Abstract
The heavy metals are exceptionally responsive at low fixations and can accumulate in the food web, causing extreme general wellbeing concerns. Remediation utilizing customary physical and substance techniques is uneconomical and creates huge volumes of compound waste. Bioremediation of risky metals has gotten impressive and developing enthusiasm throughout the years. Adsorption of Cr3+and Fe2+ from its aqueous solution on biosorbent Mango leaf powder (MLP) was prepared from matured Mango (Mangifera indica) leaves. The study was carried out by batch adsorption experiments using atomic adsorption spectroscopy upon various parameters used to assess their impact on the adsorption of metal ions. The achieved results evidenced the adsorption of Cr3+and Fe2+on MLP is better. The higher adsorption ability was shown about 237.5 and 227.5 mg/g respectively at 50 mg/L concentration of metal ions and 100 mg/L of MLP. Besides, the adsorption of Cr3+and Fe2+ on MLP was found to be fitted with the Freundlich isotherm. Thermodynamic results showed the sorption process is feasible, spontaneous, and endothermic. Kinetic parameters had shown that the adsorption of Cr3+ and Fe2+ ions found as pseudo-second-order kinetics; also this current study proposing that the adsorption process is presumably chemisorption. The consequences of this examination will be helpful for the expulsion of metals from water containing substantial metal ions. Further, this assessment is prescribed to recognize and evaluate the possible wellspring of those major and substantial metals to the wastewater. Thus, the current investigation emphatically prescribes the mango leaf powder used to expel the metals particles from wastewater.
PUBLIC INTEREST STATEMENT
Among the various types of toxic metals present in water, the chromium and iron were chosen for their bio-sorption study with regard to its wide usage in industries and potential pollution impact. So, there is considerable interest in the development of techniques to remove Cr3+ and Fe2+ from contaminated water before they are discharged into receiving bodies.
Hence, researchers have been interests in the adsorption method, to study the use of natural adsorbents as alternative adsorbents to remove the effluents from wastewater on the view of economic and environmental concern. The most current techniques employed in the removal of metals from wastewater are biological adsorption or biosorption. Plant leaves are chosen in this study as they are proposed to be natural, simple, and cheap bio-sorbents for the efficient removal of heavy metals discharged from industries as effluents.
1. Background of the study
The far-reaching penomenon which has attracted a lot of consideration is the tainting of toxic metals in the aqueous medium, which are sourced from chemical industries, for example, petrochemicals, refineries, manures, pulp, paper industries, and so on. (Kaewsarn & Yu, Citation2001). Substantial (heavy) metals are natural components of the Earth’s crust, and its availability in an aquatic environment has dramatically increased by their mining, industrial activities, and geochemical processes. They are toxic or poisonous if avail as more than the recommended amounts in water bodies (Ramesh et al., Citation2015). Also, the non-biodegradability nature of heavier metals and their affinity to bio-accumulate bases for several illnesses, disorders and mortality (Galal-Gorchev et al., Citation1993). In this way, the expulsion of poisonous substantial metals from wastewater is of essential significance regarding the assurance of general wellbeing and condition. Among the different kinds of poisonous substantial metals present in water, chromium and iron were chosen for their bio-sorption study about its extensive usage in industries and probable pollution impact (Davis et al., Citation2000).
These days chromium sullying of soil and groundwater is one of the noteworthy ecological issues. Chromium is accepted to be the second basic inorganic contaminant after lead. The poisonousness of chromium doesn’t live exclusively with the basic structure yet fluctuates extraordinarily among a wide assortment of chromium compounds. Oxidation state and solvency are pivotal factors in such manner (Salimi et al., Citation2006). Cr arises primarily in two valence states, such as the more oxidized hexavalent chromium (Cr6+), and the less oxidized trivalent chromium (Cr3+). It is deliberated essential to mammals for glucose, lipid, and protein metabolism and hence is considered as an essential dietary element (Salimi et al., Citation2006). Iron is extensively used and toxic in relatively in greater dosages. It is the prime metal responsible for initiating kidney damage, renal disorder, high blood pressure, bone fracture, and destruction of red blood cells (Drush, Citation1993). Toxic elements discharged as effluents to the aquatic environment that may absorbed and gathered by microorganisms. In this way, there is extensive enthusiasm for the turn of events of techniques to remove Fe2+ and Cr3+ from contaminated water on prior to discharging. The non-biodegradability habit of substantial metals and their propensity to bioaccumulation in living life forms cause a few sicknesses, issues, and casualty. Along these lines, the expulsion of heavy metals from modern wastewater is of essential significance regarding the security of general wellbeing and nature.
Right now, several techniques are utilized for the treatment of wastewater effluents. Among these incorporates precipitation with coagulation and flocculation, ion-exchange, membrane separation (Eltayeb & Khan, Citation2019; Khan et al., Citation2019; Kilic et al., Citation2014a, Citation2014b); complexation of dry biomass, filtration and adsorption (Çalımlı et al., Citation2019; Demirbaş et al., Citation2019a, Citation2019b). Be that as it may, a portion of these strategies, for example, precipitation and ion-exchange have critical disadvantages which are related with their significant expenses, less effectiveness, requiring a lot of reagent and dissolvable and age of harmful muck. Along these lines, there is an incredible interest for creating less expensive, eco-friendly and proficient substantial metal treatment systems to limit the measure of harmful overwhelming metals from industrial effluents (Isloor et al., Citation2019; Kolangare et al., Citation2019; Kumar et al., Citation2019).
The researchers have been interested in the adsorption method, to revising the usage of usual adsorbents as an alternative adsorbents to remove the effluents from wastewater on the view of economic and environmental concern. The most current techniques engaged in the exclusion of metals from wastewater are biological adsorption or biosorption. “Biosorption” is coined from the sorption and/or complexion of metal ions by biomass (Dang et al., Citation2008). The biosorption procedure includes a few components that contrast subjectively and quantitatively by relying upon the starting point of the biomass, the species were utilized and it’s preparedness. These systems are commonly founded on physicochemical collaborations between metal particles and utilitarian gatherings present on the cell surface, which incorporates ion-exchange, complexion, electrostatic fascination, and micro precipitation (Mack et al., Citation2008).
Bhatti et al. (Citation2020) proposed the removal of arsenic (as As3+ and As5+) by using polyacrylonitrile (PAN) fibre and iron ore as adsorbent. This study revealed that that the modified PAN had better adsorption efficiency than the iron ore adsorbent for the treatment of both As3+ and As5+ upon adsorption study. Adsorption behaviour of pistachio hull powder used as a low cost adsorbent (Beidokhti et al., Citation2019). This study focuses the adsorption of Ni (II) on pistachio hull by employing batch techniques; resulted, maximum Langmuir adsorption capacity was found as 14 mg/g, and studied results stated the optimum pH (ranged 4–6) for greater adsorption.
Organic pollutants along with substantial metals might be cause for abnormal changes in physicochemical parameters of aquatic ecosystems. In this context, Nasiri et al. (Citation2018) proposed the exclusion of chromium and phenol from aqueous solution. The most noteworthy pace of phenol and chromium expulsion was seen at a centralization of 100 mg/mL which was equivalent to 72.3% and 67.2%, separately. The examination assesses the treatment procedure both in the nearness and nonattendance of substantial metals utilizing activated sludge (Buaisha et al., Citation2020). The results disclose that the presence of substantial metal especially copper and cadmium at a concentration (0.7 mg/L) in a biological treatment system by 25 %, and 8.76 % respectively. But, no noteworthy variations in chemical oxygen demand, total suspended solids and total N2 in the final effluent in the conventional system.
Alzeyadi et al. (Citation2019) explored the proficiency of biomass bottom ash (BBA) as phosphate sorbent material from aqueous medium by utilizing batch adsorption. The outputs of the investigation revealed that the bonding between the cations, BBA surface and anions (phosphate) is essentially influenced by the pH of the medium. BBA presents phenomenal phosphate sorption, particularly, at low pH worth, and temperature around 20°C.
Adsorption utilizing actuated carbon is the most broadly utilized method for the evacuation of poisonous substantial metals. The essential huge quality of this glorious adsorbent is its greater porosity which brings about bigger surface territories to tie toxic minerals. So, adsorption is the supreme pervasive, powerful physical working strategy for the management of harmful substantial metal from polluted medium. In any case, viability and economic practicality of the action lies generally at the expense of bio-sorbent. Besides, the utilization of activated carbon isn’t picked in light of its significant expense esteems. Hence, there is a necessity in the planning and getting ready of minimal coast adsorbents utilizing locally accessible bio-sorbents. Also, they can be found easier, potentially renewable sources, harmless, and biodecomposable. Subsequently the biosorption, they are probable to precipitous and become deposits that can be inclined as safely. Furthermore, the outputs were evidenced that the concert of the dried leaves is close to the efficacy of actuated carbon (Schiewer & Volesky, Citation1995).
The available literatures are quite limited in regards to the removal of heavy metals by utilizing mango leaf powder as adsorbents (Cochrane et al., Citation2006; Hawari & Mulligan, Citation2006; Pamukoglu & Kargi, Citation2007; Sarma et al., Citation2008; Shaban et al., Citation2002; Sheng et al., Citation2004; Tiwari et al., Citation1999; Yu et al., Citation1999). Different kinds of biomass used as bio sorbent for the removal of toxic metals. Amongst, houseplant (mango) leaves are preferred as proposed as natural, simple, and cheap bio-sorbent for the effective exclusion of substantial metals (Gebretsadik et al., Citation2020; Kilic et al., Citation2014b; Ramesh et al., Citation2015; Salimi et al., Citation2006). Thus, there are no studies were found in the literature by involving mango leaf powder for the exclusion of Cr3+ and Fe2+.
The objective of the current study explored the adsorption of chromium and iron in an aqueous medium by using powdery mango leaves used as an adsorbent, which was collected from Arba Minch, Ethiopia. The present work has also been focused to explore the adsorption of Cr and Fe by following different operating constraints such as time, pH, and concentration of adsorbents, temperature, and the dose of adsorbent was considered for adsorption through the batch mode of experiment. Thermodynamic and kinetic data were further evaluated to check the feasibility of adsorption of Cr and Fe. Moreover, isotherm equilibrium was also investigated to fit the suitable isotherms.
2. Materials and methods
2.1. Biosorbent preparation
The matured and fresh mango leaves (Mangifera indica) were collected by the random method of sample collection from trees located in Arba Minch University, Technology campus, Arba Minch, Ethiopia. The leaves were cleaned systematically by steriled demineralized water that assures free from dust, mud, and soil impurities. Cleaned samples were desiccated in sunlight for few days on a perforated tray until the leaves turned a brownish colour. It was further dried in a hot air-oven for 24 hours until its became crispy. It’s powdered well using the laboratory crusher, and sieved until to achieve the particle size of adsorbent become 250 μm. According to Kumar and Sharma (Citation2014, Citation2015) the prepared sample dominated the presence of low molecular weight compounds resistant to microbial demineralization. It is a photo-chemically active material that transformed into biologically labile material.
2.2. Adsorbate solutions preparation
Stock solutions (1000 mg/L) were equipped by dissolving analytical grades of hydrated nitrate salts of chromium and iron. The solutions of heavy metal ions with necessary dilutions have been prepared from stock and used as adsorbate for this study. About 5, 20, 40, 60, 80 and 100 mg/L of Cr3+ and Fe2+ standard solutions were also prepared for calibration purpose. The required pH of the Cr3+ and Fe2+ solutions was accustomed by addition of 0.1 M HCl or NaOH, and it’s measured by employing pH meter. The prepared sample solutions freezed at 4°C; filtered the residue within 2 days from the prepared samples for avoiding the microbial growth as reported by (Chakrapani & Saini, Citation2009; Mohd Yawar & Fuqiang, Citation2018).
2.3. Biosorption evaluation
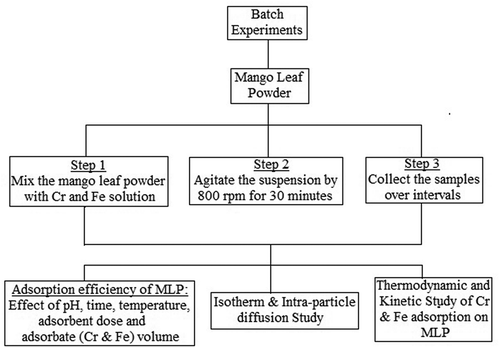
Batch adsorption tests for Cr3+ and Fe2+ ions were performed in 250 mL Erlenmeyer flask kept at 30 ± 2°C. The given amount of MLP was introduced into the metal ion (Cr3+ & Fe2+) solutions taken in the beaker, agitated with the rate of 800 rpm into 30 minutes to exist the equilibrium (shown in Figure ). Removed the residual adsorbent by filtration; experiments were conducted with duplicate under the same conditions and the average results were recorded. The effect of adsorbent dose (20–80 mg/L), contact time (15–120 minutes), metal ion solution pH (2–10), initial concentration of ions (50–100 mg/L) and temperature variation (30–60°C) was examined by changing the parameters proportionately. Calculated the adsorption amount and efficiency of adsorbates per unit mass of adsorbent (mg/g) by using EquationEquations (1)(1) and (Equation2
(2) ), that supported by Ramesh et al. (Citation2015):
Figure 1. Schematic diagram for research methodology for adsorption of Cr and Fe on 250 μm size mango leaf powder.
Where,
Co and Ce—concentration of adsorbate at initial and equilibrium
m—adsorbent mass and V—volume of adsorbate (Cr & Fe) medium.
The equilibrium (Langmuir and Freundlich isotherms) studies, kinetics and thermodynamic quantities evaluation of the present adsorption study of metal ions upon MLP pursue from the reported literature (Ramesh et al., Citation2015).
3. Results and discussions
3.1. Adsorption studies of Cr3+ and Fe2+
3.1.1. Influence of contact time
Optimization of equilibrium period for removal of studied metal ions involve in adsorption between adsorbates and biosorbent; the study was performed by keeping the concentration of Cr3+ and Fe2+ (at initial) is about 100 mg L−1 with definite amount of adsorbent (20 mg) at different time intervals (15–120 min). The percentage removal of Cr3+and Fe2+ was assessed and the obtained data given in Table .
Table 1. Adsorption studies of metal ions on Mango leaf powder
The data discloses that the rate of removal (%) of chromium and iron is greater at the beginning and the latter gets to remove the ions slightly. It is possibly due to the availability of greater biosorbent surface area at the opening of the adsorption of corresponding metal ions (Singh, Citation2006) in medium. Measurement for the removal of Estimation for the evacuation of metal ions as a component of time shows that the rate adsorption increases with an expansion in contact time and achieved equilibrium by following 80 minutes independent of the concentration of Cr3+and Fe2+. In any case, the time taken to arrive at evident equilibrium was expanded while at greater initial concentration of metal ions (Ramesh et al., Citation2015).
Outputs of the findings were noticed that most of Cr3+ and Fe2+ adsorption takes place at 120 min. of interaction time indicates the adsorption increased from 86.6% to 99.2% (for Cr3+) and 76.64 % to 96% (for Fe2+) with an elapsed time from 10–120 minutes. This might be the greater contact time among the surface of sorbent and metal solutions. Hence, the optimal interaction time for MLP adsorbent that found as 120 minutes. Greater adsorption noticed on the considered poisonous metals exhibited a benefit for crafting water treatment plants in chemical industries. Henceforth, this maximum period (120 minutes) has reflected for further processes. Furthermore, the result of the present study is well agreed with the study described by Gebretsadik et al. (Citation2020), who used the Eucalyptus Camaldulensis (as low-cost adsorbent) for the removal of heavy metals.
3.1.2. Influence of pH adsorbate medium
Influence of pH upon sorption process for removal of metal ions on MLP at different pH range (2–10) was performed and the obtained data were presented in Table . It’s clearly shown that the adsorption capcity of chromium and iron get increased as the solution pH incresed within the studied range. In strong acid medium (pH < 2.0), the whole surface charge on the dynamic sites of adsorbent became cationic (Cr3+and Fe2+ ions) and H+ competes for binding in the active spots of the biosorbent surfaces, resulted a inferior uptake of the metal ions (Sumathi et al., Citation2005).
The biosorbent surfaces turned out to be all the more contrarily charged by the pH of the medium ascents up to 10 (pH >10) and it turned out to be more deprotonated. In this context, the examined metal particles of Cr and Fe adsorbs quicker upon extnded the pH as reported (Ramesh et al., Citation2015; Sumathi et al., Citation2005). It’s seen in the consequences of the current examination (Table ). From this time forward, the measured metal particle adsorption increments was noticed as endless supply of pH, and the ideal pH was persuaded at six upon MLP utilized as an adsorbent. Among the metal ions Cr has greatly adsorbed (as 99%) than Fe (as 98%) at equilibrium pH 6. The studied results shows that the higher adsorption at higher pH esteems for both metal ions may be because of the contending of H+ with that of the metal ions for adsorption destinations which reduces their adsorption. Comparative patterns of adsorption of overwhelming metals at higher pH esteem were additionally seen by different researchers (Agbozu & Emoruwa, Citation2014). In this concern, further adsorption considers were embraced the pH 6 to be used for Cr3+ and Fe2+ removal on MLP surface.
3.1.3. Influence of adsorbent dose
Adsorption of Cr3+and Fe2+ performed by rising the dose of adsorbent (20–100 mg L−1) at 30°C. It was introduced into the 100 mg L−1 of Cr3+and Fe2+ solutions in separate reaction vessels about 120 minutes. The results were described in Table . It discloses, adsorption of metal ion gets improved as increasing of adsorbent dosage might be the greater accessibility of the adsorbing kinds (metal ions) on greater surface active sites on the adsorbent during upper dosage of adsorbent (Ajmal et al., Citation2000; Gebretsadik et al., Citation2020).
The noteworthy increment in take-up was seen when the dose was expanded. Any further addition of the adsorbent past this didn’t cause a huge change in the adsorption. This might be because of the covering of adsorption locates because of congestion of adsorbent particles (Babel & Kurniawan, Citation2004).
The outcomes were also revealed that the expansion in the biosorbent past the ideal dosage of adsorbent causes to diminish the metal take-up. This might be because of the accessible metal particles are deficient to cover all the interchangeable destinations on the biosorbent, bringing about a lower metal particle adsorption. Moreover, it may be because of the arrangement of the aggregation during biosorption causing a reduction in the viable adsorption zone when the increments of biomass concentration. The evacuation level of overwhelming metals expanded quickly with expanded in the adsorbent dose was identified with the expanded in surface territory and by expansion of the expanded number of ion-exchangeable locates accessible for interaction with metal particles. Consequently, the maximums portion of adsorbent considered for additional processing of adsorption. In this way, the evacuation limit of adsorbent, it could be presumed that the most noteworthy metal take-up happens at the greatest biosorbent, MLP dosage around 100 mg/L for both metal particles.
3.1.4. Influence of initial concentration of adsorbate solutions
The concentration of metal ions plays an important role in batch biosorption and obtained results described in Table . The outcomes demonstrated that the rate evacuation of Cr3+and Fe2+ diminishes as the concentration of metal particles expanded. This was maintained as in a few investigations have stated that an expansion in the concentration brought about an improvement in the adsorption limit at the beginning stage and an abatement in the general overwhelming metal evacuation effectiveness, which relies upon the accessibility of dynamic destinations of adsorbents. (Coruh & Ergun, Citation2009; Ören & Kaya, Citation2006). The percentage removal efficiency of MLP onto Cr3+and Fe2+ ions ranged 99.14–97.3% and 98.1–91% respectively. This might be described by the fact that the given dosage of adsorbent has limited number of active pores to remove particular amount of metal ions that could be saturated beyond a certain level of dosage.
Furthermore, the metal ion concentration functions as motivating force to overwhelmed the resistances in terms of mass transfer among the aqueous-solid phases (Anuradha et al., Citation2008; Namasivayam et al., Citation1998). Furthermore, enhancement of metal ion initial concentration may cause greater metal uptake owing to an increment of the collisions among metal ion and biosorbent, if it has enough amount (Gupta et al., Citation1997). Thus, concluded the superior metal take-up might occur at optimum metal ion concentration (i.e. 50 mg/L).
3.1.5. Effect of temperature
The experiments were employed at different temperatures (30°C, 45°C, and 60°C) and the results were presented in Table . It reveals that a clear trend could be observed by varying the results upon increasing the temperature. So, variation of temperature plays an significant role in metal ion biosorption. Increase the % adsorption upon temperature implies the proceeded adsorption process became endothermic (Ramesh et al., Citation2015). Thus, when enhancing the temperature diffusion rate will also increases and this takes the diffusion of adsorbates across the exterior periphery layer and in the interior pores of the adsorbent particle, owed to a decline in the viscosity of the solution and lead to increasing the adsorption.
3.2. Evaluation of adsorption isotherm
The best broadly used isotherms for solid-liquid adsorption schemes are Langmuir and Freundlich isotherms. These isotherms are relating qe (metal uptake/unit quantity of adsorbent) to the adsorbate concentration (at equilibrium) in bulk fluid phase, Ce which was reported by Al-Asheh and Duvnjak (Citation1996).
3.2.1. Langmuir and Freundlich isotherms
Correlation coefficient obtained from the Langmuir and Freundlich plots are presented in Table , which is > 0.828, shows the data are may be fit to Langmuir model (Kalavathy et al., Citation2005). Thus, values obtained by the linear regression correlation coefficient (R2) for Langmuir suggest that monolayer sorption may exist under that experimental condition as well. The equilibrium adsorption data obtained at a fixed concentration of metal ions and varying dose of adsorbent has been fitted into the linearized Langmuir isotherms. The vital distinctive of the Langmuir isotherm (Gaballah & Kllbertus, Citation1998) is stated by the dimensionless constant called equilibrium parameter, RL, which calculated by using EquationEquation (3)(3) :
Table 2. Results of adsorption isotherms, thermodynamic, Kinetic and Intra-particle diffusion studies on adsorption of metal ions on MLP
Where: KL—Langmuir constant; C0—initial concentration of sorbate (mol/L)
RL designate the type of isotherm. If, RL is 0 refer irreversible and 0 < RL< 1 is refer as favourable; RLis equal to 1 refer as linear, and RL>1 reflect adsorption become unfavourable. The values of separation factor (RL) of this study is between 0 and 1, and it indicates that it may be favourable condition for Langmuir isotherm with that of MLP employed as an adsorbent for exclusion of chromium and iron from an aqueous medium.
Freundlich isotherm contributes the association among the equilibrium liquid and solid surface of adsorbent propose as multilayer adsorption (heterogeneous). Correlation coefficient was found (shown in Table & Figure ) from Freundlich plot is greater than 0.8775. As seen the greater value of R2 designates the adsorption of this study is much favourable for Freundlich isotherm. Also, Freundlich constant called adsorption intensity (n) of studied metals lying in the recommended range, 1–10 (Table ), which is confirmed the classification as favourable adsorption. The value of “n” is lesser than one that indicates the unfavourable adsorption for chromium. Whereas iron has “n” value as greater than one, it shows the favourable adsorption. In the meantime, the Freundlich constant, KF found to be 0.774 (for chromium) and for iron is 0.776. These results are confirmed, that the MLP surface may behave as heterogeneous adsorbent upon adsorption of respective metals ions during in long-term, but might have a small range of consistency (Kamboh et al., Citation2011).
Figure 2. Freundlich isotherm model for adsorption of metal ions on MLP at [metal ions]: 50 mg L−1, contact time: 120 minutes, solution pH: 6.0.
![Figure 2. Freundlich isotherm model for adsorption of metal ions on MLP at [metal ions]: 50 mg L−1, contact time: 120 minutes, solution pH: 6.0.](/cms/asset/cf0ed042-47ee-42ae-9419-f79223dc81c3/oaen_a_1813237_f0002_oc.jpg)
The comparison of the regression coefficient (R2) of two isotherms is shown in Table . The order of feasibility may consider as based the value of R2. As in this context, Freundlich isotherm may have a good agreement than Langmuir due to R2value Freundlich plot have higher than R2 of Langmuir plots of both metal ions (Table ). In the case of adsorption capacity, the observation may conclude that showing good agreement with the Langmuir isotherm model. Consequently, all isotherm has proper advantages in defining the potential of mango leaf powder for the sorption of metals ions. Moreover, in terms of consideration of R2 values of isotherms and the moderate adsorption capacity of metal ions shows to be considered that the studied metal ions adsorption seems to behave good agreement with the Freundlich isotherm model known as multilayer chemisorption are takes place of Cr3+ and Fe2+ adsorption on MLP adsorbent. Furthermore, as seen the values of KF (Table ), the sorption aptitudes of the substantial metals studied was greater which might be accompanying with the physical and chemical properties of the metal. The slope (1/n) of metal ions in the presently assessed adsorbent was found as ≥ 1, which suggesting that the saturation was not attained for designated biosorbent, which are evaluated by an increment in the adsorption at a upper concentration of compounds, and makes the favoured adsorptions. Hence, the adsorption data (Table ) confirms that the removal of Cr3+ and Fe2+ by adsorption on MLP does fitted well with the Freundlich isotherm.
3.3. Thermodynamic study
According to Djeribi and Hamdauoui (Citation2008), in engineering practices entropy and energy features must be used to measure to decide about what processes does occur spontaneously. Thermodynamic parameters for the sorption systems such as change in Gibbs free energy (∆Go), change in enthalpy (∆Ho), and change in entropy (∆So) at standard state during adsorption could be evaluated. Slope and intercept of the plots of 1/T vs. ln Kc (shown in Figure ) were used and determines the values of ∆Ho and ∆So. The results were computed and the values of thermodynamic parameters described in Table . It’s showed that the ∆Go (as in negative) and ∆Ho (as in positive) demonstrate the feasibility and spontaneous, and in endothermicity of adsorption process, which is well agreed with earlier study (Djeribi & Hamdauoui, Citation2008).
Figure 3. Plot of thermodynamic study of adsorption of metal ions on MLP at [metal ion]: 50 mg L−1, contact time: 120 minutes, solution pH: 6.0.
![Figure 3. Plot of thermodynamic study of adsorption of metal ions on MLP at [metal ion]: 50 mg L−1, contact time: 120 minutes, solution pH: 6.0.](/cms/asset/a1ddbb76-fce9-4965-bacc-1ca4856a2d6d/oaen_a_1813237_f0003_oc.jpg)
According to Solangi et al. (Citation2011), the ∆Go may be up to −20 kJ/mol which show as reliable with electrostatic interface amid sorption spots and metal ion displays physical adsorption, whereas ∆Go may be greater negative than—40 kJ/mol comprise charge transfer between biomass surface into metal ion, might be form coordinate bond that illustrate chemical adsorption. Here in this current study, ∆Go obtained for both ions are < −15 kJ/mol; reveals the adsorption of studied sample shows spontaneous and also it designates that the physiorption as the predominant mechanism (Horsfall et al., Citation2004).
The positive values of ∆Ho of current study indicates the adsorption of the study follow endothermic (means takes off energy) upon the adsorption of studied metal ions on MLP. The ∆So can be used to define the randomness at the adsorbent-adsorbate interface in adsorption Additionally, the ln Kc value was found as increases by improving the the temperature, and positive value of entropy (ΔS) proposes the enlarged randomness at the solid-solution interface in the respective sorption of metal ion on mango leaf powder. The estimations of standard entropy change are not huge and demonstrate an expansion because of sorption. Regularly, adsorption of gases prompts a reduction in entropy because of precise arrangement of the gas molecules on a solid surface. Be that as it may, the equivalent may not be valid for the entangled system of sorption from solution onto mango leaf powder adsorbent. A few researchers have shown that the positive estimations of ΔS° recommended some structural changes in metal and sorbent (Ho, Citation2003; Monahar et al., Citation2002).
3.4. Adsorption kinetic modelling
Kinetic study was achieved to recognize the kinetics of Cr3+ and Fe2+ absorption on mango leaf powder in aqueous solution. Adsorption kinetic study were performed on 50 mg L−1 concentration of ionic solutions were agitated with 100 mg/L of mango leaf powder at 30°C with a pH of 6.0 and a continual agitation speed of 800 rpm about 120 minutes. Sample was filtered after shaking at different time intervals. The concentration of metal ions in the supernatant solutions was analysed using atomic absorption spectroscopy.
3.4.1. Pseudo-first-order
Generally, pseudo-first-order rate constant could be obtained from the slope of the plot between log (qe—qt) against time, t. Slopes and intercepts of plots log (qe-qt) of vs. time (t) were used to determine the pseudo-first-order rate constant (k1), regression coefficient (R2) and the equilibrium sorption capacity (qe) of metal ions are obtained and presented in Table . Though, the investigated data deviated substantially from the hypothetical data and assessment of the results with the correlation coefficients and other parameters described in Table . The correlation coefficients for the pseudo-first-order kinetic model obtained at all the studied concentrations that found as low. Also, the hypothetical qe values determines from the pseudo-first-order kinetic model did not give any reasonable values, suggested that this currently studied adsorption system does not follow the pseudo-first-order kinetic system.
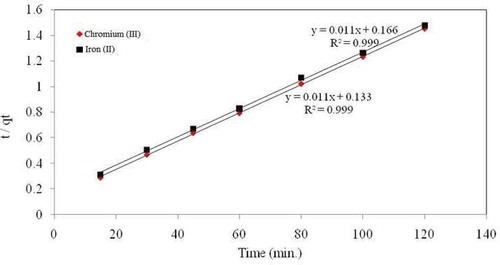
3.4.2. Pseudo-second-order
By the linear method, a hypothetical pseudo-second-order model establishes to well characterize the experimental kinetic data. If pseudo-second-order kinetics applies to the plot t/qt versus t should give linear relationship, from which qe and k can be determined from the slope and intercept of the plot respectively. The linear regression correlation coefficient R2 values are found as higher. The greater values of R2 (≥ 0.999) was found to be endorse that the adsorption data are well signified by pseudo-second-order kinetics (Abdel Ghani & Elchaghaby, Citation2007; Djeribi & Hamdauoui, Citation2008) for the complete sorption and thus supports the statement behind the model. It’s interesting to notice that the sorption of Cr and Fe ions by mango leaf powder was best described by the pseudo-second order kinetic model. The pseudo-second-order kinetic data of metal ions adsorption on MLP is presented in Figure and Table . The straight line in the plot of t/qt versus t (Figure ) well agreed with the experimental data of pseudo-second-order kinetic model. Table , lists the results obtained from the pseudo-second-order kinetic model. The correlation coefficients for the pseudo-second-order kinetic model achieved were greater than 0.999 for both metal ions. Also, the calculated qe values also agreed well with the experimental data. Furthermore, these designate that the adsorption system of studied samples follows the second-order kinetic model.
3.4.3. Intra-particle diffusion model
Intra-particle diffusion model indicates that two or more phases take place. The first, sharper portion is the exterior surface adsorption or immediate adsorption stage (Masood et al., Citation2015). The subsequent portion is the steady adsorption stage, where intra-particle diffusion is rate controlled. The third portion is the final equilibrium stage where intra-particle diffusion starts-to-slow down, this could be at extremely low adsorbate (metal ions) concentrations in the solution. In general, slope of the line in second stage is called an intra-particle diffusion rate constant, Kp. Studies were undergone, calculated the intra-particle diffusion constants for sorption of metal ions on MLP that presented in Figure and Table .
Figure 5. Intra-particle diffusion of metal ions at different time intervals at [metal ion]: 50 mg L−1, [MLP]: 100 mg L−1, solution pH: 6.0.
![Figure 5. Intra-particle diffusion of metal ions at different time intervals at [metal ion]: 50 mg L−1, [MLP]: 100 mg L−1, solution pH: 6.0.](/cms/asset/e1e16e69-8c3d-4ed3-89fc-64831c0ec562/oaen_a_1813237_f0005_oc.jpg)
Figure displays a plot of the linearized form of the intra-particle diffusion model at both the studied metal ions, and indicates external surface adsorption (stage 1) is accomplished before 15 minutes and then the stage of intra-particle diffusion control (stage 2) is achieved and continuous from 15 to 60 minutes. Finally, equilibrium adsorption (stage 3) starts after 60 minutes of the chromium and cadmium is gradually elated via intra-particle transmission into the particles and is finally reserved in the micro-pores (Masood et al., Citation2015). The slope of the straight line of the study called as intra-particle diffusion rate constant, kp is higher (shown in Table ), and it is confirmed the constructive adsorption of metal ions (Cr3+ and Fe2+) on MLP surface.
4. Conclusions
The objective of this work mainly investigation of the adsorption capacity of mango leaf powder (as bio-sorbent) for the exclusion of Cr3+ and Fe2+ from aqueous medium. Drawn the following conclusions based on the investigation undergone:
The optimum condition was drawn as the initial concentration of Cr3+ and Fe2+ was found 50 mg/L, amount of adsorbent as 100 mg L−1, the processing of contact time about 120 minutes, and at pH, 6 with agitation speed about 800 rpm at room temperature. The supreme adsorption capacity of Cr3+ and Fe2+on MLP is established 237.5 and 227.5 mg/g respectively.
The adsorption data of Cr3+ and Fe2+on MLP fitted well with Freundlich isotherm. The negative values of the free energy change indicate the feasibility and spontaneous and the positive estimations of the change in enthalpy propose the exothermic idea of the process. The positive estimations of the change in entropy reflect the affinity of the sorbents for chromium particles and compare to an expansion in irregularity at the strong/fluid interface during the sorption of metal particles onto the adsorbents. The sorption of Cr3+ and Fe2+ through a film diffusion mechanism and the kinetic outcomes were best portrayed by the pseudo-second-order kinetic model. The application to equilibrium trial consequences of the Langmuir and Freundlich isotherms shows that the Freundlich model fits the data better. Along these lines, mango leaf powder indicated high adsorption capability and it tends to be effectively utilized for the treatment of chromium and iron containing wastewater.
Subsequently this system involves the cheapest (less capital cost) and greatly efficient, also practically feasible for developing countries. The renewal of mango leaf powder is not vital because it is easily accessible material. The outcomes of this study will be beneficial for the removal of metals from water containing heavy metal ions. Further, the research work is recommended to identify and quantify the potential source of those major and heavy metals to the wastewater. Hence, the present study strongly recommends the mango leaf powder used to eradicate the metals ions from wastewater.
Nomenclature and units
Co - initial concentration of a metal ion in the samples (mg L−1),
Ce - equilibrium concentration of a metal ion in the sample (mg L−1),
m - adsorbent mass (mango leaf powder)
v - the volume of adsorbate solution (mL)
qe - the mass of heavy metal uptake/unit of MLP (mg/g)
qm - maximum sorption capacity, mg g–1
k1 - rate constant of the pseudo-first order, min–1
k2 - rate constant of the pseudo-second order, g mg–1 min–1
KL - Longmuir constant
KF- Freundlich constant related to the sorption capacity, mg1–1/n L1/n g–1
n - Freundlich constant related to sorption intensity
R2 - correlation coefficient
Acknowledgements
The authors acknowledge the Department of Chemistry, College of Natural Sciences, Arba Minch University, Ethiopia for provided the laboratory facility to accomplish this research work.
Additional information
Funding
Notes on contributors
Ramesh Duraisamy
Dr. Ramesh Duraisamy, Industrial Chemist’s working as an Associate Professor in Arba Minch University, Ethiopia. His profound expertise in the field of research includes water and wastewater treatment, waste management, sugar and food technology, textile and leather technology, Nano-material’s development. His contributions in the above said research fields evident through his publications.
Mihretu Mechoro
Mihretu Mechoro, M.Sc student (physical chemistry) of Arba Minch University. His thesis focused on the removal of heavy metals from wastewater using biosorbent activated carbon.
Tolera Seda
Dr. Tolera Seda Badessa, expertise the research fields as in Ion transport mechanism through ion exchange membrane upon electrodialysis/reversal elctrodialysis, etc. His profound contribution in the field of research has proven through the research articles were published in esteemed journals.
Masood Akhtar Khan
Dr. Masood Akhtar Khan, Analytical Chemist’s in Arba Minch University, Ethiopia. He has published many research articles in the field of ion exchange, adsorption of heavy metals and analysis of substances.
References
- Abdel Ghani, N. T., & Elchaghaby, G. A. (2007). Influence of operating conditions on the removal of Cu, Zn, Cd and Pb ion from wastewater by adsorption. International Journal of Environmental Science and Technology, 4(4), 451–17. https://doi.org/10.1007/BF03325980
- Agbozu, I. E., & Emoruwa, F. O. (2014). Batch adsorption of heavy metals (Cu, Pb, Fe, Cr and Cd) from aqueous solutions using coconut husk. African Journal of Environmental Sciences and Technology, 8(4), 239–246. https://doi.org/10.5897/AJEST2013.1577
- Ajmal, M., Rao, R. A. K., Ahmed, R., & Ahmad, J. (2000). Adsorption studies on Citrus reticulate (fruit peel of an orange): Removal and recovery of Ni (II) from electroplating wastewater. Journal of Hazardous Materials, 79(1–2), 117–131. https://doi.org/10.1016/S0304-3894(00)00234-X
- Al Rmalli, W., Dahmani, A., Abuein, M M.., Gleza, A A. Abuein, M., & Amar Gleza, A. (2002). Biosorption of mercury from aqueous solutions by powdered leaves of castor tree. Journal of Hazardous Materials, 152 (3), 955–959. https://doi.org/10.1016/j.jhazmat.2008.07.1113152
- Al-Asheh, S., & Duvnjak, Z. (1996). Adsorption of copper by canola meal. Journal of Hazardous Materials, 48(1–3), 83–93. https://doi.org/10.1016/0304-3894(95)00141-7
- Alzeyadi, A., Al-Ansari, N., Laue, J., & Alattabi, A. (2019). Study of biomass bottom ash efficiency as phosphate sorbent material. Civil Engineering Journal, 5(11), 2392–2401. https://doi.org/10.28991/cej-2019-03091419
- Anuradha, P., BeenaLahari, K., Prasanna Kumar, S. Y., & Prasad, V. S. R. K. (2008). Biosorption of zinc from aqueous solution using Azadirachta indica bark: Equilibrium and kinetic studies. J of Hazardous Materials, 152(1), 324–329. https://doi.org/10.1016/j.jhazmat.2007.06.101
- Babel, S., & Kurniawan, T. A. (2004). Cr (III) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere, 54(7), 951–967. https://doi.org/10.1016/j.chemosphere.2003.10.001
- Beidokhti, M. Z., Naeeni Mohammad, S. T., & Ghahroudi, S. A. (2019). Biosorption of Nickel (II) from aqueous solutions onto Pistachio hull waste as a low-cost biosorbent. Civil Engineering Journal, 5(2), 447–457. https://doi.org/10.28991/cej-2019-03091259
- Bhatti, Z. A., Qureshi, K., Ghulamullah, M., & Ahmed, S. (2020). Study of PAN fibre and iron ore adsorbents for arsenic removal. Civil Engineering Journal, 6(3), 548–562. https://doi.org/10.28991/cej-2020-03091491
- Buaisha, M., Balku, S., & Özalp-Yaman, S. (2020). Heavy metal removal investigation in conventional activated sludge systems. Civil Engineering Journal, 6(3), 470–477. https://doi.org/10.28991/cej-2020-03091484
- Çalımlı, M. H., Demirbaş, Ö., Aygün, A., Alma, M. H., Nas, M. S., Khan, A., & Şen, F. (2019). Equilibrium, kinetics and thermodynamics of bovine serum albumin from carbon-based materials obtained from food wastes. Bio Nano Science, 9(3), 692–701. https://doi.org/10.1007/s12668-019-00633-z
- Chakrapani, G. J., & Saini, R. K. (2009). Temporal and spatial variations in water discharge and sediment load in the Alaknanda and Bhagirathi rivers in Himalaya, India. Journal of Asian Earth Sciences, 35(6), 545–553. https://doi.org/10.1016/j.jseaes.2009.04.002
- Cochrane, E. L., Lu, S., Gibb, S. W., & Villaescusa, I. (2006). A comparison of low-cost biosorbents and commercial sorbents for the removal of copper from aqueous media. Journal of Hazardous Materials, 137(1), 198–206. https://doi.org/10.1016/j.jhazmat.2006.01.054
- Coruh, S., & Ergun, O. N. (2009). Ni2+ removal from aqueous solutions using conditioned clinoptilolites: Kinetic and isotherm studies. Environmental Progress & Sustainable Energy, 28(1), 162–172. https://doi.org/10.1002/ep.10316
- Dang, V. B., Doan, H. D., Dang-Vu, T., & Lohi, A. (2008). Equilibrium and kinetics of biosorption of cadmium (II) and copper (II) ions by wheat straw. Bioresource Technology, 100(1), 211–219. https://doi.org/10.1016/j.biortech.2008.05.031
- Davis, T. A., Volesky, B., & Vieira, R. H. S. F. (2000). Sargassum seaweed as biosorbent for heavy metals. Water Resources, 34(17), 4270–4278. https://doi.org/10.1016/S0043-1354(00)00177-9.
- Demirbaş, Ö., Çalımlı, M., Demirkan, B., Alma, M., Salih Nas, M., Khan, A., & Şen, F. (2019a). The kinetic parameters of adsorption of enzymes using carbon-based materials obtained from different food wastes. Bio Nano Science, 9(3), 749–757. https://doi.org/10.1007/s12668-019-00635-x
- Demirbaş, Ö., Çalımlı, M. H., Demirkan, B., Alma, M. H., Nas, M. S., Khan, A., & Şen, F. (2019b). Thermodynamics, kinetics, and adsorption properties of biomolecules onto carbon-based materials obtained from food wastes. Bio Nano Science, 9(3), 672–682. https://doi.org/10.1007/s12668-019-00628-w
- Djeribi, R., & Hamdauoui, O. (2008). Sorption of copper (II) from aqueous solutions by cedar sawdust and crushed brick. Desalination., 225(1–3), 95–112. https://doi.org/10.1016/j.desal.2007.04.091
- Drush, G. A. (1993). Increase of Cadmium body burden for this century. Science of the Total Environment, 26(2), 111-119. https://doi.org/10.1016/0048-9697(83)90105-5
- Eltayeb, N., & Khan, A. (2019). Design and preparation of a new and novel nanocomposite with CNTs and its sensor applications. Journal of Materials Research and Technology, 8(2), 2238–2246. https://doi.org/10.1016/j.jmrt.2019.03.002
- Gaballah, I., & Kllbertus, G. (1998). Recovery of heavy metal ions through decontamination of synthetic solutions and industrial effluents using modified barks. Journal of Geochemical Exploration, 62(1–3), 241–286. https://doi.org/10.1016/S0375-6742(97)00068-X
- Galal-Gorchev, H., Ozolins, G., & Bonnefoy, X. (1993). Revision of the WHO guidelines for drinking water quality. Annali dell’Istituto Superiore Di Sanità, 29(2), 335–345. https://apps.who.int/iris/handle/10665/52485
- Gebretsadik, H., Abraha, G., & Libargachew, D. (2020). Removal of heavy metals from aqueous solutions using Eucalyptus Camaldulensis: An alternate low-cost adsorbent. Cogent Chemistry, 6(1), 1720892. https://doi.org/10.1080/23312009.2020.1720892
- Gupta, V. K., Srivastava, S. K., & Mohan, D. (1997). Equilibrium uptake, sorption dynamics, process optimization, and column operations for the removal and recovery of malachite green from wastewater using activated carbon and activated slag. Industrial & Engineering Chemistry Research, 36(6), 2207–2218. https://doi.org/10.1021/ie960442c
- Hawari, A. H., & Mulligan, C. N. (2006). Biosorption of lead (II), cadmium (II), copper (II) and nickel (II) by anaerobic granular biomass. Bioresource Technology, 97(4), 692–700. https://doi.org/10.1016/j.biortech.2005.03.033
- Ho, Y. S. (2003). Removal of copper ions from aqueous solution by tree fern. Water Resource, 37(10), 2323–2330. https://doi.org/10.1016/S0043-1354(03)00002-2
- Horsfall, M., Spiff, A. I., & Abia, A. A. (2004). Studies on the influence of mercaptoacetic acid (MAA) modification of cassava (Manihot sculentacranz) waste biomass on the adsorption of Cu2+ and Fe2+ from aqueous solution. Bulletin of Korean Chemical Society, 25(7), 969–976. https://doi.org/10.5012/bkcs.2004.25.7.969
- Isloor, A. M., Chandrashekhar Nayak, M., Inamuddin Prabhu, B., Ismail, N., Ismail, A. F., & Asiri, A. M. (2019). Novel polyphenylsulfone (PPSU)/nano tin oxide (SnO2) mixed matrix ultrafiltration hollow fiber membranes: Fabrication, characterization and toxic dyes removal from aqueous solutions. Reactive & Functional Polymers, 139, 170–180. https://doi.org/10.1016/j.reactfunctpolym.2019.02.015
- Kaewsarn, P., & Yu, Q. (2001). Cadmium (II) removal from aqueous solutions by pre-treated biomass of marine alga padina sp. Environmental Pollution, 112(2), 209–213. https://doi.org/10.1016/S0269-7491(00)00114-7
- Kalavathy, M. H., Karthikeyan, T., Rajgopal, S., & Miranda, L. R. (2005). Kinetic and Isotherm studies of Cu (II) adsorption onto H3PO4 activated rubberwood sawdust. Journal of Colloid Interface Science, 292(2), 354–362. https://doi.org/10.1016/j.jcis.2005.05.087
- Kamboh, M. A., Solangi, I. B., Sherazi, S. T. H., & Memon, S. (2011). A highly efficient calix [4] arene based resin for the removal of azo dyes. Desalination, 268(1–3), 83–89. https://doi.org/10.1016/j.desal.2010.10.001
- Khan, A., Parwaz Khan, A., Khan, I., Oves, M., Khan, S., Asiri, A., & Facchetti, A. (2019). Facial synthesis of highly active polymer vanadium molybdate nano- composite: Improved thermoelectric and antimicrobial studies. Journal of Physics and Chemistry of Solids, 13, 148–155. https://doi.org/10.1016/j.jpcs.2019.03.022
- Kilic, Z., Atakol, O., Aras, S., Cansaran-Duman, D., Celikkol, P., & Emregul, E. (2014a). Biosorption properties of zinc(II) from aqueous solutions by Pseudevernia furfuracea (L.) Zopf. Journal of the Air & Waste Management Association, 64(10), 1112–1121. https://doi.org/10.1080/10962247.2014.926299
- Kilic, Z., Atakol, O., Aras, S., Cansaran-Duman, D., Celikkol, P., & Emregul, E. (2014b). Evaluation of different isotherm models, kinetic, thermodynamic and copper biosorption efficiency of Lobaria pulmonaria. Journal of the Air & Waste Management Association, 64(1), 115–123. https://doi.org/10.1080/10962247.2013.831383
- King, P., Anuradha, K., Lahari, S. B., Prasanna Kumar, Y., & Prasad, V. S. (2007). Biosorption of zinc from aqueous solution using Azadirachta indica bark: Equilibrium and kinetic studies. Journal of Hazardous Materials, 152(1), 324–329. https://doi.org/10.1016/j.jhazmat.2007.06.101
- Kolangare, I. M., Isloor, A. M., Asiri, A. M., & Ismail, A. F. (2019). Improved desalination by polyamide membranes containing hydrophilic glutamine and glycine. Environmental Chemistry Letter, 17(2), 1053–1059. https://doi.org/10.1007/s10311-018-00825-1
- Kumar, A., & Sharma, M. P. (2014). Review of methodology for estimation of labile organic carbon in reservoirs and lakes for GHG emission. Journal of Materials and Environmental Sciences, 5(3), 653–660. https://www.jmaterenvironsci.com/Document/vol5/vol5_N3/80-JMES-601-2014-Kumar.pdf
- Kumar, A., & Sharma, M. P. (2015). Estimation of carbon stocks of Balganga Reserved Forest, Uttarakhand, India. Forest Science and Technology, 11(4), 177–181. https://doi.org/10.1080/21580103.2014.990060
- Kumar, M., Rao, S., Isloor, A. M., Ibrahim, G. P. S., Inamuddin Ismail, N., & Asiri, A. M. (2019). Use of cellulose acetate/polyphenylsulfone derivatives to fabricate ultrafiltration hollow fiber membranes for the removal of arsenic from drinking water. International Journal of Biological Macromolecules, 129, 715–727. https://doi.org/10.1016/j.ijbiomac.2019.02.017
- Mack, C., Wilhelmi, B., Duncan, J. R., & Burgess, J. E. (2008). Biosorption of precious metals. Biotechnology Advances, 25(3), 264–271. https://doi.org/10.1016/j.biotechadv.2007.01.003
- Masood, A. K., Amanual, A., Ramesh, D., & Abiyu, K. B. (2015). Removal of Lead ion from aqueous solution by bamboo activated carbon. International Journal of Water Research, 5(2), 33–46.
- Mohd Yawar, A. K., & Fuqiang, T. (2018). Understanding the potential sources and environmental impacts of dissolved and suspended organic carbon in the diversified Ramganga River, Ganges Basin, India. Proceedings of the International Association of Hydrological Sciences, 379, 61–66. https://doi.org/10.5194/piahs-379-61–2018
- Monahar, D. M., Anoop Krishnan, K., & Anirudhan, T. S. (2002). Removal of mercury (II) from aqueous solutions and chlor-alkali industry wastewater using 2-mercaptobenzimidazole-clay. Water Resources, 36(6), 1609–1619. https://doi.org/10.1016/s0043-1354(01)00362-1
- Namasivayam, C., Prabha, D., & Kumutha, M. (1998). Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresource Technology, 64(1), 77–79. https://doi.org/10.1016/S0960-8524(97)86722-3
- Nasiri, E. F., Yousefi, D. K., & Qaderi, F. (2018). An experimental study on the simultaneous phenol and chromium removal from water using titanium dioxide photocatalyst. Civil Engineering Journal, 4(3), 585–593. https://doi.org/10.28991/cej-0309117
- Ören, A. H., & Kaya, A. (2006). Factors affecting adsorption characteristics of Zn2+ on two natural zeolites. Journal of Hazardous Materials, 13(1–3), 59–65. https://doi.org/10.1016/j.jhazmat.2005.09.027
- Pamukoglu, Y. M., & Kargi, F. (2007). Effects of operating parameters on kinetics of copper (II) ion biosorption onto pretreated waste sludge (PWS). Enzyme and Microbial Technology, 42(1), 76–82. https://doi.org/10.1016/j.enzmictec.2007.08.004
- Ramalli, S. W., Dahmani, A. A., Abuein, M. M., & Gleza, A. A. (2002). Biosorption of mercury from aqueous solutions by powdered leaves of castor tree (Ricinus communis L). Journal of Hazardous Materials, 152(3), 955–959. https://doi.org/10.1016/j.jhazmat.2007.07.111
- Ramesh, D., Kiruthiga, P. M., Belete, Y. H., & Abiyu, K. B. (2015). Adsorption of Azure B dye on rice husk activated carbon: Equilibrium, kinetics and thermodynamic studies. International Journal of Water Research, 5(2), 18–28. Corpus ID:212509208.
- Salimi, A., Amini, N., Danyali, H., & Hallaj, R. (2006). Electrocatalytic reduction of Cr6+ by thionine: Electrochemical properties and mechanistic study. Electroanalysis, 18(17), 1664–1671. https://doi.org/10.1002/elan.200603568
- Sarma, J., Sarma, A., & Bhattacharyya, K. G. (2008). Biosorption of commercial dyes on Azadirachta Indica leaf powder: A case study with a basic dye Rhodamine B. Industrial & Engineering Chemical Research, 47(15), 5433–5440. https://doi.org/10.1021/ie071266i
- Schiewer, S., & Volesky, B. (1995). Modelling the proton-metal ion exchange in biosorption. Environmental Science Technology, 29(12), 3049–3058. https://doi.org/10.1021/es00012a024
- Sheng, P. X., Ting, Y. P., Chen, J. P., & Hong, L. (2004). Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. Journal of Colloidal Interface Science, 275(1), 131–141. https://doi.org/10.1016/j.jcis.2004.01.036
- Singh, D. (2006). Biosorption of Cu (II) from aqueous solution by Spirogyra species. Journal of Environmental Research Division, 1, 227–231.
- Solangi, I. B., Bhatti, A. A., Kamboh, M. A., Menon, S., & Bhaange, M. I. (2011). Comparative fluoride sorption study of new calyx [4] arene based resins. Desalination, 272(1–3), 98–106. https://doi.org/10.1016/j.desal.2011.01.005
- Sumathi, K. M. S., Mahimairaja, S., & Naidu, R. (2005). Use of low-cost biological wastes and vermiculite for removal of chromium from tannery effluent. Bioresource Technology, 96(3), 309–316. https://doi.org/10.1016/j.biortech.2004.04.015
- Tiwari, D., Mishra, S. P., Mishra, M., & Dubey, R. S. (1999). Biosorptive behaviour of mango (Mangifera indica) and neem (Azadirachta indica) bark for Hg2+, Cr3+ and Fe2+ toxic ions from aqueous solutions: A radiotracer study. Applied Radiation and Isotopes, 50(4), 631–642. https://doi.org/10.1016/S0969-8043(98)00104-3
- Yu, Q., Matheickal, J. T., Yin, P., & Kaewsarn, P. (1999). Heavy metal uptake capacities of common marine macroalgal biomass. Water Research, 33(6), 1534–1537. https://doi.org/10.1016/S0043-1354(98)00363-7

![Figure 4. Pseudo second-order of metal ions at different time intervals at [metal ion]: 50 mg L−1, [MLP]: 100 mg L−1, solution pH: 6.0.](/cms/asset/048bb8a4-dd73-4950-b476-52cb761c6cbf/oaen_a_1813237_f0004_oc.jpg)