?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Application of plant extracts for corrosion inhibition of carbon steels continues to increase due to their nontoxic nature and presence of phytochemical components. The corrosion inhibition performance of Apium graveolens, Punica granatum, and Camellia sinensis plant extracts on plain carbon steel in 0.5 M sulfuric acid was assessed with coupon measurement, potentiodynamic polarization technique and optical microscopy characterization. The plant extracts effectively inhibited the carbon steel from 60% to 90% concentration with optimal inhibition efficiency values of 95.83%, 87.08%, and 92.37% at 90% concentration from coupon measurement. The corrosion inhibition capability of the plant extracts proves it to be time and concentration dependent, though time dependence decreases with increase in concentration and was negligible at 90% concentration. Polarization studies showed the extracts exhibited cathodic inhibition mechanism from the displacement of the polarization plots and significant decrease in cathodic portion of the polarization plots. Optical image of the steel without the extracts exhibited general corrosion with macro-pits which significantly differ from the image of the inhibited steel.
PUBLIC INTEREST STATEMENTS
http://coventuniversity.edu.ng The economic impact and problems resulting from corrosion has drawn strong attention from scientists and engineers worldwide. Corrosion of mild steel in industrial environments is a major concern in chemical processing plants, oil and gas industry, manufacturing, automobile industry, marine operations, boiler plants, and power generation plants due to the considerable cost involved in the replacement of metallic parts in their various applications. The consequence often leads to plant shutdowns, breakdown of industrial equipment, reduced efficiency, industrial downtime, high maintenance cost due to replacement of damaged part, wastage of valuable resources, and expensive overdesign. Corrosion inhibition by environmentally sustainable biodegradable compounds is of great practical importance compared to conventional toxic chemical compounds due to the long-term benefits to the environmental and personnel safety.
1. Introduction
Corrosion is the degradation of the metallurgical, physical, microstructural, and mechanical properties of metallic alloys due to chemical reaction processes between the alloys and their surrounding (Gilbert, Citation2012; Rajendrachari, Citation2018). It is an inherent, spontaneous and thermodynamic process whereby the materials return to their more chemically stable forms, i.e. corrosion products such as oxides, hydroxides, sulfides, etc. at lower energy state (Mokhatab et al., Citation2019). The corrosion products is due to the ionization of the metallic ions which diffuses into the corrosive environment and probably undergoes secondary reaction; chemically combining with other ions present to form an insoluble molecular species such as rust (Alcántara et al., Citation2017). This phenomenon is common in industrial processes such as refinery operations in crude distillation units, production of intermediate chemical compounds, fertilizer production, pickling operations, etc. where acidic solutions are extensively applied thus increasing the vulnerability of metallic components within this conditions to corrosion (Fiori-Bimbi et al., Citation2015). The effect of corrosion on domestic economies worldwide reaches about 3.1% of national GDP (Gilbert, Citation2012). According to Shreir (Citation2010), destruction of refined materials due to corrosion is ranked third after conflict and epidemic. Corrosion effect on metals is more conspicuous and deleterious compared to nonmetallic materials resulting in metallic wastage due to the action of corrosive agents. Mild steel is a ferrous alloy composed of 0.1–0.25% C content with versatile application in civil and industrial construction (Kilcherman, Citation2015). Petrochemical pipelines, some automobile parts, marine structures, and industrial machinery are constructed with mild steel. The steel is relatively inexpensive compared to stainless steels. It is weldable and recyclable coupled with high machinability (Lgaz et al., Citation2018). Electrochemical deterioration of mild steel occurs readily in electrolyte solution during pickling and scale-removal, transportation of acids, boiler operations, heat transfer in nuclear reactions, and other industrial processes. Its poor corrosion resistance results in accelerated general corrosion propagated throughout the entire exposed surface. General corrosion is the most destructive cause of mild steels degradation on tonnage basis, as a result the steel requires continual maintenance, repairs, and replacement leading to huge overhead and operating costs (Aminu, Citation2015). There are a number of corrosion control methods such as materials selection, changes in design philosophies and the adoption of varied prevention techniques, which are in common use. Usage of chemical derivatives known as corrosion inhibitors is an economic means of corrosion mitigation and control. These chemical compounds alter the reduction-oxidation reaction mechanism through modification of the corrosive media and alteration of the electrochemical reaction between the steel and the corrosive media (Amitha & Basu, Citation2012). Most corrosion inhibitors being of organic and inorganic origin are highly effective but toxic to the environment (Marko & Chigondo, Citation2016; Sığırcık et al., Citation2016). Previous research has shown plant extracts to be promising compounds that suppresses metal-electrolyte reactions (El-Etre, Citation1998, Citation2003, Citation2006; Ebenso et al., Citation2004; Ebenso & Ekpe, 1996; Ibrahim et al., Citation2012; Li, Deng, et al., Citation2012; Li , Deng, Fu, et al., Citation2014; Li, Deng, Xie, et al., Citation2014; Li, Zhang et al., Citation2012; C.A. Loto et al., Citation2011; Loto & Olowoyo, Citation2018; R.T. Loto et al., Citation2018; Loto, Citation2018; Mourya et al., Citation2014). The need for nontoxic and cost-effective compounds emphasizes the importance of environmental protection, sustainability and human health concerns. The wide spread availability of plant extracts makes them appropriate alternatives in contrast to the costly and harmful convention organic and inorganic corrosion inhibitors. Plant extracts are biodegradable, ecologically acceptable, cost effective, and renewable. In contribution to the research on application of plants extracts for corrosion prevention and control, this article evaluates the corrosion prevention properties of Apium graveolens, Punica granatum, and Camellia sinensis plant extracts on plain carbon steel in 0.5 M H2SO4 solution. The major chemical compositions of the extracts are shown in Figure .
2. Experimental methods
2.1. Materials and preparation
Plain carbon steel (PCS) rod (6 mm length and 12 mm diameter) has nominal content (wt. %) of 0.05% S, 0.04% P, 0.8% Mn, 0.16% C, and 98.95% Fe. The steel rod was cut and machined into 21 samples, and subsequently washed with deionized H2O and CH3COCH3. Apium graveolens (APG), Punica granatum (PCG), and Camellia sinensis (CLS) were purchased from Shoprite, Lagos, Nigeria. 1.2 Kg of APG and PCG were separately cut to very small pieces together with the bark and blended. The grinded solutions were sifted to get the fluid concentrate. CLS was extricated utilizing a rotational evaporator in 3 L of deionized H2O. APG, PCG, and CLS were separately prepared in volumetric concentrations of 10%, 15%, 30%, 45%, 60%, 75%, and 90% per 200 mL of 0.5 M H2SO4 solution.
2.2. Coupon measurement
PCS samples were independently deposited in 200 mL of 0.5 M H2SO4 solution and weighed at 24 h interval for 240 h. Corrosion rate, Ĉ (mm/y) was obtained from the following equation;
ω represents weight loss (g), D represents density (g/cm3), A represents visible surface area of PCS specimen (cm2), 87.6 represents corrosion rate constant, and t represents duration of the experiment (h). Inhibition efficiency η (%) was obtained from the formula below;
ω1 and ω2 represents weight loss of PCS at predetermined APG, PCG, and CLS concentrations. Inhibitor coverage of the steel was obtained as follows:
where θ represents inhibitor coverage on PCS.
2.3. Potentiodynamic polarization
Electrochemical evaluation of PCS was performed at 30◦C surrounding temperature. A triple electrochemical cell consisting of platinum counter electrical conductor, silver chloride reference electrical conductor containing 3 M KCl solution at pH of 6.5 and PCS working electrical conductor were submerged inside a specialized cell filled 200 mL of H2SO4/plant extract (APG, PCG and CLS) solution. The electrode configuration was interfaced with potentiostatic device (Digi-Ivy 2311) and computer. PCS working electrode was encased in hardened Versocit metallographic mounts with visible surface dimension of 1.13 cm2. Polarization curves were produced at scan rate of 0.0015 V/s from −0.75 V to +1.5 V. Corrosion current density CJ (A/cm2) and corrosion potential CP (V) values were gotten from Tafel extrapolation. Corrosion current CI (A) was obtained from the intercept between the anodic-cathodic portions of the polarization curves. Corrosion rate, Ĉ (mm/y) was calculated as follows;
Ev represents PCS equivalent weight (g), 0.00327 represents constant for corrosion calculation, and D represents the density (g/cm3).
2.4. Optical macroscopy analysis
Optical macrographs of degraded PCS surface from noninhibited electrolyte was analyzed and matched with APG, PCG, and CLS inhibited PCS surface after corrosion tests using Omax trinocular metallurgical microscope.
3. Results and discussion
3.1. Coupon measurement
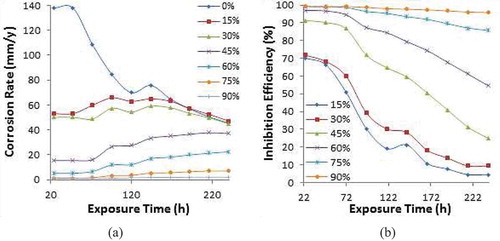
Figure ) - ) shows the plots of PCS corrosion rate and plant extracts (APG, PCG, and CLS) inhibition efficiency against exposure time. Table shows the data for weight loss, corrosion rate and inhibition efficiency from coupon measurement at 240 h. Results for corrosion rate of PCS at 0% APG, PCG and CLS concentrations contrast the values at 45%, 60%, 75%, and 90% inhibitor concentration due to the degradation effect of SO42- anions on ionized PCS surface in the acid solution. PCS corrosion rate values at 24 h (137.92 mm/y, 113.22 mm/y and 138.47 mm/y) decreased to 45.49 mm/y, 45.51 mm/y, and 46.3 mm/y at 240 h. The SO42- anion caused the oxidation of PCS surface and release of Fe2+ anions into the solution, effectively degrading the entire steel surface throughout the exposure period. Addition of APG, PCG and CLS inhibitor compounds at 15% and 30% concentration caused significant decrease in corrosion rate to values between 71.66 mm/y and 53.09 mm/y at 24 h. However, the overall effect of the inhibitor compounds (15% and 30% concentrations) at 240 h is negligible with PCS corrosion rate values not differing from its value at 0% inhibitor concentration. At 15% and 30% concentration, the molecules of the inhibitor compounds were insufficient to counteract the degradation effect of the corrosive anions and possibly modify the corrosive medium through the formation of complexes less harmful to the steel surfaces. At 45% inhibitor concentration, the inhibition performance of APG, PCG, and CLS where under 40% while at 60% concentration only the inhibition performance of APG compound crossed the 50% barrier to 54.55%. The performance of APG, PCG and CLS compounds significantly improved at 75% concentration to values of 85.74%, 82.85%, and 62.7%. These observations show the inhibition performance of APG, PCG, and CLS compounds are significantly concentration dependent, though changes in the plots configuration (Figure )-)) confirm time dependence of the inhibitor. However, the inhibition performance of the inhibitors gradually reduces with rise in inhibitor concentration and time. At 90% inhibitor concentration, the performance index of APG, PCG, and CLS (95.83%, 87.08%, and 92.37%) at 240 h of exposure results from plot configurations whose time dependence is negligible. At 75% concentration, APG and CPG inhibitor were time dependent to a small degree while CLS inhibitor still showed relatively significant time dependence. Results exhibited from the performance of the plant extracts in the acid electrolyte show effective inhibition performance begins at 60%.
Table 1. Data of PCS weight loss and corrosion rate, and APG, PCG, and CLS inhibition efficiency at 240 h
3.2. Optical microscopy analysis
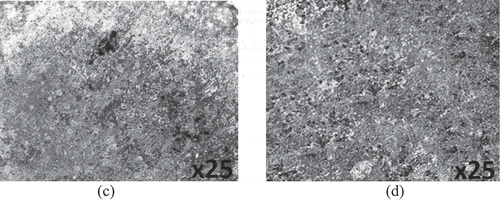
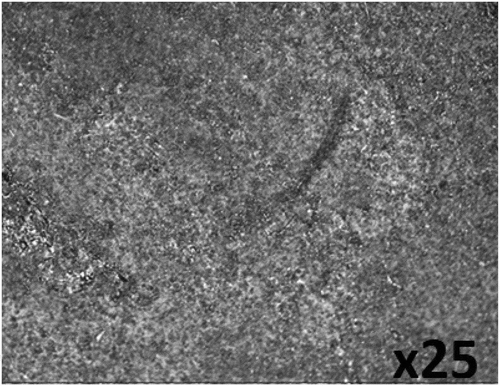
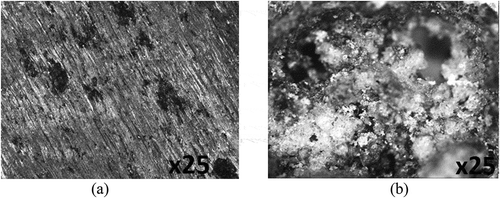
Optical representations of PCS surface prior to corrosion test, and after corrosion in 0.5 M H2SO4 solution with and without the addition of plant extracts are displayed from Figure ) to Figure at mag. ×25. Figure ) exhibits the morphology of PCS before corrosion. The serrated morphology is due to machining of the steel surface. The morphology in Figure ) is due to the oxidation effect of SO42- anion reactions, which is the causative factor for the degradation of the steel surface. The corroded morphology results from general corrosion in addition to the growth of macro-pits. The oxidation of the steel surface caused the release of iron (II) oxide into the acid solution. Figures -) and displays the morphology of PCS after corrosion test in the presence of APG, CPG, and CLS inhibitor. The improved morphologies are due to the inhibiting action of the plant extracts. The mild surface deterioration is due to the gradual inhibitive action of the plant extracts. Further analysis from potentiodynamic polarization, which will be discussed later showed the plant extracts displayed dominant cathodic inhibition. Hence the inhibition mode of the plant extracts results from the modification of the corrosive media, whereby the molecules of the plant extracts reacts with the anions of the corrosive solution, hence limiting the cathodic reaction mechanism.
Figure 5. Surface morphology of (a) PCS before corrosion and (b) PCS after corrosion in 0.5 M H2SO4 solution.
3.3. Potentiodynamic polarization studies
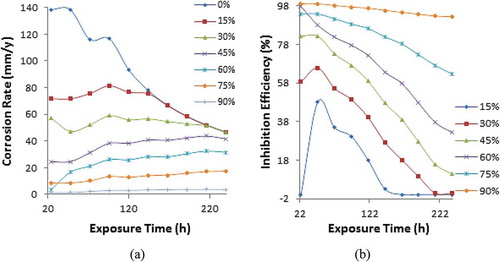
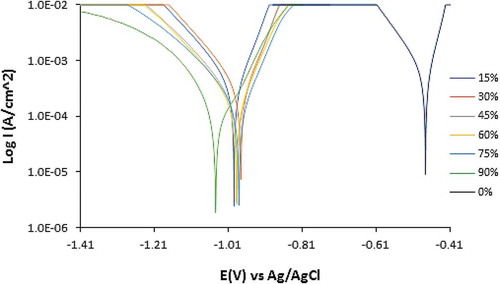
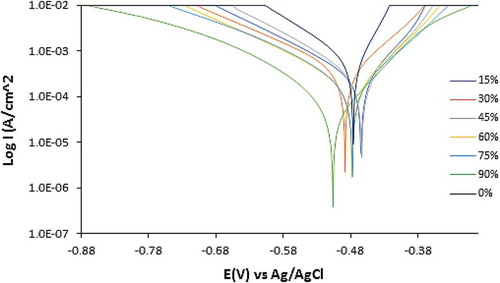
Potentiodynamic polarization curves displaying the inhibition effect of APG, PCG, and CLS on PCS corrosion in 0.5 M H2SO4 solution are shown from Figures -1. Tables – show the polarization data for PCS corrosion in 0.5 M H2SO4 solution at 0%−90% APG, PCG, and CLS concentration. The trend in corrosion rate results and inhibition efficiency are generally similar to the data observed from coupon measurement. The anodic cathodic polarization plots for APG inhibition performance on PCS corrosion indicates mixed inhibition effect with respect to the shift in corrosion potential values in Table . The maximum variation in corrosion potential of the control sample is less than 85 mV. Hence APG molecules influenced the oxidation reaction mechanism on the steel surface where loss of valence electrons leading to the formation of Fe2+ is suppressed. H2 evolution and consequentially O2 reduction cathodic reactions are simultaneously hindered. Observation of the polarization plot in Figure confirms this assertion; the presence of APG inhibitor (15%–45% concentration) decreased the anodic-cathodic polarization plots of PCS corrosion. However, the change has negligible influence on the inhibition of PCS corrosion considering the inhibition efficiency values obtained (15.05%, 28.66%, and 37.93%). Further increase in APG concentration (from 60% to 90%) decreased the slopes further with improved inhibition efficiency values of 60.24%, 90.03%, and 96.70%. At 90% APG concentration the cathodic slope of PCS is more significantly influence indicating dominant cathodic inhibition despite its mixed inhibition property.
Table 2. Potentiodynamic polarization data of PCS corrosion in 0.5 M H2SO4 solution at 0%−90% APG concentration
Table 3. Potentiodynamic polarization plots of PCS corrosion in 0.5 M H2SO4 solution at 0%−90% PCG concentration
Table 4. Potentiodynamic polarization plots of PCS corrosion in 0.5 M H2SO4 solution at 0%−90% CLS concentration
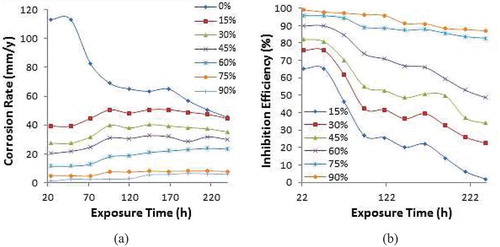
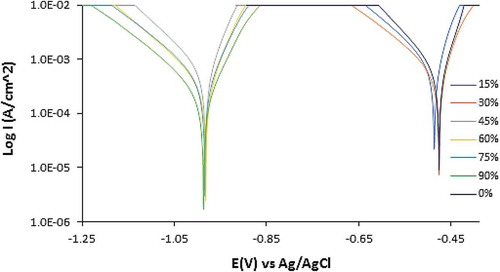
PCG and CLS exhibited significant cathodic inhibition effect at most concentrations from observation of the polarization curves depicted in Figures and . The polarization plots in Figure shifted significantly from −0.476 V at 0% PCG to values between −0.994 V and −1.044 V. The potential difference is significantly above 85 mV signifying it belongs to the cathodic class of corrosion inhibitors. PCG decreased the cathodic and anodic polarization plots of PCS corrosion after 15% concentration. At 90% concentration, the cathodic polarization plot decreased further due to cessation of the H2 evolution and O2 reduction reactions on PCS surface. The inhibition efficiency results of PCG attained effective values from 60% concentration similar to the observation for APG inhibitor. The performance of CLS inhibitor was significantly poor at 15% and 30% concentration relative to APG and CPG inhibitor. Between 45% and 60% concentration, relative improvement in inhibition efficiency occurred but the value is below effective inhibition performance until 75% and 90% inhibition efficiency where the values are 75.37% and 87.10% respectively.
Figure 8. Potentiodynamic polarization plots of PCS corrosion in 0.5 M H2SO4 solution at 0%−90% APG concentration.
4. Conclusion
Apium graveolens, Punica granatum, and Camellia sinensis plant extracts effectively inhibited the corrosion of plain carbon steel in dilute acid solution at high concentrations. The performance of the extracts was significantly concentration dependent and to a lesser extent time dependent. The extracts exhibited cathodic inhibition effect suppressing the H2 evolution and O2 reduction reactions. In the absence of plant extracts, plain carbon steel underwent general surface degradation coupled with significant macro-pits. The steel morphology significantly improved in the presence of the plant extracts in the corrosive media.
Acknowledgments
The author acknowledges the support of Covenant University, Ota, Nigeria for the provision of research facilities for this work and the laboratory contribution of Fope Oyedeko.
Disclosure statement
Authors declare no conflict of interest.
Additional information
Funding
Notes on contributors

Roland Tolulope Loto
Prof. Roland Tolulope Loto is a lecturer and researcher at Covenant University. He is proven scholar in the field of metallic corrosion prevention and control. He has over one hundred and eighty (180) research publications. He has consistently served as reviewer in respectable journals due to his intensive knowledge and technical expertise. Roland has undertaking a number renowned engineering research in collaboration with research/educational institutions. His in-depth experience in research experimentation basically aimed at proffering solutions to the current depreciating effect of metallic degradation and failure in service in various engineering and industrial applications. He has a Doctor of Technology (DTech) in Metallurgical and Materials Engineering (2014) from Tshwane University of Technology, Pretoria, South Africa, Master’s Degree (MSc) in Metallurgical and Materials Engineering (2007) from University of Lagos, Lagos, Nigeria and a Bachelor of Technology in Mechanical Engineering (2002) from Ladoke Akintola University of Technology, Oyo State, Nigeria.
References
- Alcántara, J., Fuente, D., Chico, B., Simancas, J., Díaz, I., & Morcillo, M. (2017). Marine atmospheric corrosion of carbon steel: A Review. Materials (Basel), 10(4), 406. https://doi.org/10.3390/ma10040406
- Aminu, U. D. (2015). Mild steel corrosion in different oil types. International Journal of Scientific Research and Innovative Technologies, 2(2).
- Amitha, B. E., & Basu, B. J. (2012). Green inhibitors for corrosion protection of metals and alloys: An overview. International Journal of Corrosion, 380217. https://doi.org/10.1155/2012/380217
- Ebenso, E. E., Ibok, U. J., Ekpe, U. J., Umoren, S., Jackson, E., Abiola, O. K., Oforka, N. C., & Martinez, S. (2004). Corrosion inhibition studies of some plant extracts on aluminium in acidic medium. Transactions of the SAEST, 39(4), 117–15.
- El-Etre, A. Y. (1998). Natural honey as corrosion inhibitor for metals and alloys. I. Copper in neutral aqueous solution. Corrosion Science, 40(11), 1845–1850. https://doi.org/10.1016/S0010-938X(98)00082-1
- El-Etre, A. Y. (2003). Inhibition of aluminum corrosion using opuntia extract. Corrosion Science, 45(11), 2485–2495. https://doi.org/10.1016/S0010-938X(03)00066-0
- El-Etre, A. Y. (2006). Khillah extract as inhibitor for acid corrosion of SX 316 steel. Applied Surface Science, 252(24), 8521–8525. https://doi.org/10.1016/j.apsusc.2005.11.066
- Fiori-Bimbi, M. V., Alvarez, P. E., Vaca, H., & Gervasi, C. A. (2015). Corrosion inhibition of mild steel in HCl solution by pectin. Corrosion Science, 92, 192–199. https://doi.org/10.1016/j.corsci.2014.12.002
- Gilbert, P. T. (2012). What is corrosion? British Corrosion Journal, 13(4), 153. https://doi.org/10.1179/000705978798276131
- Ibrahim, T., Alayan, H., & Mowaqet, Y. A. (2012). The effect of Thyme leaves extract on corrosion of mild steel in HCl. Progress in Organic Coatings, 75(4), 456–462. https://doi.org/10.1016/j.porgcoat.2012.06.009
- Kilcherman, L. 2015. What is mild steel? http://www.wisegeek.org/what-is-mild-steel.htm.
- Lgaz, H., Chung, I., Albayati, M. R., Chaouiki, A., Salghi, R., & Mohamed, S. K. (2018). Improved corrosion resistance of mild steel in acidic solution by hydrazone derivatives: An experimental and computational study. Arabian Journal of Chemistry, 13(1), 2934-2954. https://doi.org/10.1016/j.arabjc.2018.08.004
- Li, X., Deng, S., & Fu, H. (2012). Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corrosion Science, 62, 163–175. https://doi.org/10.1016/j.corsci.2012.05.008
- Li, X., Deng, S., Fu, H., & Xie, X. (2014). Synergistic inhibition effects of bamboo leaf extract/major components and iodide ion on the corrosion of steel in H3PO4 solution. Corrosion Science, 78, 29–42. https://doi.org/10.1016/j.corsci.2013.08.025
- Li, X., Deng, S., Xie, X., & Fu, H. (2014). Inhibition effect of bamboo leaves’ extract on steel and zinc in citric acid solution. Corrosion Science, 87, 15–26. https://doi.org/10.1016/j.corsci.2014.05.013
- Li, X. L., Zhang, X., Lei, J., He, J., Zhang, S., & Pan, S. (2012). Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel. Corrosion Science, 63, 82–90. https://doi.org/10.1016/j.corsci.2012.05.026
- Loto, C. A., Loto, R. T., & Popoola, A. P. I. (2011). Electrode potential monitoring of effect of plants extracts addition on the electrochemical corrosion behaviour of mild steel reinforcement in concrete. International Journal of Electrochemical Science, 6(8), 3452–3465.
- Loto, R. T. (2018). Surface coverage and corrosion inhibition effect of Rosmarinus officinalis and zinc oxide on the electrochemical performance of low carbon steel in dilute acid solutions. Results in Physics, 8, 172–179. https://doi.org/10.1016/j.rinp.2017.12.003
- Loto, R. T., Leramo, R., & Oyebade, B. (2018). Synergistic combination effect of salvia officinalis and lavandula officinalis on the corrosion inhibition of low-carbon steel in the presence of SO4 2−- and Cl−-Containing aqueous environment. Journal of Failure Analysis and Prevention, 18(6), 1429–1438. https://doi.org/10.1007/s11668-018-0535-0
- Loto, R. T., & Olowoyo, O. (2018). Corrosion inhibition properties of the combined admixture of essential oil extracts on mild steel in the presence of SO42− anions. South African Journal of Chemical Engineering, 26, 35–41. https://doi.org/10.1016/j.sajce.2018.09.002
- Marko, M., & Chigondo, F. (2016). Recent natural corrosion inhibitors for mild steel: An Overview. Journal of Chemistry, 6208937. https://doi.org/10.1155/2016/6208937
- Mokhatab, S., Poe, W. A., & Mak, J. Y. (2019). Raw gas transmission, handbook of natural gas transmission and processing, principles and practices (4th ed). Gulf Professional Publishing. https://doi.org/10.1016/C2017-0-03889-2
- Mourya, P., Banerjee, S., & Singh, M. M. (2014). Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corrosion Science, 85, 352–363. https://doi.org/10.1016/j.corsci.2014.04.036
- Rajendrachari, S. (2018). Investigation of electrochemical pitting corrosion by linear sweep voltammetry: A fast and robust approach. IntechOpen. https://doi.org/10.5772/intechopen.80980
- Shreir, L. L. (2010). Basic concepts of corrosion (Vol. 1, 3rd ed). Elsevier B.V.
- Sığırcık, G., Tüken, T., & Erbil, M. (2016). Assessment of the inhibition efficiency of 3,4-diaminobenzonitrile against the corrosion of steel. Corrosion Science, 102, 437–445. https://doi.org/10.1016/j.corsci.2015.10.036