (MgO) and calcium oxide (CaO) are usually employed for heterogeneous hydrolysis of TG. The reaction rate can also be increased by immobilized lipase biocatalysts such as Mucor miehei, Rhizopus oryzae, Candida antarctica and Pseudomonas cepacia. Also, sulphuric acid (H2SO4), sulphonic acid (H2SO3), phosphoric acid (H3PO4) and hydrochloric acid (HCl) are the most commonly used acid catalysts for biodiesel synthesis (Ma & Hanna, Citation1999; Meher, Sagar, & Naik, Citation2006).
One of the optimum conditions put forward by various researchers towards efficient conversion of fatty acids to methyl esters are: catalyst concentration of 1.5% for potassium hydroxide (KOH) and 1.0% for sodium hydroxide (NaOH), alcohol to oil molar ratio of 6:1 for methanol and 9:1 for ethanol, reaction temperature of 60–65°C and reaction time of 2–3 h (Al Naggar, Ashour, Ettouney, & El Rifai, Citation2017; Anawe & Folayan, Citation2018c; Encinar, Gonzalez, & Rodriguez-Reinares, Citation2007).
2. Salicornia plants morphological and physiological characteristics
2.1. Salicornia bigelovii
Salicornia bigelovii is a halophytic plant (salt tolerant plant) that belongs to the family chenopodiaceae and grows in saline semi-deserts, mangrove swamps, salt marshes, mudflats, sloughs and seashores. They are adapted to low water environment by their capacity for osmotic adjustment and survive through irrigation with saline water (Noorollahi, Sokhansefat, Sokhansefat, Rahmani, & Jalilinaasrabady, Citation2015). The plant is a leafless, succulent, small seeded annual salt-marsh plant that can be used as an oilseed, a forage and a grain crop (Glenn et al., Citation2013). It is a potential oil seed and a promising candidate for carbon sequestration. It is very rich in linoleic acid (about 70%) – a poly unsaturated fatty acid and with a fatty acid profile that is similar to sunflower oil. The seed contains about 26–33% oil content (similar to safflower oil), 35% protein and a salt content of about 3% (Garcia, Citation2010; Glenn & Brown, Citation1999).
The oil has a taste and its texture is similar to that of olive oil and it grows in an area where the annual rainfall is between 80 and 300 mm/year (Falasca, Ulberich, & Acevedo, Citation2014).
2.2. Salicornia brachiata
Salicornia brachiata is an annual erect herb that can be used as vegetable, animal fodder, herbal salt and oil yielding crops for biodiesel. It is a salt water irrigated crop that belongs to the family Chenopodiaceae. It is a seasonal herb that completes its life cycle within eight to nine months with leafless bush plants and an average height of 32 cm (Geddada & Pragada, Citation2013). It is a green, jointed, vascular flowering and leafless halophytic plant that carries articulated succulent systems (Deepa, Ramesh, Swarna, Raghava, & Bangaru, Citation2014).
3. Materials and method
3.1. Oil extraction process by super critical fluid extraction process (SFE)
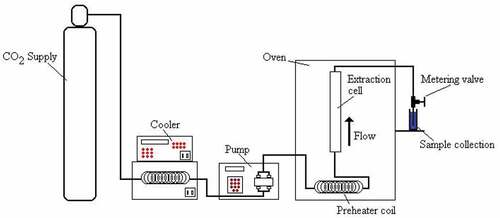
Super critical fluid extraction (SFE) (Figure ) is the process of separating one component otherwise known as the extractant from another component called the matrix by using supercritical fluids as the extracting solvent. Carbon dioxide (CO2) and water (H2O) are the most commonly employed supercritical solvents with CO2 offering more excellent super critical fluid characteristics due to its non-flammability, non-toxicity, lack of chemical residual problem and low critical temperature (Park et al., Citation2007). It is a viable alternative to liquid extraction that uses hexane, petroleum ether or dichloromethane as the extraction solvent. SFE process is of high demand where product purity is of utmost importance because unlike other extraction process where some residual solvent is left in the extract and matrix with some level of environmental contamination, SFE process leaves no residue behind. Moreover, CO2 is very easy to remove by reducing the pressure without any residue left behind, non-toxic, non-flammable, odourless, tasteless, inert and inexpensive with critical temperature and pressure of 31°C and 74 bar, respectively. The essential steps in SFE process are transport by diffusion of the solid particles to the surface, dissolution of the solutes in the supercritical fluids, diffusion into the particle by the super critical fluid and desorption.
Figure 1. Schematic diagram of supercritical fluid extraction process (Sapkale, Patil, Surwase, & Bhatbhage, Citation2010)
Mature seeds of Salicornia bigelovii and Salicornia brachiate were collected from the botanical garden of International Institute of Tropical Agriculture, Nigeria. The seeds were cleaned and separated by sieves before it was washed to remove contaminants and dried in a cabinet dryer at 50°C for 12 h. Dried seeds were then weighed, crushed and stored under cold temperature condition for seven days to form molten matrix.
The molten Salicornia oil seeds were then transferred into the extractor vessel of the SFE apparatus (Figure ) and all outlet and inlet valves were immediately closed. The SFE apparatus contains reciprocating CO2 pump, a pressure cell to contain the sample, pressure maintenance apparatus, inlet and outlet valves, oven, vent valve, cooler, pre-heater coil and a collecting vessel. The extraction solvent was carbon iv oxide modified with ethanol as co-solvent due to ability of ethanol to form hydrogen bonding with solutes and enhance the density of supercritical CO2 resulting in higher miscibility and solubility of the solvent in the solutes (Shahid, Citation2014). The CO2 was pumped in liquid form at 41°F and pressure of 50 bar to an oven where it was heated to supercritical temperature of 60°C because if it were pumped in supercritical conditions, much of the pump stroke would be used up in compressing the fluid rather for pumping. When the required temperature of 60°C in the oven and pressure pump of 450 bar was reached, the CO2 inlet and outlet valves were opened so that super critical CO2 could flow into the extractor vessel where it rapidly diffuses into the solid matrix and dissolves the materials to be extracted. A pump was used to maintain the pressure requirement and continuous supply of CO2 to the system. The dissolved materials were swept from the extraction chamber into a separator at lower pressure for the extracted materials to settle out. At the end of the process, the outlet and exit valves were opened to collect the extracted oil in a 500 mL measuring cylinder and the percentage yield of oil was deduced. The CO2 was then cooled, recompressed and discharged into the atmosphere.
3.2. Determination of fatty acid profile of the Salicornia oil
The fatty acid compositional analysis was done by using Agilent, HP 6890 Gas Chromatograph with Flame ionization detector and 6890 Auto Sampler that connects with a controller box (Figure ). Gas chromatography technique is used for separation and detection of various molecular weight compounds in the gas phase. The HP 6890 gas chromatograph equipped with flame ionization detector has a robust mechanism for correct analysis of fatty acids present in the Salicornia oil samples. It has an electronic pneumatic control system for all gas pressures and flow rates and an on-board sensor that compensate for ambient temperature changes in the range of +4–450°C. The automatic injector system and oven cryocooling gives room for exact measurement of retention attributes of volatile compound for accurate estimation of their physical properties.
Figure 2. Agilent, HP 6890 gas chromatograph with flame ionization detector and 6890 auto sampler (Gentechscientific.com, Citation2018)

The TG in the oil was esterified by using an alkylation derivatization reagent (sodium methoxide) because fatty acids present in oils may be difficult to analyze in their free state due to their high polarity and formation of hydrogen bonds that consequently result in adsorption problems. Hence, methylation reduces fatty acids polarity and methyl esters offer better stability and quick quantitative samples for gas chromatography analyses. The FAME was re-dissolved in 100 µL hexane, and 5 µL volume of the samples were injected into GC-FID for separation and quantification. The carrier gas consists of a low molecular weight helium that is chemically inert and it serves as the mobile phase with flow rate of 1 mL/min.
The separation was done by HP-5 mm column coated with a stationary phase. When pressure was applied, the carrier gas helped to transfer the sample from the injector through the column into the flame ionization detector. The initial temperature was set at 100°C and adjusted until the injector and detector temperatures were at 260°C and 280°C, respectively. In the ionization detector, the sample was burnt to produce ion and free electrons, which produces measurable current flow in the gap between the two electrodes inside the detector. The percentage of FAME was calculated from the gas chromatography graph by running the internal standard with the samples and taken into consideration the response factor of the different FAME.
3.3. Free fatty acids value determination
The free fatty acid (FFA) in the vegetable oil was determined by chemical titration method (AOAC, Citation1990).
Fifty-six grams of well-mixed sample of each Salicornia oil species was accurately measured into a 300ML Erlenmeyer flask. Also, 50 mL of ethyl alcohol (95% ethanol) containing 2 mL of phenolphthalein indicator was heated to a temperature of 60°C by using water bath to prevent evaporation because ethanol boils at 78°C. The mixture of hot, neutralized ethanol was then added to Salicornia oil in the flask and titrated with 0.1 N sodium hydroxide solution (NaOH). The mixture was shaken constantly until a pink colour which persisted for 30s was observed in the alcohol layer above the sample.
4. Calculations
The Salicornia oil– FFAs (%) were calculated in terms of % oleic by using EquationEquation (1(1)
(1) )
Where V = volume in ml of standard sodium hydroxide solution used
N = normality of standard sodium hydroxide solution used
W = mass (g) of oil sample used.
4.1. Enzymatic transesterification process overview
Enzymes are biological catalysts that speed up the rate of chemical reactions without undergoing a permanent change in its structure. Lipase is an enzyme that catalyzes the hydrolysis of fats or lipids (Svendsen, Citation2000). Enzymatic biodiesel production process involves the use of the following micro-organisms: Candida antarctica, Candida rugose, Candida cylindrical, Aspergillus niger and Pseudomonas species among others. However, Candida antarctica has been reputed to show highest reactivity in methanol and ethanol transesterification reactions resulting in more than 90% conversion efficiency in both tert-butanol and solvent free environment (Gog, Roman, Tosa, Paizs, & Irimie, Citation2012; Luque, Cervero, & Alvarez, Citation2014; Modi, Reddy, Rao, & Prasad, Citation2007; Tupu, Jae, Marquis, Adesina, & Rogers, Citation2013). Lipases are insensitive to FFAs concentration of the plant oils at any level and hence, does not require a pre-treatment and both the acid esterification and the transesterification process can occur concurrently to form the required FAAEs (Sebastian, Muraleedharan, & Santhiagu, Citation2016). Also, the moisture content in the raw plant oil does not degrade the reaction and as a matter of fact, it enhances lipase reactivity. In enzymatic transesterification reaction, the process can be carried out with low operating conditions such as temperature range between 35 and 45°C (Kumar, Madras, & Modak, Citation2004; Szczesna, Kubiak, Antczak, & Bielecki, Citation2009). High product purity can also be guaranteed with the process because contrary to chemical catalysts, lipase does not form soaps and it catalyzes esterification of FFA and TG in a single step.
However, longer reaction period and high cost of enzymes have always been the major drawbacks for enzymatic biodiesel production (Caballero et al., Citation2009; Noureddini, Gao, & Philkana, Citation2005; Robles-Medina, Gonz´alez-Moreno, Esteban-Cerd´an, & Molina-Grima, Citation2009; Sharma, Singh, & Upadhyay, Citation2008). These problems can be mitigated by recycling the enzyme through immobilization (Hanifar, Al-Zuhair, Ali, Al-Marzouqi, & Mohammed, Citation2011). Enzyme immobilization is a technique where free movement of the enzyme is restricted and localized to an inert, insoluble support or carrier. This technique provides us with a number of essential benefits such as enzyme re-usability, ease of enzyme removal from products, reduction in inhibition rate and enhanced activity and stability (De Paola, Ricca, Calabr`, Curcio, & Iorio, Citation2009). By immobilization, enzymatic transesterification process can be carried out at higher temperatures and thus increases the rate of reaction (Fjerbaek, Christensen, & Norddahl, Citation2009). The immobilized enzymes are specifically designed to perform two essential functions. These are: the non-catalytic functions that are designed to help separation and the catalytic functions that are concerned with substrates conversion to the desired products within a stipulated reaction duration (Cao, Citation2005).
Enzyme immobilization technique can be physical adsorption that involves the adhesion of the enzyme lipase to insoluble resins, nanospheres, beads, nanofibers and membranes. Examples include poly-methyl methacrylate resins (PMMA), poly-2-hydroxy-ethyl methacrylate-co-N-methacryloyl-(1)-phenyl alanine-methyl ester (poly-HEMA-MAPA) nanospheres, chitosan beads and chitosan membranes, polypropylene (PP) membranes and poly acrylonitrile-2-methacryloyloxyethyl phosphoryl choline (PANCMPC) nanofibers. Physical adsorption mechanism offers various advantages such as feasibility of regeneration, absence of expensive and toxic chemicals and ease of immobilization. However, adsorption is not a chemical reaction and the immobilized lipase active site can be blocked by the matrix or bead and thus reduce enzyme activity.
Physical entrapment or encapsulation is another method of lipase immobilization where the enzyme is trapped in insoluble beads or microspheres such as calcium alginate beads. This insoluble substance may inhibit the arrival of the substrate and the exit of the products. Cross linkage technique of immobilization is a process whereby the lipase molecules are covalently bonded to each other to create an enzyme matrix that helps to ensure that the binding site does not block the enzyme’s active site and the activity of the enzyme is only affected by immobility. Finally, the enzyme can also be covalently bonded to an insoluble support such as silica gel or macroporous polymer beads with epoxide groups and thus brings about the strongest enzyme/support interaction (Zucca & Sanjust, Citation2014). Examples of chemical attachment include: eupergit resins, poly (HEMA-MAPA) membranes, poly-acrylonitrile-co-methyl methacrylate (PANCMMA) membranes and nanofibers, chitosan nanofibers and poly-acrylonitrile-co-2-hydroxyethyl methacrylate (PANCHEMA) nanofibers.
Finally, immobilized enzymes are subjected to internal and external diffusion limitations and inactivation mostly by methanol. These anomalies can be annulled by addition of inert solvent such as tert-butyl alcohol (TBA).
4.2. Salicornia oil enzymatic biodiesel production process
Eight hundred millilitres of filtered Salicornia oil (Bigelovii and Brachiata) were measured separately by using measuring cylinder and transferred into a 2,000 mL PYREX Erlenmeyer flask with glass stopper. The oil was heated to a temperature of 50°C with the aid of water bath. This was followed by the addition of 200 mL of methanol (analytical grade) which was initially preheated and maintained at a temperature of 50°C. About 13.60 g (30 wt.%) of Candida antarctica lipase catalyst immobilized on a macroporous acrylic resin particles (polyhydroxyethylmethacrylate) were added to the Oil–Alcohol mixture inside the Erlenmeyer flask. However, because immobilized enzymes are subjected to inactivation mostly by methanol (Drapcho, Nhuan, & andWalker, Citation2008), hence 400 mL of an inert-solvent (TBA) was added to the mixture to annul the effect of immobilized enzyme inactivation.
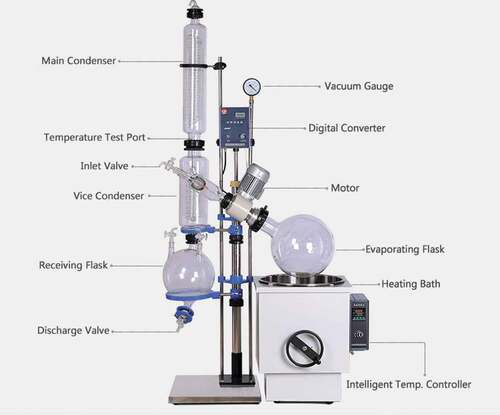
Thus, the reaction mixture contains 57.14% Salicornia oil, 28.57% TBA solvent and 14.29% methanol. The mixture in the flask was then placed in an electric orbital shaker at temperature of 50°C and rotor speed of 300 rpm for 24 h. After the completion of the reaction duration, the reaction mixture was then filtered by vacuum filtration in order to retain the enzyme for subsequent recovery and use. The solvent in the resulting filtrate was recovered together with methanol by using a rotary vacuum evaporator (Figure ) consisting of water bath, condenser and vacuum pump with polytetrafluoroethylene (PTFE) rubber sealing. The water bath temperature was set at 50°C (a temperature that is lower than the boiling point of methanol (64.7°C) and tert-butanol (83°C) with 710 pressure of 75 torr (99 mbar). Otherwise, the condenser could be overloaded because the evaporation rate will exceed the condensation capacity. The rotary evaporator has evaporating and receiving flask capacities of 5 and 2 L, respectively. While the evaporation rate was approximately 1.5 L/h.
Figure 3. Zhengzhou Keda RE-501 vacuum rotary evaporator (Zzkdinstrument.com, Citation2019)
Finally, the reaction products were transferred into a separating funnel and allowed to settle for 12 h under gravity and two distinct layers of upper FAME and a denser lower layer of monoglyceride, diglyceride, unreacted catalysts and other impurities were clearly seen. The produced ester was then purified by step wise washing process and deodorized to form a pure biodiesel.
4.3. Fuel properties evaluation methods
The Saponification number (S.N) and Iodine value (I.V) of the Salicornia oils and their FAMEs were calculated from EquationEquations (2)(2)
(2) and Equation(3)
(3)
(3) proposed by Kalayasiri et al. (Kalayasiri, Jayashoke, & Krisnangkura, Citation1996) and validated by Mohibbe et al. (Mohibbe, Amtul, & Nahar, Citation2005).
The advantage of these equations is that the relative contributions of each fatty acid to the total S.N and I.V can be easily evaluated.
Where Ai is the percentage composition of each fatty acid in the vegetable oil or its ester, D is the number of double bonds present in each unsaturated fatty acid or its ester and Mwi is the molecular weight of each fatty acid or its ester component.
Similarly, the cetane number (CN) and higher heating values (HHVs) were also evaluated by using EquationEquations (4)(4)
(4) and Equation(5)
(5)
(5) as reported by Krisnangkura (Citation1986) and Demirbas (Citation1998), respectively
5. Results and discussion
5.1. Oil yield evaluation
The percentage yield of Salicornia bigelovii and Salicornia brachiata oil obtained from the SFE process was determined by using EquationEquation (6)(6)
(6) . The percentage yield of Salicornia bigelovii was 32.14% while a yield of 26.58% was obtained for Salicornia brachiata. The results showed a close proximity to that obtained by Anwar, Anger, Nasir, and Ismail (Citation2002), where a yield of 27.2–32.0% was obtained for Salicornia bigelovii with hexane as extraction solvent. Similarly, a yield of 29% was also obtained by Al-Rasheed, Ibrahim, Maysa, and El-Gaaly (Citation2016) through hexane extraction method for Salicornia bigelovii. Meanwhile, Eganathan, SR-Subramanian, Latha, and Rao (Citation2006) recorded a maximum yield of 22.4% for Salicornia brachiata by using hexane as extraction solvent and 10.5% when petroleum ether was used as extraction solvent. The high yield of Salicornia oil obtained from this study shows the higher efficiency of SFE method over the solvent extraction method for Salicornia oil extraction.
5.2. Fatty acids compositional analyses of Salicornia oils
The results of the fatty acid components and percentage composition of each Salicornia oil species as determined from gas-chromatograph equipped with flame ionization detector are shown in Table . While the distribution of saturated and unsaturated fatty acids in the oil samples is presented in Table . The Salicornia brachiata consists of larger percentage of saturated fatty acids (palmitic and myristic) than the bigelovii oil and hence a lower degree of unsaturation (Table ). While linoleic acid (poly unsaturated fatty acid) constitutes the major source of unsaturation in S. bigelovii oil, undec-10-enoic acid and oleic acid which are mono-unsaturated account for the bulk of unsaturation in S. brachiata. Moreover, from the carbon length distribution of various fatty acids in the extracted oil samples (Table ), the S. bigelovii contains absolutely of long chain fatty acids (LCFAs) (99.90%) whereas S. brachiata has 37.60% of medium chain fatty acids (MCFAs) and 62.39% of LCFAs. The degree of unsaturation and carbon length distribution of fatty acids present in the oil samples is a primary determinant of the cold flow and critical properties of the oils and their FAME (biodiesel). From gas chromatography–mass spectroscopy analysis, Al-Rasheed et al. (Citation2016) quantified the fatty acid composition of S. bigelovii in the following manner: palmitic acid (7.40%), stearic acid (2.40%), oleic acid (13.30%), linoleic acid (72.50%) and linolenic acid (2.30%). While Llah, Ssan, Murui, and Ami (Citation1994) recorded 66.50% linoleic acid and 1.4% linolenic acid for Salicornia bigelovii. However, the fatty acid profile of S. brachiata from the GC-Ms analysis by Eganathan et al. (Citation2006) shows that the oil contains 16.48% palmitic acid, 12.88% myristic acid, 37.85% 10-undecenoic acid and 32.79% mono-unsaturated oleic acid.
Table 1. Fatty acid composition of S. bigelovii and S. brachiata oil from GC-FID analysis
Table 2. Distribution of saturated and unsaturated fatty acids in the oil samples
Table 3. Carbon length distribution of fatty acids in the oil samples
The FFA of S. bigelovii was found to be 0.90%, while S. brachiata has a higher FFA level of 4.35%.
5.3. Percentage yield of biodiesel in solvent-free system and with tert-butyl alcohol solvent
The yield of the biodiesel was deduced by using EquationEquation (7)(7)
(7) . While the yield of biodiesel produced at various time intervals and with or without TBA as solvent is presented in Table . The use of non-toxic TBA as a solvent significantly improved the enzymatic process by dissolving both methanol and the glycerol. Lipase does not act on tertiary alcohols. Hence t-butanol is not a substrate for the enzyme. The highest yield of 92.80% and 81.30% were obtained from S. bigelovii and S. brachiata when tert-butyl-alcohol was used as solvent. These results are comparable with a yield of 97% that was obtained by Royon, Daz, Ellenrieder, and Locatelli (Citation2007) during enzymatic methanolysis of cotton seed oil with t-butanol solvent. The reaction mixture consists of t-butanol (32.5%), methanol (13.5%), cotton seed oil (54%), enzyme loading of 0.017 g/g of oil, temperature of 50°C and reaction duration of 24 h. However, short chain alcohols most especially methanol has low solubility in oils and thus result in the appearance of a new liquid phase in the system at moderate concentrations leading to enzyme inactivation and decreased yield of biodiesel (Shimada, Watanabe, & Samukawa et al., Citation1999). Similarly, enzyme activity can be reduced by the low solubility of glycerol that forms coatings on the immobilized enzyme (Dossat, Combes, & Marty, Citation1990).
Table 4. Percentage yield of biodiesel in solvent free system and with tert-butyl alcohol solvent
Similarly, Salicornia oil biodiesel yield increased as the reaction time increases (Table ). However, for chemical transesterification at reaction times higher than the optimum value, a decrease in biodiesel yield can occur with sodium or potassium hydroxide as catalyst owing to higher rate of soap formation that consequently leads to gel formation which traps a large amount of FAAE within the glycerol layer (Hossain, Boyce, Salleh, & Chandran, Citation2010; Stavarache, Vinatoru, Nishimura, & Maeda, Citation2005; Vicente, Martinez, & Aracil, Citation2004).
5.4. Cold flow properties of S. bigelovii and S. brachiata oil and its biodiesel
The cold flow properties of the Salicornia oil and its biodiesel are presented in Table . Cloud point represents the temperature at which a cloud of waxy crystals first appears in a liquid when it is cooled under standard conditions while the pour point is the lowest temperature at which the liquid can flow. Cold flow properties must be taken into consideration if the fuel is to be used in cold environment. The cold flow properties of the Salicornia raw oil are poor and thus threatens the direct application of the oil as fuel in diesel engines due to anticipated formation of gum and crystallization of fuel particles that eventually affect fluid flow, clogging of fuel pipes, causes pumping issues and injector problems (Bello, Adekanbi, & Akinbode, Citation2015). The S. bigelovii biodiesel has better cold flow properties because it contains higher percentage of polyunsaturated fatty acid. In S. brachiata, the presence of more saturated components is responsible for poor cold flow behaviour that was observed in both the oil and its methyl ester (biodiesel). Saturated fatty acid crystallizes at higher temperature region while unsaturated fatty acid ester crystallizes at lower temperature region (Chandravati, Saini, Bera, & Maji, Citation2017). Unsaturated fatty acid ester molecules most especially the cis configuration possess very weak intermolecular force of attraction and hence crystallize at lower temperature (Borugadda & Goud, Citation2014; Dunn, Citation1999). Whereas saturated alkyl ester components have very strong intermolecular attractive forces and thus higher crystallization temperature (Zuleta, Rios, & Benjumea, Citation2012). The common standard methods of determining the cloud point and pour point characterizes only the beginning of crystallization process and initiation of operative problems (Perez, Casas, Fernandez, Ramos, & Rodriguez, Citation2010). Hence, full range of crystallization can only be accurately monitored by cold filter plugging point (CFPP) and low temperature filterability test (LTFT) and thus provide us with better biodiesel cold flow behaviour prediction.
Table 5. Cold flow properties of salicornia oil and its biodiesel
5.5. Fuel properties of S. bigelovii and S. brachiata oil and its biodiesel
5.5.1. Kinematic viscosity
Kinematic viscosity measures the ease with which a fluid will flow under force. The Salicornia oils have higher kinematic viscosities at 40°C (Table ) and thus make the oil to be problematic and unsuitable for direct use as petroleum diesel replacement in compression ignition engines because of the following impending catastrophes: cold weather starting issues, thick exhaust smokes and emission, coking of injectors and head of engines as a result of poor or incomplete combustion and excessive engine wear (Cherng & Lin, Citation2012; Knothe & Steidley, Citation2005). However, these abnormally high kinematic viscosities were able to be reduced to acceptable values of 3.92 and 3.46 mm2/s (Table ) for S. bigelovii and S. brachiata biodiesel respectively after enzymatic transesterification and thus annul the aforementioned catastrophes that may arise from the direct use of the oil without any physical and or chemical modification. For ASTM, a kinematic viscosity value of 1.9–6.0 mm2/s is required at 40°C for biodiesel to function efficiently in diesel engine while the European union recommends a value of 3.5–5.0 mm2/s.
The S. bigelovii and its biodiesel have higher viscosity than the S. brachiata because viscosity increases with chain length and degree of unsaturation of the fatty acid (Knothe, Citation2005a).
Table 6. ASTM D6751-07b and EN 14,214 standard specification (American Society for Testing and Materials, Citation2007; European committee for standardization, Citation2003)
Table 7. Fuel properties of S. bigelovii and S. brachiata oil
Table 8. Fuel properties of S. bigelovii and S. brachiata biodiesel
5.5.2. Specific gravity
Fuel specific gravity is primarily concerned with the air–fuel ratio and the enhancement of fuel atomization efficiency (Alptekin & Canakci, Citation2008; Lee, Han, Lee, & Hong, Citation2010). The S. bigelovii plant oil has a higher density of 0.918 kg/m3 than the S. brachiata with density of 0.914 kg/m3 (Table ). This is partly due to higher degree of saturation in the former (Rafaat, Citation2009). This high density of the oil is not unconnected with the natural gums (Phosphatides) that is present in the oil and thus initiate operational issues such as plugging and gumming of filters, liners and injector and invariably rendered the oil useless for direct application in diesel engines. However, when the oil was refined through enzymatic transesterification, these high specific gravities were significantly lowered to 0.878 for S. biglovii and 0.861 for S. brachiata and thus conform to standard biodiesel requirement.
5.5.3. Flash point
Flash point is very vital when considering the transportation and storage requirement of biofuels. It represents the lowest temperature at which a liquid will form an ignitable mixture in air. The Salicornia oils and their biodiesel fall within the American and European standard flash point requirements (Tables –). These moderately high flash points can ensure safe operation and reduced vaporization within the minimum operating temperature.
5.5.4. Cetane number
The cetane index is a dimensionless scale to numerically quantify fuel ignition quality. Higher cetane index undoubtedly corresponds to shorter ignition delay time and better ignition quality (Knothe, Citation2005b). The S. brachiata oil and its biodiesel has cetane number of 50 and 52.60, respectively (Tables and ) and thus better ignition quality (Azam, Waris, & Nahar, Citation2005). Whereas, the S. bigelovii and its biodiesel have ignominiously lower cetane number of 38.93 and 42.05, respectively. These values totally violate the European and American standard minimum requirements of 51 and 47, respectively (Table ). This lower cetane number can be attributed to higher degree of unsaturation in the S. bigelovii fatty acid profile. S. bigelovii is principally composed of 75.14% of poly-unsaturated linoleic acid (Table ). Meanwhile linoleic acid has ridiculously low cetane value of 36.8 and 38.2 as measured by Bangboye and Hansen (Citation2008) and Knothe, Matheaus, and Ryan (Citation2003), respectively. While saturated palmitic acid methyl ester has a value of 85.9 and stearic is 101. Fuels with low cetane number usually cause improper injection timing and knocking of the engine (Harwood, Citation1984).
5.5.5. Iodine value
The iodine value measures the degree of unsaturation of oil and biodiesel and this has great influence on the fuel’s oxidation stability (Adekunle, Oyekunle, Obisesan, Ojo, & Ojo, Citation2016). High iodine values of 154.05 and 146.47 gI2/100 g of oil were recorded for S. bigelovii oil and its biodiesel respectively. This higher iodine value is not ideal for fuel because when biodiesel containing higher amount of unsaturated fatty acid is heated, polymerization of glycerides occurs which may result in deposit formation and thus deteriorates the fuel’s lubrication property (Salles et al., Citation2010). High iodine value fuel has a tendency of oxidizing and drying into a tough, insoluble plastic like solid. However, the S. brachiata has iodine value of 81.77 in its raw oil and 76.92 in its methyl ester (biodiesel). These lower iodine values will make the biodiesel to be more combustible and efficient fuel (Uriate, Citation2010).
5.5.6. Higher heating values (HHVs)
This is the heat energy that is released when a unit volume of fuel is burnt and it has a significant influence on engine fuel consumption. The Salicornia oil and its biodiesel have moderately high energy content and thus lower fuel consumption.
5.5.7. Total and free glycerine content
The total glycerine refers to the sum of free glycerol and glycerol bounded in the form of mono, di and TG present in the biodiesel. The produced biodiesel fuels have low percentages of total and free glycerine which are sufficiently below the maximum standard requirements (Table ). Higher values may cause coking and formation of deposits on injector nozzles, pistons, valves and filter plugging (Bello, Akinola, Otu, & Owoyemi, Citation2013). Also, higher free glycerine in biodiesel can increase its viscosity and thus plug fuel filters and causes combustion problems in diesel engines. Total glycerol may also affect biodiesel quality when stored over a long period of time.
5.5.8. Sulphated ash percentage
Salicornia bigelovii and S. brachiata biodiesel have very low ash percentages of 0.0091% and 0.0084% (Table ). These are sufficiently lower than the maximum standard requirements by ASTM and European Union. According to these biodiesel regulatory bodies, a maximum percentage of 0.020% is ideal for any fuel that wants to function as biodiesel. Sulphated ash in biodiesel can occur as a result of abrasive solids, unremoved catalysts and presence of soluble metallic soap. The ash content has an inverse relationship with the heating value. Heating value decreases with increasing ash content. The Salicornia FAME (biodiesel) has very low percentage of ash content that enhances fuel better performance. High ash concentration as a result of presence of abrasive solid in fuel can result in engine deposits, fuel pump wear, piston and ring wear and injection system wear while metallic soap presence may cause filter plugging and engine deposits (ASTM, Citation2009).
5.5.9. Carbon residue
Carbon residue measures the carbon-depositing tendencies of the biodiesel. The synthesized biofuels have very low carbon residue of 0.038% and 0.030% for S. bigelovii and S. brachiata methyl ester, respectively. These values were determined by using the ASTM D4530 procedure. The American standard for testing and materials specified a maximum carbon residue of 0.050% for 100% oil sample.
5.5.10. Water and sediment percentage
Higher water and sediment content in fuel facilitates its hydrolysis and thus deteriorates the biodiesel fuel properties for efficient performance, combustion and emission characteristics. It can also promote microorganism’s growth (algae) which can clog fuel filters and increase the rate of metallic engine parts corrosion (Aktas et al., Citation2010). Biodiesel must be dried after water washing in order to keep the water specification below 0.050%. Accumulation of moisture over time can make the biodiesel to become rancid and has its chemical structure altered by increasing its acid level and invariably leads to corrosion of metal parts in fuel lines. The Salicornia biodiesel have water and sediment percentages of 0.013 and 0.010 for bigelovii and brachiate, respectively.
6. Conclusion
The fuel characteristic potential of Salicornia bigelovii and Salicornia brachiata halophytic plant oils and its biodiesel as possible alternative to diesel fuel in compression ignition engines was studied. The plant oil was extracted by SFE process while its biodiesel was produced through enzymatic transesterification reaction by using Candida antarctica lipase catalyst immobilized on a macro-porous, acrylic, resin particles.
In summary, the following important findings can be established.
Enzymatic transesterification reaction is a technically viable and reliable process for modification of plant oil characteristics with a view to eliminating undesirable properties that threatens the use of plant oil as alternative fuel in diesel engines. For instance, a significant improvement in the oil’s cold flow properties such as cloud, pour, CFPP and low LTFT was experienced after enzymatic tans-esterification.
Similarly, about 87.59% and 4.35% reduction in kinematic viscosity and specific gravity respectively of the S. bigelovii oil was experienced after transesterification. While S. brachiata oil has a viscosity reduction of 88.19% and specific gravity reduction of 5.7%. These exceptionally lower viscosities will make the biodiesel to have good lubrication and excellent fuel atomization behaviour. While lower density will reduce filter plugging and gumming issues.
The S. bigelovii biodiesel has better cold flow properties but with lower cetane number of 42.05 and higher iodine value of 146.47 gI2
. Lower cetane index results in longer ignition delay time and poor ignition quality. While higher iodine value makes the biodiesel to be susceptible to oxidation and polymerization.
S. brachiata has significantly better biodiesel fuel characteristics with higher cetane number, lower iodine values and high calorific value. This makes the biodiesel to be more combustible and efficient fuel than S. bigelovii biodiesel.
The amount of sulphated ash, carbon residue, free glycerine, total glycerine and water- sediment in the synthesized Salicornia oil biodiesel are significantly lower than the required standard maximum values by various biodiesel regulatory bodies. Hence, the fuel is free of any unreacted catalyst, abrasive solids, soluble metallic soap and glycerol and thus prevent issues such as injector plugging, injector fouling, filter plugging and corrosion of injector metallic parts.
Finally, the use of non-toxic TBA as a solvent significantly improved the enzymatic transesterification process by dissolving both methanol and the glycerol. Thus producing high biodiesel yield than solvent-free system.
List of abbreviations
| ASTM | = | American Society for Testing and Materials |
| CFPP | = | Cold filter plugging point |
| C.N | = | Cetane number |
| CO2 | = | Carbon dioxide |
| EN 14,214 | = | European committee for standardization |
| FFA | = | Free fatty acid |
| GC-FID | = | Gas chromatograph with flame ionization detector |
| I.V | = | Iodine value |
| K.V | = | Kinematic viscosity |
| SFE | = | Supercritical fluid extraction |
| SFS | = | Solvent-free system |
| TBA | = | Tert-butyl alcohol |
Conflict of interest disclosure
The authors are happy to say that there is no conflict of interest regarding the publication of this paper publication. The paper represents a unanimous purpose of interest.
Acknowledgements
The authors are very grateful to Chancellor of Covenant University and the university management team for their support in providing research enabling environment and condition.
Data availability statement
The authors can boldly say that all the data used in this study were gotten from rigorous experimental research in the laboratory and not from any journal either in print or on line. We also declare that the data will be available for public use once the paper is published.
Additional information
Notes on contributors
Adewale Johnson Folayan
Adewale Johnson Folayan is a young and vibrant academic of international repute. He has a second class upper, bachelor’s degree in chemical engineering from Ladoke Akintola University of Technology, Ogbomoso, Nigeria and a master’s degree in petroleum engineering from university of Ibadan with distinctions. He worked briefly as a research assistant in petroleum engineering at university of Ibadan and he was a former lecturer in petroleum engineering at covenant university, Ota, Nigeria.
Paul Apeye Lucky Anawe
Paul Apeye Lucky Anawe is an associate professor of Petroleum Engineering at Covenant University, Ota, Nigeria. His research interests include: clean energy (biogas, bioethanol and biodiesel), petroleum production and refining and corrosion engineering among others.
Augustine Omoniyi Ayeni
Augustine Omoniyi Ayeni holds a PhD degree in chemical engineering. His research areas include sustainable and renewable energy, statistical modelling and optimization and bioprocess engineering. He is currently the head of Chemical Engineering Department at Covenant University, Ota, Nigeria.
References
- Adekunle, S. A., Oyekunle, A. O., Obisesan, R. O., Ojo, O. S., & Ojo, S. O. (2016). Effects of degumming on biodiesel properties of some non-conventional seed oils. Energy Reports, 2(2016), 188–20. doi:10.1016/j.egyr.2016.07.001
- Aktas, D. F., Lee, J. S., Little, B. J., Ray, R. I., Davidova, I. A., Lyles, C. N., & Suflita, J. M. (2010). Anaerobic metabolism of biodiesel and its impact on metal corrosion. Energy Fuels, 24, 2924–2928. doi:10.1021/ef100084j
- Al Naggar, M. M., Ashour, F. H., Ettouney, R. S., & El Rifai, M. A. (2017). Production of biodiesel from locally available spent vegetable oil. Journal of Renewable Energy and Sustainable Development, 3(2), 189–195. doi:10.21622/resd.2017.03.2.189
- Alptekin, E., & Canakci, M. (2008). Characterization of the key fuel properties of methyl ester biodiesel-diesel fuel blends. Renewable Energy, 33, 2623–2630. doi:10.1016/j.renene.2008.02.020
- Al-Rasheed, S. A., Ibrahim, M. M., Maysa, M. A., & El-Gaaly, G. A. (2016). Biodiesel production and antioxidant capability from seeds of Salicornia bigelovii collected from Al-Jubail eastern province, Saudi Arabia. Pakistan Journal of Botany, 48(6), 2527–2533.
- Al-Zuhair, S. (2007). Production of biodiesel: Possibilities and challenges. Biofuels, Bioproducts and Biorefining, 1(1), 57–66. doi:10.1002/(ISSN)1932-1031
- Al-Zuhair, S., Ling, F. W., & Jun, L. S. (2007). Proposed kinetic mechanism of the production of biodiesel from palm oil using lipase. Process Biochemistry, 42(6), 951–960. doi:10.1016/j.procbio.2007.03.002
- American Society for Testing and Materials. (2007). ASTM standard specification for biodiesel fuel (B100): Annual book of ASTM standards. West Conshohocken: ASTM International. Method D6751-07b.
- Anawe, P. A. L., & Folayan, J. A. (2018a). Data on physico-chemical, performance, combustion and emission characteristics of persea Americana biodiesel yield and its blends on direct injection, compression ignition engines. Data in Brief, 21(2018), 1533–1540. doi:10.1016/j.dib.2018.10.166
- Anawe, P. A. L., & Folayan, J. A. (2018b). Novel synthetic based drilling fluid through enzymatic Inter-esterification of canola oil. International Journal of Chemical Engineering. Article ID 6418090. Hindawi.
- Anawe, P. A. L., & Folayan, J. A. (2018c). Data on optimization of production parameters on persea Americana (Avocado) plant oil biodiesel yield and quality. Data in Brief, 20, 855–863. doi:10.1016/j.dib.2018.08.064
- Anwar, F. B., Anger, M., Nasir, M., & Ismail, S. (2002). Analytical characterization of Salicornia bigelovii seed oil cultivated in pakistan. Journal of Agricultural and Food Chemistry, 50(15), 4210–4214.
- AOAC. (1990). Official methods of analysis (15TH). Virginia, USA: Author. p. 69–84: 951–979.
- ASTM. (2009). Standard specification for biodiesel fuel (B100) blend stock for distillate fuels. 1131–1136.
- Azam, M. M., Waris, A., & Nahar, N. M. (2005). Prospects and potential of fatty acid methyl esters of some non-tradition seed oils for use as biodiesel in India. Biomass and Bioenergy, 29(4), 293–302. doi:10.1016/j.biombioe.2005.05.001
- Bajpai, D., & Tyagi, V. K. (2006). Biodiesel: Source, production, composition, properties and its benefits. Journal of Oleo Science, 55(10), 487–502. doi:10.5650/jos.55.487
- Bangboye, A. I., & Hansen, A. C. (2008). Prediction of cetane number of biodiesel fuel from the fatty acid methyl ester (FAME) composition. Int. Agrophysics., 22(1), 21–29.
- Bello, E. I., Adekanbi, I. I., & Akinbode, F. O. (2015). Production and characterization of coconut (Cocos nucifera) oil and its methyl ester. European Journal of Engineering and Technology, 3(3), 25–35.
- Bello, E. I., Akinola, A. O., Otu, F., & Owoyemi, J. J. (2013). Fuel and physico-chemical properties of cashew (Anarcardium occidentale). Nut oil,its biodiesel and blends with diesel. British Journal of Applied Science and Technology, 3(4), 1055–1069. doi:10.9734/BJAST/2013/1680
- Borugadda, V. B., & Goud, V. V. (2014). Thermal,oxidative and low temperature properties of methyl esters prepared from oils of different fatty acids composition: A comparative study. Thermochimica Acta, 577(2014), 33–40. doi:10.1016/j.tca.2013.12.008
- Caballero, V., Bautista, F. M., Campelo, Campelo, J. M., Luna, D., Marinas, J. M., … Giordano, G. (2009). Sustainable preparation of a novel glycerol-free biofuel by using pig pancreatic lipase: Partial 1,3-regiospecific alcoholysis of sunflower oil. Process Biochemistry, 44(3), 334–342. doi:10.1016/j.procbio.2008.11.015
- Cao, L. (2005). Immobilised enzymes: Science or art. Current Opinion in Chemical Biology, 9(2), 217–226. doi:10.1016/j.cbpa.2005.02.014
- Chandravati, V., Saini, A., Bera, M., & Maji, P. K. (2017). Thermo-analytical characterization of biodiesel produced from edible and non-edible oils. Fuel Processing Technology, 167(2017), 395–403.
- Cherng, Y. L., & Lin, Y.-W. (2012). Fuel characteristics of biodiesel produced from a high acid oil of soyabean soap stock by supercritical-methanol transesterification. Energies, 5(2012), 2370–2380. doi:10.3390/en5072370
- De Paola, M. G., Ricca, E., Calabr`, O., Curcio, S., & Iorio, G. (2009). Factor analysis of transesterification reaction of waste oil for biodiesel production. Bioresource Technology, 100(21), 5126–5131. doi:10.1016/j.biortech.2009.05.027
- Deepa, S., Ramesh, K. P., Swarna, V. K., Raghava, R. J., & Bangaru, C. (2014). Studies on the physiological and biochemical characteristics of Salicornia brachiate: Influence of saline stress due to soaking waste water of tannery. Desalination and Water Treatment, 52(31–33), 6022–6029. doi:10.1080/19443994.2013.812987
- Demirbas, A. (1998). Fuel properties and calculation of higher heating values of vegetable oils. Fuel, 77(9–10), 1117–1120. doi:10.1016/S0016-2361(97)00289-5
- Demirbas, A. (2007). Importance of biodiesel as transportation fuel. Energy Policy, 35(9), 4661–4670. doi:10.1016/j.enpol.2007.04.003
- Dossat, V., Combes, D., & Marty, A. (1990). Continuous enzymatic transesterification of high oleic sunflower oil in a packed bed reactor: Influence of the glycerol production. Enzyme Microb. Technol, 25, 194–200. doi:10.1016/S0141-0229(99)00026-5
- Drapcho, C. M., Nhuan, N. P., & andWalker, T. H. (2008). Biofuels engineering process technology. McGraw-Hill Companies.
- Dunn, R. O. (1999). Thermal analysis of alternative diesel fuels from vegetable oils. Journal of American Oil Chemists Society, 76(1999), 109–115. doi:10.1007/s11746-999-0056-9
- Eganathan, P., SR-Subramanian, H. M., Latha, R., & Rao, C. S. (2006). Oil analysis in seeds of Salicornia brachiate. Industrial Crops and Product, 23(2006), 177–179. doi:10.1016/j.indcrop.2005.05.007
- Encinar, J. M., Gonzalez, J. F., & Rodriguez-Reinares, A. (2007). Ethanolysis of used frying oil biodiesel preparation and characterization. Fuel Processing Technology, 88(5), 513–522. doi:10.1016/j.fuproc.2007.01.002
- European committee for standardization. (2003). Committee for standardization of automotive fuels-fatty acid FAME for diesel engines requirements and test methods. Brussels: Author. EN 14214.
- Falasca, S. L., Ulberich, A., & Acevedo, A. (2014). Identification of Argentinian saline drylands suitable for growing Salicornia bigelovii for bioenergy. International Journal of Hydrogen Energy, 39(16), 8682–8689. doi:10.1016/j.ijhydene.2013.12.061
- Fellows, P. (2000). Food processing technology: Principles and practice. Woodhead Publishing Limited and CRC Press LLC, 2000.
- Fjerbaek, L., Christensen, K. V., & Norddahl, B. (2009). A review of the current state of biodiesel production using enzymatic transesterification,”. Biotechnology and Bioengineering, 102(5), 1298–1315. doi:10.1002/bit.v102:5
- Fukuda, H., Kondo, A., & Noda, H. (2001). Biodiesel fuel production by transesterification of oils. Journal of Bioscience and Bioengineering, 92(5), 405–416. doi:10.1016/S1389-1723(01)80288-7
- Garcia, R. M. (2010). Physiological studies of the halophyte Salicornia bigelovii: A potential food and biofuel crop for integrated aquaculture- agriculture systems. The University of Arizona, 2010.
- Geddada, M. N., & Pragada, P. M. (2013). Morphological and anatomical features of Salicornia brachiata. Journal of Biological and Chemical Research, 30(2), 887–891.
- Gentechscientific.com (2018). Gas Chromatography. http://gentechscientific.com/gas chromatography. (accessed 5TH August 2018).
- Glenn, E. P., Anday, T., Chaturvedi, R., Martinez-Garcia, R., Pearlstein, S., Soliz, D., … Felger, R. S. (2013). Three halophytes for saline-water agriculture: An oilseed, a forage and a grain crop. Environmental and Experimental Botany, 92, 110–121. doi:10.1016/j.envexpbot.2012.05.002
- Glenn, E. P., & Brown, J. J. (1999). Salt tolerance and crop potential halophytes. Critical Reviews in Plant Sciences, 18(2), 227–255. doi:10.1080/07352689991309207
- Gog, A., Roman, M., Tosa, M., Paizs, C., & Irimie, F. D. (2012). Biodiesel production using enzymatic transesterification–Current state and perspectives. Renewable Energy, 39(10–16). doi:10.1016/j.renene.2011.08.007
- Hanifar, T., Al-Zuhair, S., Ali, H., Al-Marzouqi, Y. F., & Mohammed, M. F. (2011). A review of enzymatic transesterification of microalgal oil-based biodiesel using supercritical technology. Enzyme Research, 2011. doi:10.4061/2011/468292
- Harwood, H. J. (1984). Oleochemicals as a fuel. Mechanical and economic feasibility. Jaocs, 61, 315–324. doi:10.1007/BF02678788
- Hossain, A. B. M., Boyce, A. N., Salleh, A., & Chandran, S. (2010). Impact of alcohol type, ratio and stirring time on the biodiesel production from waste canola oil. African Journal of Agricultural Research, 5(14), 1851–1859.
- Kalayasiri, P., Jayashoke, N., & Krisnangkura, K. (1996). Survey of seed oils for use as diesel fuels. Journal of the American Oil Chemists Society, 73(4), 471–474. doi:10.1007/BF02523921
- Knothe, G. (2005a). Viscosity of biodiesel. Chapter 6.2. In The biodiesel handbook. Champaign, Il, USA: AOCS press.
- Knothe, G. (2005b). Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Processing Technology, 86(2005), 1059–1070. doi:10.1016/j.fuproc.2004.11.002
- Knothe, G., Matheaus, A. C., & Ryan, T. W. (2003). Cetane numbers of branched and straight-chain fatty esters determined in an ignition quality tester. Fuel, 82(8), 971–975. doi:10.1016/S0016-2361(02)00382-4
- Knothe, G., & Steidley, K., . R. (2005). Kinematic viscosity of biodiesel fuel components and related compounds: Influence of compound structure and comparison to petrodiesel fuel components. Fuel, 84(9), 1059–1065. doi:10.1016/j.fuel.2005.01.016
- Krisnangkura, K. (1986). A simple method for estimation of cetane index of vegetable oil methyl esters. Journal of the American Oil Chemists Society, 63(4), 552–553. doi:10.1007/BF02645752
- Kumar, R., Madras, G., & Modak, J. (2004). Enzymatic synthesis of ethyl palmitate in supercritical carbon dioxide. Industrial and Engineering Chemistry Research, 43(7), 1568–1573. doi:10.1021/ie034032h
- Lee, S. B., Han, K. H., Lee, J. D., & Hong, I. K. (2010). Optimum process and energy density analysis of canola oil biodiesel synthesis. Journal of Industrial and Engineering Chemistry, 16, 1006–1010. doi:10.1016/j.jiec.2010.09.015
- Llah, E. M., Ssan, M. H., Murui, T., & Ami, E. S. (1994). Detailed studies on seed oil of Salicornia SOS, 7, cultivated at the Egyptian boarder of red sea. Grasas Y Aceites, 45(6), 385–389.
- Luque, S., Cervero, J. M., & Alvarez, J. R. (2014). Novozym 435-catalyzed synthesis of fatty acid ethyl esters from soybean oil for biodiesel production. Biomass and Bioenergy, 61, 131–137. doi:10.1016/j.biombioe.2013.12.005
- Ma, F., & Hanna, M. A. (1999). Biodiesel production: A review. Bioresource Technology, 70(1), 1–15. doi:10.1016/S0960-8524(99)00025-5
- Meher, L. C., Sagar, D. V., & Naik, S. N. (2006). Technical aspects of biodiesel production by transesterification: A review. Renewable and Sustainable Energy Reviews, 10(3), 248–268. doi:10.1016/j.rser.2004.09.002
- Modi, M. K., Reddy, J. R. C., Rao, B. V. S. K., & Prasad, R. B. N. (2007). Lipase-mediated conversion of vegetable oils into biodiesel using ethyl acetate as acyl acceptor. Bioresource Technology,98, 1260–1264. doi:10.1016/j.biortech.2006.05.006
- Mohibbe, A., Amtul, W., & Nahar, N. M. (2005). Prospect and potential of fatty acid methyl esters of some non-traditional seeds oil for use as biodiesel in India. Biomass Bioenergy, 29(2005), 293–302. doi:10.1016/j.biombioe.2005.05.001
- Murugesan, A., Umarani, C., Chinnusamy, T. R., Krishnan, M., & Subramania, R. (2009). Production and analysis of biodiesel from non-edible oils: A review. Renewable Sustainable Energy Reviews, 13, 825–834. doi:10.1016/j.rser.2008.02.003
- Noorollahi, Y., Sokhansefat, S., Sokhansefat, T., Rahmani, K., & Jalilinaasrabady, S. (2015). Biodiesel resources assessment and evaluation of the production capacity from Salicornia plant in Golestan province, north-east, Iran. International Journal of Renewable Energy Research, 5(3), 847–858.
- Noureddini, H., Gao, X., & Philkana, R. S. (2005). Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresource Technology, 96(7), 769–777. doi:10.1016/j.biortech.2004.05.029
- Park, H. S., Lee, H. J., Shin, M. H., Lee, K. W., Lee, H., Kim, Y. S., … Kim, K. H. (2007). Effects of co-solvents on the definition of green tea of supercritical CO2. Food Chemistry, 105(3), 1011–1017. doi:10.1016/j.foodchem.2007.04.064
- Perez, A., Casas, A., Fernandez, C. M., Ramos, M. J., & Rodriguez, I. (2010). Winterization of peanut biodiesel to improve the cold flow properties. Bioresource Technology, 101(2010), 7375–7381. doi:10.1016/j.biortech.2010.04.063
- Rafaat, A. A. (2009). Correlation between the chemical structure of biodiesel and its physical properties. International Journal of Environment Science and Technology, 6(4), 677–694. doi:10.1007/BF03326109
- Ranganathan, S. V., Narasimhan, S. L., & Muthukumar, K. (2008). An overview of enzymatic production of biodiesel. Bioresource Technology, 99(10), 3975–3981. doi:10.1016/j.biortech.2007.04.060
- Rashid, U., Anwar, F., Moser, B. R., & Samia, A. (2008). Production of sunflower methyl esters by optimized alkali-catalyzed methanolysis. Biomass Bioenergy, 32(12), 1202–1205. doi:10.1016/j.biombioe.2008.03.001
- Robles-Medina, A., Gonz´alez-Moreno, P. A., Esteban-Cerd´an, L., & Molina-Grima, E. (2009). Biocatalysis: Towards ever greener biodiesel production. Biotechnology Advances, 27(4), 398–408. doi:10.1016/j.biotechadv.2008.10.008
- Royon, D., Daz, M., Ellenrieder, G., & Locatelli, S. (2007). Enzymatic production of biodiesel from cotton seed oil using t-butanol as a solvent. Bioresource Technology, 98(2007), 648–653. doi:10.1016/j.biortech.2006.02.021
- Salles, K., Meneghetti, S. M. P., Ferreira de la, S. W., Meneghetti, M. R., Dos Santos, L. C. F., Da Silva, J. P. V., … Soletti, J. I. (2010). Characterization of Syagrus coronate (Mart) Becc. Oil and properties of methyl esters for use as biodiesel. Ind.Crops Prod, 32(2010), 518–521. doi:10.1016/j.indcrop.2010.06.026
- Sapkale, G. N., Patil, S. M., Surwase, U. S., & Bhatbhage, P. K. (2010). Supercritical fluid extraction. International Journal of Chemical Science, 8(2), 729–743.
- Schumacher, L. G., Marshall, W., Krahl, J., Wetherell, W. B., & Grabowski, M. S. (2001). Biodiesel emissions data from series 60 DDC engines,”. Transactions of the American Society of Agricultural Engineers, 44(6), 1465–1468. doi:10.13031/2013.6999
- Sebastian, J., Muraleedharan, C., & Santhiagu, A. (2016). A comparative study between chemical and enzymatic transesterification of high free fatty acid contained rubber seed oil for biodiesel production. Cogent Engineering.3, 1178370. doi:10.1080/23311916.2016.1178370
- Shahid, H. (2014). Enhanced oil recovery using supercritical carbondioxide with and without co-solvents. International Journal of Petroleum and Gas Engineering, 2(1), 1–12.
- Sharma, Y. C., Singh, B., & Upadhyay, S. N. (2008). Advancements in development and characterization of biodiesel: A review. Fuel, 87(12), 2355–2377. doi:10.1016/j.fuel.2008.01.014
- Shimada, Y., Watanabe, Y., Samukawa, T., et al (1999). Conversion of vegetable oil to biodiesel using immobilized Candida Antarctica lipase. Journal of the American Oil Chemists’ Society, 76, 789–793. doi:10.1007/s11746-999-0067-6
- Stavarache, C., Vinatoru, M., Nishimura, R., & Maeda, Y. (2005). Fatty acids methyl esters from vegetable oil by means of ultrasonic energy. Ultrasonics Sonochemistry, 12(5), 367–372. doi:10.1016/j.ultsonch.2004.04.001
- Svendsen, A. (2000). Lipase protein engineering. Biochimica Et Biophysica Acta, 15(43), 223–238. doi:10.1016/S0167-4838(00)00239-9
- Szczesna, A., . M., Kubiak, A., Antczak, T., & Bielecki, S. (2009). Enzymatic biodiesel synthesis: Key factors affecting efficiency of the process. Renewable Energy, 34(5), 1185–1194. doi:10.1016/j.renene.2008.11.013
- Tupu, S. C., Jae, Y., Marquis, C., Adesina, A. A., & Rogers, P. L. (2013). Enzymatic conversion of coconut oil for biodiesel production. Fuel Processing Technology, 106, 721–726. doi:10.1016/j.fuproc.2012.10.007
- Uriate, F. A. (2010). Biofuels from Plant Oils: A book for practitioners and professionals involved in biofuels, to promote a better and more accurate understanding of the nature, production and use of biofuels from plant oils. National Academy of Science and Technology. Government of Japan. Japan ASEAN. Solidarity Fund
- Vicente, G., Martinez, M., & Aracil, J. (2004). Integrated biodiesel production: A comparism of different homogenous catalyst system. Bioresource Technology, 92, 297–305. doi:10.1016/j.biortech.2003.08.014
- Zhou, W., Konar, S. K., & Boocock, D. G. B. (2003). Ethyl esters from the single-phase base-catalyzed ethanolysis of vegetable oils. Journal of American Oil Chemist Society, 80(4), 367–371. doi:10.1007/s11746-003-0705-1
- Zucca, P., & Sanjust, E. (2014). Inorganic materials as supports for covalent enzyme immobilization: Methods and mechanism. Molecules.19, (9), 14139–14194. doi:10.3390/molecules190914139
- Zuleta, E. C., Rios, L. A., & Benjumea, P. N. (2012). Oxidative stability and cold flow behaviour of palm, sacha-hinchi, Jatropha and castor oil biodiesel blends. Fuel Processing Technology, 102(2012), 96–101. doi:10.1016/j.fuproc.2012.04.018
- Zzkdinstrument .com (2019). Zhengzhou Keda RE-501 vacuum rotary evaporator. Retrieved from http://zzkdinstrument.com/RE-501 vacuum rotary evaporator
