?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, the adsorption behavior of Cu2+, Pb2+ and Cd2+ from synthetic metal solutions using natural zeolites was studied in order to investigate the efficiency of adsorbents of heavy metals from industrial wastewater. The kinetic study indicated the suitability of the zeolite for the removal of Cu2+, Pb2+ and Cd2+ ions from synthetic wastewater; batch experiments were used to identify the effect of parameters that affect the rate of adsorption such as the adsorbent mass, the initial solution concentration, the initial solution pH, the adsorbent particle size, and the agitation speed and evaluated their impact on the removal efficiency of heavy metals from industrial wastewater using the natural zeolite. Zeolite samples with masses between 1 g and 10 g were contacted with constant volume (100 ml) of multicomponent synthetic solutions containing Cu2+, Pb2+ and Cd2+ ions. They were agitated at agitation speeds in the range of 100–300 rpm for agitation times from 1 hr to 8 hrs in a magnetic stirrer at room temperature, the pH values were monitored and adjusted regularly. Every hour, 15 ml of the samples was analyzed using the Atomic Absorption Spectroscopy. The results show that the removal efficiency of Cu2+ increase from 60% for 1 gr to 99% for 10 gr of the mass of absorbent, increase from 62% for 1% to 94% for 7 of the initial solution pH, increase from 90% for 100 rpm to 94% for 300 rpm of the agitation speed, the amount adsorbed increase from 0.5 mg/g for 100 mg/l to 2.1% for 400 mg/l of the initial solution concentration. Similar results are obtained for the two other heavy metals (Pb2+, Cd2+) and showed that the capacity of the adsorbents for the removal of heavy metals is directly proportional to the mass of absorbent, initial solution pH, agitation speed, and initial solution concentration. The highest adsorption rate of Cu2+, Pb2+ and Cd2+ ions took place in the first hours followed by a slower adsorption rate later on.
PUBLIC INTEREST STATEMENT
The presence of accumulated heavy metals in the environment constitutes is a serious threat to human life and the environment due to their toxicity. The use of natural zeolite to remove heavy metals from wastewater has attracted much attention due to its availability, low cost and its economic advantages and high removal efficiency.
This study indicated the suitability of the zeolite for the removal of Cu2+, Pb2+ and Cd2+ ions from synthetic wastewater; batch experiments were used to identify the effect of parameters that affect the rate of adsorption such as the adsorbent mass, the initial solution concentration, the initial solution pH, the adsorbent particle size, and the agitation speed and evaluated their impact on the removal efficiency of heavy metals from industrial wastewater using the zeolite.
1. Introduction
The presence of accumulated heavy metals in the environment constitutes is a serious threat to human life and the environment due to their toxicity. Cu2+, Pb2+ and Cd2+ are the common metals that tend to accumulate in organisms, causing numerous diseases (Ahali Abadeh & Irannajad, Citation2017; Buaisha et al., Citation2020; Salman et al., Citation2017). Several techniques such as adsorption, extraction, phytoextraction, ultrafiltration, reverse osmosis, electrodialysis, ion-exchange, and membrane processes are proposed for the handling of wastewater pollution and removing heavy metals from industrial wastewater required high energy or special operational requirements (Ayoub et al., Citation2016; Ebrahimi et al., Citation2014; Kwon et al., Citation2010). The selection of alternative low-cost materials such as sorbents for the removal of heavy metals has emphasized. Recently and the adsorption method using zeolite is considered to be an appropriate method for wastewater handling because of its cost-effectiveness and simplicity (Gupta et al., Citation2009; Scharnberg et al., Citation2020).
The efficiency of zeolite for the removal of three heavy metal Cu2+, Pb2+ and Cd2+ ions from a synthetic solution was considered. These three ions were chosen as abundance more pollutants that are present in industrial wastewaters.
The first part discusses the experimental materials and methods used for the adsorption of heavy metals from synthetic wastewaters. The experiments included are the most widely used techniques for the batch system. The second part describes the kinetic studies in order to investigate the behaviour of adsorbent and understand the removal mechanisms involved in the adsorption process, in this part, a number of these factors that affect the efficiency of natural zeolite in removing of Cu2+, Pb2+ and Cd2+ from solution are investigated in detail. In conclusion, the specific aims of this study are reviewed to present an overview of the conclusions of this study and to make a critical evaluation and discusses the recommendations for further work.
2. Materials and methods
The experiments have focused on the efficiency of zeolite for the removal or reduction of Cu2+, Pb2+ and Cd2+ ions from synthetic industrial wastewater to achieve allowable limits. Synthetic multicomponent solutions of Cu2+, Pb2+ and Cd2+ ions were prepared.
In all experiment stages deionised water was used, which had almost all of its mineral ions removed. The pH of the starting solution was pH 5 to get the best adoration rate. The pH values were monitored and adjusted using a pH meter (Thermo Scientific Orion Star A111 Benchtop). The pH was adjusted to as 1, 5, and 7, respectively, by adding hydrochloric acid or bases sodium hydroxide. To observe the effect of agitation speed; agitation in a beaker was obtained by using a magnetic stirrer (IKA, Germany, RH basic 2, 3,339,000) at a speed of 100 rpm, 200 rpm and 300 rpm. The mesh sizes of the zeolite particle used in this study was 100 µm < dp < 200 µm, unless stated. A Thermo Scientific model PR305220G, Gravity Convection stainless steel universal oven was used to dry and heat the samples.
Zeolite samples with masses 1 g, 5 g and 10 g were contacted with constant volume (100 ml) of multicomponent synthetic solutions containing Cu2+, Pb2+ and Cd2+ ions. They were agitated at agitation speeds of 100, 200 and 300 rpm for agitation times from 1 hr to 8 hrs in a magnetic stirrer at room temperature, the pH values were monitored and adjusted regularly. Every hour, 15 ml of the samples was filtered and taken for metal ion concentration analysis using the Atomic Absorption Spectroscopy (AA6800Shimadzu) with oxygen air-acetylene flame analysis technique was used to analyse the digested solutions, the wavelength range was between 190 and 900 nm. A flame temperature is continuously adjustable between 2300°C and 2950°C, which makes it possible to choose the best atomization temperature for different elements. Integrated flame/graphite furnace atomization system, changeable with flame emission burner, was involved. Hollow cathode lamps were used as sources of radiation and the background correction was provided by a deuterium lamp.
The experiments were duplicated four times in order to examine the reproducibility of the results, while the mean value was used for all taken data, the deviation between the duplicate samples in analysing the cations was ± 5.1%, 4.5% and 5.1% for Cu2+, Pb2+ and Cd2+ ions, respectively.
3. Results and discussion
The adsorption of heavy metal cations from solution using zeolite materials was affected by several factors; examples of such factors were the adsorbent mass, initial solution concentration, initial solution pH, agitation speed and adsorbent particle size. The effect of these factors on the efficiency of zeolite in removing Cu2+, Pb2+ and Cd2+ from solution will be investigated in order to determine the behaviour of adsorbents and understand the removal mechanisms involved in the any adsorption experiment in wastewater treatment (Amarasinghe & Williams, Citation2004; Ennigrou et al., Citation2015; Myroslav et al., Citation2006; Inglezakis et al., Citation2004).
3.1. Kinetic study results
Multicomponent solutions were mixed with zeolite and agitated for an hour at room temperature and the initial concentrations of the mixed component solutions were 100 mg/l for the Cu2+, Pb2+ and Cd2+ ions.
The data obtained from the kinetic adsorption tests were used to determine the removal capacity, qe (mg/g) of the different adsorbents using the following EquationEquation (1)(1)
(1) and (Equation2
(2)
(2) ):
The percentage removal of metal ions from solution was also determined using the equation below:
3.2. Factors influencing the rate of adsorption
A number of parameters influencing the rate of adsorption were studied and described, these parameters include the effect of adsorbent mass, effect of initial solution concentration, effect of initial solution pH, effect of adsorbent particle size, and effect of agitation speed.
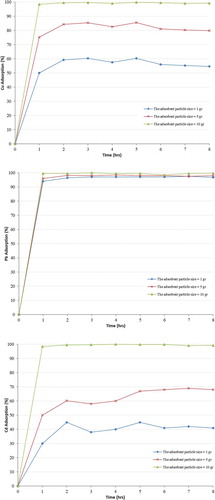
3.2.1. Effect of adsorbent mass
The effect of adsorbent mass on the adsorption capacities from solutions was investigated. Three different masses were used in this study, 1 g, 5 g and 10 g of zeolite in 100 ml solutions. The particle size of zeolite used was 200 µm, the pH of the solution was adjusted to 5, the agitation speed was 200 rpm at room temperature; the samples were collected every hour and analysed using the Atomic Absorption Spectroscopy (AA6800Shimadzu) with flame and graphite techniques. The experiment was carried-out at fixed initial metal concentrations.
The results in Figure clearly show that when the adsorbent mass was increased, this resulted in an increase in the adsorption of the heavy metal ions. The main reason for this is that as the adsorbent mass increases, more adsorption sites are available per mass of adsorbent surface and thus the total amount of metal that is removed increases. This result indicates that the removal of Cu2+, Pb2+ and Cd2+ ions mostly occurs in the early stage and the mineral mass in the solution can affect the adsorption capacity for the removal of heavy metals as it determines the availability of adsorption sites.
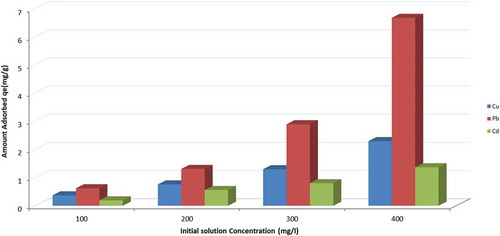
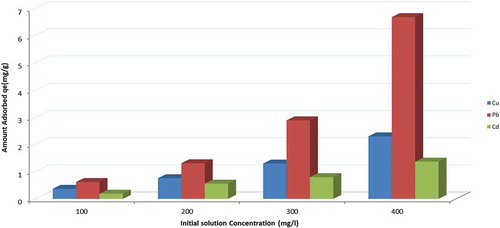
3.2.2. Effect of initial solution concentration
The initial concentration of the solution significantly impacts on the heavy metal removal process, several studies have reported that an increase in the initial concentration resulted in an improvement in the adsorption capacity at the initial stage and a decrease in the overall heavy metal removal efficiency, which depends on the availability of active sites (Coruh & Ergun, Citation2009; Ören & Kaya, Citation2006).
The effect of the initial metal concentration on the efficiency of zeolite in the removal of heavy metals from the solution was determined using multicomponent solution concentrations ranging from 100 to 400 mg/l. 100 ml solutions at pH 5 were contacted with 5 g of zeolite samples of size 200 μm, agitation speed of 200 rpm was used at room temperature.
The results in Figure show that generally any increase in the initial solution concentration results in an increase in the heavy metal removal efficiency and the rate of adsorption. As the initial concentration increases, the driving force increases, which in turn leads to an improvement in the efficiency of the heavy metal removal process. Then, after the system reaches equilibrium, the initial solution concentration does not show any significant change due to a decrease in the number of active sites (Ibrahim & Mutawie, Citation2013; Ibrahimi & Sayyadi, Citation2015; Taamneh & Sharadqah, Citation2017; Visa & Popa, Citation2015).
3.2.3. Effect of initial solution pH
The initial solution pH parameter has a significant impact on heavy metal removal processes since it can impact on adsorbent ability to remove metals with the competition of hydrogen ions with heavy metal cations for active sites on the adsorbent surface (Dimirkou, Citation2007; Inglezakis et al., Citation2002). An acidic solution can impact on both the character of the exchanging ions and the character of the adsorbent.
Three different pH values of initial solution were preferred 1, 5 and 7 for the multicomponent solutions, the pH of the solution was adjusted using sodium hydroxide solution and hydrochloric acid solution. Thus, 100 ml of the solution samples were in contact with 5 g of zeolite for 1 hour, the particle size of zeolite used was 200 µm, the agitation speed was 200 rpm at room temperature, samples using fixed metal concentrations of Cu2+, Pb2+ and Cd2+ cations were collected every hour and analysed using the Atomic Absorption Spectroscopy (AA6800Shimadzu) with flame and graphite techniques.
The results are presented in Figure , shown that the solution pH increases, the heavy metal removal efficiency also increases. This is due to the competition between the hydrogen ions and heavy metal cations for the same exchange sites and electrostatic repulsion between the heavy metal cations in solution; as more hydrogen ions are adsorbed, the number of protonated zeolite surfaces increases (Alvarez-Ayuso et al., Citation2003; Hamedreza et al., Citation2015; Moreno et al., Citation2001; Wingenfelder et al., Citation2005).
Figure 3. Variation of heavy metal removal (Cu2+, Pb2+ and Cd2+) as function of initial solution pH.
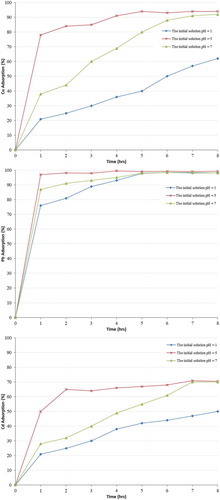
Also, the results show that the ion exchange process increases with an increase in pH up to a maximum value. It can be concluded that the best heavy metal removal efficiency value was obtained between pH values of 5 and 7, while pH values below pH = 5 or above pH = 7 was found to decrease the heavy metal removal efficiency. These results are in agreement with outcomes obtained from the literature review (Argun, Citation2008; DalBosco et al., Citation2005; Mier et al., Citation2001).
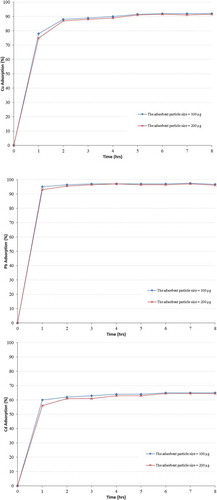
3.2.4. Effect of adsorbent particle size
The effect of adsorbent particle size on adsorption process was investigated: two different sizes were used, 100 and 200 μm. 5 g of zeolite was mixed with 100 ml solution of the appropriate multicomponent solution at an agitation speed of 200 rpm, the pH of the solution was adjusted to 5 and samples were collected every hour, then filtered to remove solids and analysed using the Atomic Absorption Spectroscopy (AA6800 Shimadzu) with flame and graphite techniques. This experiment was carried using fixed initial metal concentrations.
The results depicted in Figure show that the smaller particle-sized samples adsorbed more of the Cu2+, Pb2+ and Cd2+ ions and indicate that any decrease in adsorbent particle size causes an increase in the adsorption of the heavy metals ions. This is because as the adsorbent particles get smaller more adsorption sites are available for metal uptake and more contacts are taking place. On the other hand, smaller particle sizes result in the shortening of the diffusion distance that ions have to travel in order to get to an active site. This is in agreement with the results obtained from literature review (Erdem et al., Citation2004; Malliou et al., Citation1994; Myroslav et al., Citation2006), they concluded that the larger particle size adsorbent had lower adsorption capacities than the smaller particle sizes.
The particle size can affect the adsorption capacity mostly at the initial stage. As the contact time increases, in the level of the effect of particle size on adsorption decreases, and as a result, the adsorption process gets slower and the use of very fine particles can also cause some operational problems such as difficulty in the filtration of the zeolite from solution in batch studies (Erdem et al., Citation2004; Malliou et al., Citation1994; Inglezakis et al., Citation2001).
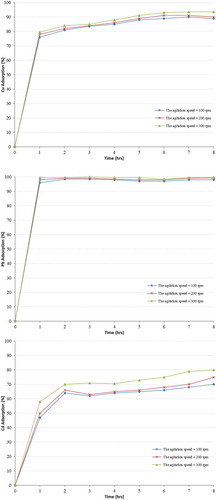
3.2.5. Effect of agitation speed
The effect of agitation speed on the removal of the cations heavy metal from the solution by zeolite was determined using a magnetic stirrer at a speed of 100, 200 and 300 rpm, the mixture was contacted with 5 g of zeolite samples and agitated, the particle size of the zeolite samples used was 200 μm at room temperature, the pH was adjusted to 5. The samples were collected every hour and analysed using the Atomic Absorption Spectroscopy (AA6800Shimadzu) with flame and graphite techniques and the results are presented in Figure .
The results show that the metal removal efficiency increased as the speed of agitation increased, Agitation of the mixture results in abrasion and the production of more broken zeolite particles. This means that fresh smaller size zeolite particles are produced and more activate sites are available on the surface (Khachatryan, Citation2014; Mehdizadeh et al., Citation2014). Thus, this phenomena leads to an increase in the surface area, which significantly improves the efficiency of the heavy metal removal from solution. This is in agreement with the results obtained by Ören and Kaya (Citation2006), they concluded that an increase in the speed of agitation resulted in higher adsorption capacities and the agitation helps in overcoming the external mass transfer resistance, which controls the rate of adsorption.
4. Conclusion
Kinetic studies and experiments were carried out in order to investigate the impact of several parameters on the efficiency of zeolite for the removal of heavy metals from industrial wastewater, the results obtained may be summarized as follows:
The highest adsorption rate of Cu2+, Pb2+ and Cd2+ ions took place in the first hours followed by a slower adsorption rate later on. The first hour is an initial stage of adsorption when higher rates of adsorption take place; this may be due to the availability of more adsorption sites and the fact that the metal ions exchange easily on the surface of the zeolite grains. After that, a slower adsorption rate follows due to slower diffusion of the metal ions into the interior channels.
The study indicated the suitability of the zeolite used for the removal of Cu2+, Pb2+ and Cd2+ ions from synthetic wastewater, while considering the economic aspects of wastewater treatment.
The adsorbent mass, adsorbent particle size, initial solution pH, initial solution concentration and agitation speed are usually the most influential parameters.
The efficiency of heavy metal removal was enhanced with increased initial solution pH, increased agitation speed, increased solution concentration, decreased particle size and greater mass of absorbent.
The adsorption is a heterogeneous process as the removal rate of Cu2+, Pb2+ and Cd2+ ions mostly occurred early on, but as the contact time increased, there was a decrease in the level of the effect of the parameters on adsorption and the adsorption process became slower.
The recent studies show that precipitation affects the rate of removal and the quantities of heavy metals in solution and the adsorption capacity of zeolite that has been regenerated using NaCl is either the same or it changed slightly.
The natural zeolite is promising sorbent due to its availability and its low cost and can substitute effectively the use of activated carbon as adsorbent for the removal of metal cations from wastewater.
Nomenclature and units
| m | = | The mass of the zeolite used (mg), |
| V | = | The aqueous volume (L), |
| Co | = | The initial metal ion concentrations in the samples (mg/L), |
| Ce | = | The equilibrium metal ion concentrations in the samples (mg/L), |
| qe | = | The mass of heavy metal uptake per unit of natural zeolite (mg/g). |
Acknowledgements
The author is grateful to the editor and the referees for carefully reading the paper and for their comments and suggestions that have improved the paper.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
Notes on contributors
Noureddine Elboughdiri
Dr Noureddine Elboughdiri is an associate professor and currently the head of Chemical Engineering Department, College of Engineering, Hail University, Saudi arabia. He received his B.Sc., Master and Ph. D. degree in Chemical Engineering from the National School of Engineering Gabes, University of Gabes, Tunisia. He joined the Central Laboratory for Analysis and Testing LCAE-Tunisia in 2004 and SGS Group in 2008 and held different teaching and administrative positions including optimization methods, statistical analysis, academic accreditation (NCAAA/ABET) and industrial accreditation (ISO 17025/ISO 9001) to provide reliable solutions to many of the contemporary problems facing the chemical industries.
His research interest is in the area of Removal and valorization of polyphenols from Olive Mill WasteWaters (OMWW), Optimization by response surface methodology (RSM) and wastewaters treatment.
References
- Ahali Abadeh, Z., & Irannajad, M. (2017). Removal of Ni and Cd ions from aqueous solution using iron dust-zeolite composite: Analysis by thermodynamic, kinetic and isotherm studies. Chemical Research in Chinese Universities, 33(2), 318–13. https://doi.org/10.1007/s40242-017-6150-x
- Alvarez-Ayuso, E., Garcia-Sanchez, A., & Querol, X. (2003). Purification of metal electroplating waste waters using zeolites. Water Research, 37(20), 4855–4862. https://doi.org/10.1016/j.watres.2003.08.009
- Amarasinghe, B. W., & Williams, R. A. (2004). Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chemical Engineering Journal, 132(1–3), 299–309. https://doi.org/10.1016/j.cej.2007.01.016
- Argun, M. E. (2008). Use of clinoptilolite for the removal of nickel ions from water: Kinetics and thermodynamics. Journal of Hazardous Materials, 150(3), 587–595. https://doi.org/10.1016/j.jhazmat.2007.05.008
- Ayoub, M., Rashed, I., & El-Morsy, A. (2016). Energy production from sewage sludge in a proposed wastewater treatment plant. Civil Engineering Journal, 2(12), 637–645. https://doi.org/10.28991/cej-2016-00000064
- Buaisha, M., Balku, S., & Özalp, S. (2020). Heavy metal removal investigation in conventional activated sludge systems. Civil Engineering Journal, 6(3), 470–477. https://doi.org/10.28991/cej-2020-03091484
- Coruh, S., & Ergun, O. N. (2009). Ni 2+++ removal from aqueous solutions using conditioned clinoptilolites: Kinetic and isotherm studies. Environmental Progress & Sustainable Energy, 28(1), 162–172. https://doi.org/10.1002/ep.10316
- DalBosco, S. M., Jimenez, R. S., & Carvalho, W. A. (2005). Removal of toxic metals from wastewater by Brazilian natural scolecite. Journal of Colloid and Interface Science, 281(2), 424–431. https://doi.org/10.1016/j.jcis.2004.08.060
- Dimirkou, A. (2007). Uptake of Zn2+++ ions by a fully iron-exchanged clinoptilolite. Case study of heavily contaminated drinking water samples. Water Research, 41(12), 2763–2773. https://doi.org/10.1016/j.watres.2007.02.045
- Ebrahimi, R., Zandi, S., & Gharibi, F. (2014). Removal of nickel and total chromium using Escherichia coli biofilm supported on clinoptilolite. Journal of Advances in Environmental Health Research, 2(2), 126–133. https://doi.org/10.22102/JAEHR.2014.40153
- Ennigrou, D. J., Ali, M. B., Dhahbi, M., & Ferid, M. (2015). Removal of heavy metals from aqueous solution by polyacrylic acid enhanced ultrafiltration. Desalination and Water Treatment, 56(10), 2682. https://doi.org/10.1080/19443994.2014.982958
- Erdem, E., Karapinar, N., & Donat, R. (2004). The removal of heavy metal cations by natural zeolites. Journal of Colloidal and Interface Science, 280(2), 309–314. https://doi.org/10.1016/j.jcis.2004.08.028
- Gupta, V. K., Carrott, P. J. M., Ribeiro, M. M. L., & Suhas, T. L. (2009). Low-cost adsorbents: Growing approach to wastewater treatment-a review. Critical Reviews in Environmental Science and Technology, 39(10), 783–842. https://doi.org/10.1080/10643380801977610
- Hamedreza, J., Ghorbani, F., Tayebi, H., & Asl, S. (2015). Study of the adsorption of Cd (II) from aqueous solution using zeolite-based geopolymer, synthesized from coal fly ash; kinetic, isotherm and thermodynamic studies. Arabian Journal of Chemistry, 8(6), 837–849. https://doi.org/10.1016/j.arabjc.2013.02.018
- Ibrahim, W. M., & Mutawie, H. H. (2013). Bioremoval of heavy metals from industrial effluent by fixed-bed column of red macroalgae. Toxicology and Industrial Health, 29(1), 38–42. https://doi.org/10.1177/0748233712445044
- Ibrahimi, M. M., & Sayyadi, A. S. (2015). Application of natural and modified zeolites in removing heavy metal cations from aqueous media: An overview of including parameters affecting the process. International Journal of Geology, Agriculture and Environmental Sciences, 3, 1–7. https://www.woarjournals.org/admin/vol_issue1/upload%20Image/IJGAES031201.pdf
- Inglezakis, V. J., Hadjiandreou, K. J., Loizidou, M. D., & Grigoropoulou, H. P. (2001). Pretreatment of natural clinoptilolite in a laboratory-scale ion exchange packed bed. Water Research, 35(9), 2161–2166. https://doi.org/10.1016/S0043-1354(00)00500-5
- Inglezakis, V. J., Loizidou, M. M., & Grigoropoulou, H. P. (2002). Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Research, 36(11), 2784–2792. https://doi.org/10.1016/S0043-1354(01)00504-8
- Inglezakis, V. J., Loizidou, M. M., & Grigoropoulou, H. P. (2004). Ion exchange studies on natural and modified zeolites and the concept of exchange site accessibility. Journal of Colloid and Interface Science, 275(2), 570–576. https://doi.org/10.1016/j.jcis.2004.02.070
- Khachatryan, S. V. (2014). Heavy metal adsorption by Armenian natural zeolite from natural aqueous solutions. Proceedings of the Yerevan State University Chemistry and Biology., 2, 31–35. http://ysu.am/files/8.%20HEAVY%20METAL%20ADSORPTION%20BY%20CLINOPTILOLITE%20FROM.pdf
- Kwon, J. S., Yun, S. T., Lee, J. H., Kim, S. O., & Jo, H. Y. (2010). Removal of divalent heavy metals (Cd, Cu, Pb, and Zn) and arsenic (III) from aqueous solutions using scoria: Kinetics and equilibria of sorption. Journal of Hazardous Materials, 174(1–3), 307–313. https://doi.org/10.1016/j.jhazmat.2009.09.052
- Maleki, A., Mahvi, A. H., Zazouli, M. A., Izanloo, H., & Barati, A. H. (2011). Aqueous cadmium removal by adsorption on barley hull and barley hull ash. Asian Journal of Chemistry, 23(3), 1373–1376. http://www.asianjournalofchemistry.co.in/User/ViewFreeArticle.aspx?ArticleID=23_3_100
- Malliou, E., Loizidou, M., & Spyrellis, N. (1994). Uptake of lead and cadmium by clinoptilolite. Science of the Total Environment, 149(3), 139–144. https://doi.org/10.1016/0048-9697(94)90174-0
- Mehdizadeh, S., Sadjadi, S., Ahmadi, S. J., & Outokesh, M. (2014). Removal of heavy metals from aqueous solution using platinum nanoparticles/Zeolite-4A. Journal of Environmental Health Science & Engineering, 12(1), 7. https://doi.org/10.1186/2052-336X-12-7
- Mier, M. V., Callejas, R. L., Gehr, R., Cisneros, B. E., & Alvarez, P. J. (2001). Heavy metal removal with Mexican clinoptilolite: Multi-component ionic exchange. Water Research, 35(2), 373–378. https://doi.org/10.1016/S0043-1354(00)00270-0
- Moreno, N., Querol, X., & Ayora, C. (2001). Utilization of zeolites synthesised from coal fly ash for the purification of acid mine waters. Environmental Science & Technology, 35(17), 3526–3534. https://doi.org/10.1021/es0002924
- Myroslav, S., Boguslaw, B., Artur, T. P., & Jacek, N. (2006). Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+ and Cd2+) adsorption on clinoptilolite. Journal of Colloid and Interface Science, 304(1), 21–28. https://doi.org/10.1016/j.jcis.2006.07.068
- Ören, A. H., & Kaya, A. (2006). Factors affecting adsorption characteristics of Zn2+ on two natural zeolites. Journal of Hazardous Materials, 13(1–3), 59–65. https://doi.org/10.1016/j.jhazmat.2005.09.027
- Salman, H., Shaheen, H., Abbas, G., & Khalouf, N. (2017). Use of Syrian natural zeolite for heavy metals removal from industrial wastewater: Factors and mechanism. Journal of Entomology and Zoology Studies, 5(4), 452–461. http://www.entomoljournal.com/archives/2017/vol5issue4/PartF/5-3-217.pdf
- Scharnberg, A., Adrison, C. L., & Annelise, K. (2020). Optical and structural characterization of Bi2FexNbO7 nanoparticles for environmental applications. Emerging Science Journal, 4(1), 11–17. https://doi.org/10.28991/esj-2020-01205
- Taamneh, Y., & Sharadqah, S. (2017). The removal of heavy metals from aqueous solution using natural Jordanian zeolite. Applied Water Science, 7(4), 2021–2028. https://doi.org/10.1007/s13201-016-0382-7
- Visa, M., & Popa, N. (2015). Adsorption of heavy metals cations onto zeolite material from aqueous solution. Journal of Membrane Sciences and Technology, 5, 133. https://doi.org/10.4172/2155-9589.1000133
- Wingenfelder, U., Hansen, C., Furrer, G., & Schulin, R. (2005). Removal of heavy metals from mine waters by natural zeolites. Environmental Science & Technology, 35(12), 4606–4613. https://doi.org/10.1021/es048482s