?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The red kidney beans found in the Esanland of Edo State, Nigeria, popularly known as Ikpakpa, are a variety of Phaseolus vulgaris. However, a lack of awareness about this indigenous legume and a lack of easy culinary applications are factors that lead to its low use. The very long hours of cooking, coupled with frequent changing of the cooking water, makes it very unattractive to the present generation. In this work, a novel attempt is made to have a culinary product from ikpakpa via spontaneous and controlled fermentation of the beans (Ikpakpa) and investigate their impact on the product’s nutritional value. Endogeneous microorganisms were used for spontaneous fermentation, while Bacillus subtilis was used for the control fermentation. Two routes of Ikpakpa preparation were followed: viz; beans were boiled for 8 hours to be soft, dehulled, and fermented; and the raw beans soaked overnight and dehulled, boiled for 4 hours fermented. After 5 days of fermentation, the proximate analysis results showed better results for the spontaneous fermentation via route 1, increasing protein and carbohydrate content by 18% and 36%, respectively. Both spontaneous and controlled fermentation were not favoured via route 2 as a decrease in protein and carbohydrate content by 13.5%/77.4% and 54.2%/66,4%, respectively. The duration for fermentation may have been too long to sustain the protein and carbohydrate content. It is, therefore, recommended that more research on the optimal fermentation duration be investigated and shelf-life studies conducted on the products.
PUBLIC INTEREST STATEMENT
Beans is a known staple and protein-dense food for most parts of the world. There are many species of legume and ikpakpa is one of them. It is grown and consumed by the people in the Esan part of South-South Nigeria. It is an underutilized legume, as it is only consumed by boiling. No other consumption route is known for this legume. This research seeks to expand its consumption while maintaining the nutritional value of the legume. Fermentation of the ikpakpa legume was carried out and the nutrients, like carbohydrate, protein, fat and fibres were determined. Results showed the possibility of a new product from fermentation with improved nutritional values.
1. Introduction
Peas, beans, lentils, peanuts, and other poded plants used for food are examples of legumes. Legumes have long been an essential part of many people’s traditional diets worldwide (Messina, Citation1999). For millennia, legumes have been an important part of the human diet, and they are still consumed all around the globe. They are a varied group of plants that belong to the Fabaceae family (also known as Leguminosae) and are believed to have around 20,000 species divided into 700 genera. However, only a handful of these species are available in common culture. The high protein content of legume meals, at least double that of cereal seeds in most legume seeds, is an essential nutritional feature. Humans have used the nutritional and phytochemical reserves in legume seeds for dietary needs and health benefits (Messina, Citation1999).
Ikpakpa comes under underutilized legumes such as Bambara groundnut, African locust bean, African yam bean, pigeon pea, kidney bean, which flourish in harsh environments and are nutritionally superior (Ebert, Citation2014). The high nutritional value of these beans could help to battle malnutrition (Kalidass & Mahapatra, Citation2014). The restricted utilization of legumes is due to several issues like the presence of anti-nutrients, their link to bloating and flatulence, and their difficult-to-cook nature are just a few of the concerns. They also contain substances that interact with the digestive tract, such as phytate, tannins, and oxalates which diminish protein digestibility and amino acid absorption, and lower nutritional quality (Asogwa & Onweluzo, Citation2010).
Anti-nutrients are inactivated by processing methods such as dehulling, soaking, fermentation, cooking, and roasting, which can improve the nutritional quality of the beans (Thani et al., Citation2018). Soaking the seeds before cooking softens them and cuts down on cooking time. Rinsing legumes and changing the boiling water multiple times minimizes the number of oligosaccharides in legumes, relieving the discomfort and embarrassment associated with bloating and flatulence caused by oligosaccharides.
Fermentation is a branch of biotechnology that rapidly evolves and absorbs a growing number of processes and products (Choudhary et al., Citation2018). Fermentation as innovation has a more promising future and more extended history than organic sciences by recovering critical spaces of food and medication in human administrations. Fermentation innovation has been broadly utilized in food, drugs, and alcohols for immense scope (Frost & Sullivan Research Service, Citation2015). Most fermented meals are made up of a complex mixture of carbohydrates, proteins, lipids, and other ingredients modified simultaneously or sequentially by various microbes and enzymes.
Open fermentation had been done utilizing endogenous microorganisms or those present in the air. In contrast, the controlled fermentation has been done using Aspergillus Niger, Aspergillus sojae, Aspergillus ficuum (Olukomaiya et al., Citation2020) Lactobacillus Plantarum MRS1, and Lactobacillus Brevis MRS4 (Pasquale et al., Citation2020) as starters to improve the nourishing properties of legumes and their flours. The impact of cooking length on proximate and metabolizable energy content of Kidney bean seeds revealed an increased protein content from 18.34% to 18.49%, a 2.24% to 3.78% drop in fat content, and an increase in Crude fiber, ash content, moisture, and Nitrogen free extract constituents expanded, going from 1.86% to 2.84%, 1.31% to 3.68%, 6.78 to 10.71% and 66.19 to 72.55% respectively (Thani et al., Citation2018). Fermentation of locust beans (Ojewumi et al., Citation2018) expanded the protein content in the range of 32%.
Low returns, a lack of market, a lack of awareness about indigenous legumes, and a lack of easy culinary applications are all factors that lead to low legume use. The invention of new legume products may result in stronger demand for legumes, motivating local farmers to enhance their commercial production of these legumes (Maphosa & Jideani, Citation2015).
Ikpakpa beans are a variety of Phaseolus vulgaris (red kidney beans) grown in the South-South area in Nigeria. It is a commonly consumed legume with the same components as most beans. There is, therefore, a need to accurately determine the effect of spontaneous and controlled fermentation (using Bacillus subtilis) as a food processing method on its proximate composition, especially as this procedure has not been fully explored, to the best of our knowledge, to this legume.
2. Methodology
2.1. Sample sourcing and preparation
The Ikpakpa beans were purchased from the Uromi market in Esan Central Local Government Area of Edo State. They were handpicked to eliminate debris and beans destroyed by insects.
Two routes were followed in preparing the legume for fermentation, viz:
Route 1: 400 g of Ikpakpa was rinsed and boiled with distilled water for 8 hours, with cooking water changed every 2 hours. After boiling, the beans were strained out and manually dehulled. Samples were withdrawn prior to fermentation for proximate analysis and labeled A1. 150 g each was scooped into beakers—for spontaneous and controlled fermentation. The beakers were covered with foil paper and fermented for 5 days. These were labeled samples A2 and A3, respectively. The raw Ikpakpa bean was labeled Sample C.
Route 2: 400 g of Ikpakpa was soaked overnight, manually dehulled, and boiled for 4 hours using distilled water. Samples were also withdrawn for proximate analysis and labeled B1. This was also split into two batches of 150 g each in beakers—for spontaneous and controlled fermentation. The beakers were covered with foil paper and fermented for 5 days. These were labeled B2 and B3, respectively.
2.2. Preparation of inoculants
The bacteria isolates used for the fermentation procedure were Bacillus subtilis from the Applied Biology Laboratory in Covenant University was isolated and reconfirmed using standard methods. Sterile distilled water was produced by autoclaving 500 ml at 120°C for 20 minutes in a 500 ml conical flask tightly covered with aluminum foil. The sterile water was allowed to cool and used for preparing the Bacillus broth according to the method of Ojewumi et al. (2021).
2.3. Fermentation of legumes
Spontaneous fermentation was done using the endogenous microorganisms in the legume for 5 days at room temperature. The controlled fermentation used Bacillus Subtilis as a starter for 5 days and at room temperature. The beakers were well covered with aluminum foil to achieve anaerobic fermentation.
2.4. Analyses of samples
Proximate analysis was done on the raw legume before and after the fermentation of the legumes. Moisture content, ash content, protein, crude fibre, and fat content were determined according to AOAC Association of Official Analytical Chemists and Food composition; additives; natural contaminants (Citation2000). The raw and prior fermentation samples were stored in the refrigerator at 4 °C before analyses were carried out on them. The calculations involved in the proximate analyses are:
2.4.1. Test for moisture content(MC)
To ensure that the crucibles were completely dry, they were baked in an oven at a temperature of 105°C for 30 minutes. The desiccator containing silica gel (desiccant) was then used to cool the dried crucibles for around 30 minutes. After that, the crucibles were weighed on an electronic weighing scale, and the weight labeled as W1. Samples weighing between 2 and 5 grams from each fermented bean sample were weighed into the clean, dried, and previously weighed crucible and labeled W2. The crucibles and their contents were oven dried at 105°C until they reached a constant weight. The crucibles were then placed in the desiccator to cool for another 45 minutes before being weighed and labeled W3.
Where W1 = Weight of empty crucibles
W2 = Weight of crucible with samples
W3 = Final weight of crucible with dried samples
2.4.2. Test for ash content (AC)
Clean crucibles were dried thoroughly in hot air ovens at 105°C for 30 minutes before being placed in a desiccator to cool for another 30 minutes. Weight (W1) was assigned to the crucibles after they had been cleaned and dried. About 5 grams of homogenized (ground) beans samples (Ws) were put to these weighed crucibles. The crucibles containing the weighed samples were sealed and placed in a muffle furnace heated to 600°C for 4 hours. Using a tong, they were removed and placed in the desiccator to cool. W2 was recorded as the ultimate weight of the crucible containing the ash.
The following formula was used to compute the percentage of ash:
W1 = Weight of empty crucibles.
W2 = Weight of crucible with ash
Ws = Weight of sample
2.4.3. Test for carbohydrate content (CC) by anthrone method
1 gram of each fermented bean sample was measured into a conical flask with 100 ml distilled water added, then stirred. The samples were left to soak in the liquid for 2 hours after which they were stirred again, and then 1 ml of each fermented sample liquid was pipetted into test tubes. Working standard solution samples ranging from 0 to 1 ml (with a 0.2 ml gap) were poured into five separate test tubes and then mixed with distilled water to bring the volume up to 1 ml in each test tube. After that, 5 ml of anthrone reagent was added into all the test tubes (working standard and bean samples alike), properly mixed, and the test tubes were placed in a steam bath for 10 minutes.
The absorbances were measured using an Ultraviolet spectrophotometer with a wavelength of 620 nm after the test tube was cooled to room temperature. For absorbance measurement, 0.5 ml of each of the fermented samples was diluted in the ratio 1:9 with concentrated sulphuric acid before entering the spectrophotometer. 1 ml distilled water and 5 ml anthrone reagent were used to make a blank. The glucose concentration was plotted on the y-axis and the absorbance at 620 nm was put on the x-axis to create a standard glucose calibration curve. The carbohydrate content of the samples were then read off the graph.
2.4.4. Test for crude fat content (CFC)
The soxhlet method was used for the determination of the crude fat in the samples.
All glass wares were rinsed with petroleum spirit, drained and dried in an oven at 102°C for 30 minutes and cooled in a desiccator. The weights of the round bottom flasks to be used were accurately weighed and recorded as W1. Sample weights (Ws) ranging between 3 grams to 5 grams were taken and put into the filter paper/ thimble which were inserted into the soxhlet liquid/solid extractor. ¾ of the volume of each flask to be used was filled with the extracting solvent (petroleum ether) and water was passed using hoses through the condenser. The extraction unit was assembled over a heating mantle. The solvent in the flask was heated until it boiled and the heat source adjusted till the condensed solvent dropped into the thimble at the rate of 6 drops per second.
The extraction continued for 4 hours after which the extraction unit was removed from the heat source, the extractor and condenser detached. The flask was replaced on the heat source to evaporate any of the solvent left. The flasks were put in the oven at 102°C and dried until a constant weight was reached. After cooling, the flask was weighed and recorded as W2. The percentage crude fat is computed as:
W1 = Weight of empty flasks.
W2 = Weight of flasks with fat
Ws = Weight of sample
2.4.5. Test for crude fibre (CF)
Samples ranging between 2 grams and 5 grams were weighed (Ws) and put into 500 ml conical flasks. 200 ml of 1.25% sulphuric acid was measured and poured into the flasks and boiled on an electric hotplate for thirty minutes with periodic agitation, after which the acid was drained off using a muslin cloth on a funnel into a conical flask labeled as discard. 500 ml of hot distilled water was used to rinse the residue on the muslin cloth and the flask used for boiling to remove any acid residue to avoid any unnecessary reactions in the next step. 200 ml of 1.25% sodium hydroxide was measured and used in rinsing the residue on the muslin cloth back into the conical flask. The solution was boiled for thirty minutes with periodic agitation, after which the base was drained off using a muslin cloth on a funnel into the conical flask labeled as discard. 500 ml of hot distilled water was used to rinse the sample’s fibre residue on the muslin cloth to remove any sodium hydroxide residue on the sample.
Using clean and dry crucibles, the samples were scraped from the muslin cloths. They were placed on the electric heating plates to evaporate water from the samples before being placed in 109°C oven to dry for two hours. After drying, the crucibles containing the dried fibre samples were weighed (W1) and placed into the muffle furnace for incineration at 550°C for two hours. The sample was left to cool after which the weight of crucible with ash was taken (W2). The calculation for crude fibre is given as:
W1 = Weight of crucible with dried fibre
W2 = Weight of crucible with ash
Ws = Weight of Sample
2.4.6. Test for protein content (PC)
The protein content for each fermented bean sample was computed using the calculation method given below:
Protein Content, PC (%) (3.5)
Where MC is Moisture Content, AC is Ash Content, CC is Carbohydrate content, CFC is Crude Fibre and CF is Crude fat/Lipid Content, and.
2.5. Results and discussion
2.6. Moisture content
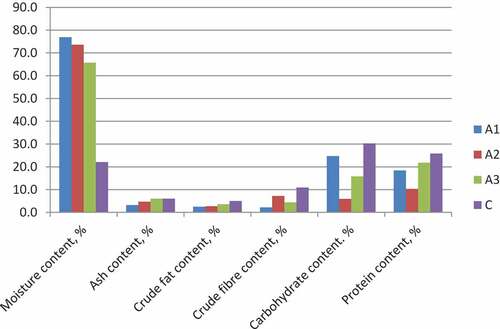
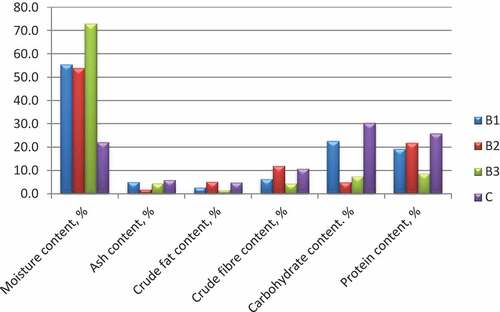
An increase in percentage moisture content was observed for both controlled and spontaneous fermentation, which is in line with Ojewumi et al. (Citation2018). Samples A1, A2, and A3 had a very high moisture content of more than 180% of the initial moisture in the raw Ikpakpa (Figure ). On the other hand, samples B1, B2, and B3 also had high moisture content but were in the range of 120%—150% of the initial moisture (Figure ). This observed increased moisture suggests that Ikpakpa absorbed more moisture during the soaking and boiling processes. Omafuvbe et al. (Citation2004) detailed that metabolic exercises of certain microorganisms during fermentation time give out moisture as one of their finished results. Increment could likewise be due to the activities of the inoculum on the fermented samples because of extracellular enzymes creation, as observed in Figures .
2.7. Ash content
The ash content dropped sharply by about 46% in the samples before fermentation. Still, it was observed to have increased in samples A2 and A3 by 46.9 and 87.5%, respectively, while for the soaked and boiled samples, the ash content dropped by 13.3% with preparation, but the fermentation process further dropped the ash by 63.5% and 11.5% in samples B2 and B3 respectively. The drop in percent ash content might be due to using some vital salts during fermentation by the fermenting metabolites for their metabolic activities (Thani et al., Citation2018).
2.8. Crude fat content
Extended cooking time is known to extract oil from legumes by fractionating a portion of the oil bodies and rupturing the cellular structure (Ojewumi et al., Citation2018; Omafuvbe et al., Citation2004). This activity may be responsible for the 50% and 44% crude fat content drop of samples for fermentation, A1 and B1, respectively. Spontaneous fermentation favoured the crude fat with an increase of 89.3%, while a sharp decrease of 42.9% was observed in the controlled fermentation. Sample B3 deviates from other samples with respect to crude fat content. Also, it contradicts the findings of Kasprowicz-Potocka et al. (Citation2016), where the crude fibre increased with fermentation using different yeast strains.
2.9. Crude fibre content
The indigestible plant material is referred to as crude fiber. It reduces the risk of diabetes, hypertension, and hypercholesterolemia in humans by lowering blood cholesterol levels, preventing cancer, and lowering the chance of acquiring diabetes, hypertension, and hypercholesterolemia (Thani et al., Citation2018). The fiber content recorded showed a drop in the crude fibre content by 79.8% in A1 and 41.3% in B1, as shown in Figures . Fermentation favoured the crude fibre content as it increased by 227.3% and 100% in spontaneous (A2) and controlled (A3) fermentation, respectively. Conversely, while spontaneous fermentation increased the crude fibre by 87.5% in sample B2, a drop by 29.7% was observed in sample B3 (controlled fermentation). The observed pattern agrees with that reported by Moses et al. (Citation2012).
2.10. Carbohydrate content
During both spontaneous and controlled fermentation, Table indicates that the carbohydrate content of the red kidney bean seed decreased in comparison with the raw sample. This may have been caused by the leaching of soluble carbohydrates such as sugars into the boiling and soaking water. The loss during fermentation could be due to the organisms’ use of some sugars for development and metabolism for 5 days under study. When Bacillus acts as an anaerobe (that is, in an anaerobic environment as was used in the fermentation presented in this work- by covering the beakers with aluminum foil), the substrate becomes the predominant source of energy for growth, survival, and metabolism. Bacillus species produce amylase, galactanase, glucosidase, fructofuranosidase, and galactosidase, among other enzymes (Moses et al., Citation2012) that aid the degradation of carbohydrates as seen in Table , where it was observed that neither preparation by soaking/boiling nor fermentation favoured the carbohydrate content of ikpakpa.
Table 1. Table of results
2.11. Protein content
The protein content dropped during preparation by 28.7% (in A1) and 25.6% (in B1). Still, fermentation of the boiled and dehulled samples experienced a boost in the protein content by 10.3% and 18.5% in samples A2 and A3, respectively. The secretion of extracellular enzymes by Bacillus subtilis throughout the fermentation may also account for some of the rise in protein content as observed in A2 and A3. Fermentation of the soaked and boiled sample, on the other hand, recorded an increase in the spontaneous fermentation by 21.8% (B2) but a sharp drop by 54.2% in the controlled fermentation (B3). The extreme decrease in crude protein content may be linked to the observed rise in carbohydrate and crude fibre (Olukomaiya et al., Citation2020).
3. Conclusion
Since ikpakpa is a very hard seed, it was prepared for the fermentation routes via boiling and soaking until it was soft enough. Dehulling of the legume exposed the seeds to fermentation agents in both spontaneous and controlled fermentation. Novel fermented products were obtained from the study, and results obtained showed that Route A3 favoured protein content by an 18.5% increase, unlike a 44.4% drop via Route A2. On the other hand, Route B2 recorded a 13.5% increase in protein, while a 54.2% drop was recorded in Route B3. The carbohydrate content was depleted in all routes. The long duration of fermentation may be responsible for the heavy drop in the contents of interest to human nutrition. It is, therefore, recommended that lower fermentation duration be researched upon and the optimal duration identified for the fermentation of ikpakpa.
Acknowledgements
The Team would like to thank Covenant University Ota for financial support in publishing this manuscript and appreciate the collaborative support of Benson Idahosa University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
E.E Alagbe
The Team’s research activities include but not limited to fermentation technology, water and environmental engineering, waste to wealth projects. The work on the fermentation of ikpakpa is the first step in an attempt to produce new products from the legume that would meet the nutritional needs of humans.
References
- AOAC Association of Official Analytical Chemists. (2000). Official methods of analysis of AOAC International. In H. William Ed., AOAC Association of Official Analytical Chemists. Official method (pp. 15–10). Vol. 942. Gaithersburg (Maryland): AOAC International.
- Choudhary, M., Joshi, S., Bhagyawant, S., & Srivastava, N. (2018). Advances in fermentation technology: Principles and their relevant applications. In A. Kuila & V. Sharma (Eds.), Principles and applications of fermentation technology (pp. 53–63). Scrivener Publishing.
- Ebert, A. (2014). Potential of underutilized traditional vegetables and legume crops to contribute to food and nutritional security, income, and more sustainable production systems. Sustainability, 6(1), 319–335. https://doi.org/10.3390/su6010319
- Frost & Sullivan Research Service. (2015). Advances in fermentation technologies: An industry overview technical insights.
- Kalidass, C., & Mahapatra, A. K. (2014). Evaluation of the proximate and phytochemical compositions of an underexploited legume Mucuna pruriens var. utilis (Wall ex Wight). International Food Research Journal, 21(1), 303–308.
- Kasprowicz-Potocka, M., Zaworska, A., Gulewicz, P., Nowak, P., Borowczyk, P., & Frankiewicz, A. (2016). The effect of dry yeast fermentation on chemical composition and protein characteristics of blue lupin seeds. Food Technology and Biotechnology, 3, 360–366. https://doi.org/10.17113/ftb.54.03.16.4459
- Messina, M. J.(1999). Legumes and Soybeans: Overview of their nutritional profiles and health effects. The American journal of clinical nutrition, 270(3), 439s–450s. https://doi.org/10.6133/apjcn.2016.25.1.23
- Maphosa, Y., & Jideani, V. A. (2015). Dietary fiber extraction for human nutrition–A review. Food Reviews International, 32(1), 98–115. https://doi.org/10.1080/87559129.2015.1057840
- Moses, O., Olawuni, I., & Iwouno, J. O. (2012). The proximate composition and functional properties full fat flour and protein isolates of Lima Bean (Phaseolus lunatus) flour. SkyJournal of Food Science, 4(2), 24–29. http://dx.doi.org/10.4172/scientificreports.349
- Ojewumi, M. E., Omoleye, J. A., Emetere, M. E., Ayoola, A., Obanla, O., & Babatunde, E. (2018). Effect of various temperatures on the nutritional compositions of fermented African locust bean (Parkia biglobosa) seed. International Journal of Food Science and Nutrition, 3(1), 117–122. http://www.foodsciencejournal.com
- Ojewumi, M., Omoleye, J. A., & Nyingifa, A. (2018). Biological and chemical changes during the aerobic and anaerobic fermentation of African Locust Bean. InternationalJournal of Chemistry Studies, 2(2), 25–50. http://eprints.covenantuniversity.edu.ng/10369/1/AREOBIC%20PUBLISHED.pdf
- Olukomaiya, O. O., Aidamo, O. Q., Fernando, W. C., Mereddy, R., Li, X., & Sultanbawa, Y. (2020). Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chemistry, 315, 126238. https://doi.org/10.1016/j.foodchem.2020.126238
- Omafuvbe, B. O., Olumuyiwa, S., Falade, B. A., Osuntogun, S., & Adewusi, R. A. (2004). Chemical and biochemical changes in African Locust Beans (Parkia biglobosa) and Melon (Citrullus vulgaris) seeds during fermentation to condiments. Pakistan Journal of Nutrition, 3(3), 140–145. https://doi.org/10.3923/pjn.2004.140.145
- Pasquale, I., Pontonio, E., Gobbetti, M., & Rizello, C. (2020). Nutritional and functional effects of the lactic acid bacteria fermentation on gelatinized legume flours. Elsevier.
- Sogwa, I. S., & Onweluzo, J. C. (2010). Effects of processing methods on the chemical composition of flour, MoinMoin, and Akara from Mucunapruriens. Journal of Tropical Agriculture Food and Environment, 9(3), 200–208. https://doi.org/10.4314/as.v9i3.65760
- Thani, R. J., Alu, S. E., & Yakubu, A. (2018). Evaluation of differently processed kidney bean seeds on nutrient and anti-nutrient compositions: Implications for monogastric animal feeding. Nigerian Journal of Animal Science, 20(1), 134–144. eISSN: 1119-4308.