?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Among the various types of recently released composite materials, particle-reinforced metal matrix composites (MMCs), in particular aluminum as the matrix material, have been shown to provide substantial industrial benefits in the automotive and aerospace sectors. The current research focused on the corrosion behavior of an Al7075 hybrid composite in 0.1 M hydrochloric acid (HCl) and 3.5% NaCl media at different temperatures. Electrochemical techniques, such as Tafel polarization (TP) and electrochemical impedance spectroscopy (EIS), were employed to study the corrosion behavior in the respective solutions. The results showed that the corrosion rate of the studied specimen increased from 31.77 to 47.61 mmy−1 in 0.1 M HCl and from 0.26 to 1.28 mmy−1 in 3.5% NaCl with increasing temperature. The corrosion current density of the Al7075 hybrid composite in 0.1 M HCl is on the order of 10−3 Acm2, and that in 3.5% NaCl is on the order of 10−5 Acm2. This confirms the increased rate of composite corrosion in the 0.1 M HCl medium compared to that in the 3.5% NaCl medium. A suitable mechanism was proposed for the corrosion of the Al7075 hybrid composite in both media.
Introduction
The versatility and widespread adoption of aluminum alloys and their composites can be attributed to their outstanding mechanical properties. Aluminum matrix composites containing particle reinforcements have advantages in various engineering applications. This distribution of particles was generated during the fabrication process using various techniques. Aluminum alloys are a good choice for matrix materials due to their light weight, resistance to the environment and beneficial mechanical qualities like modulus, strength, toughness and impact resistance (Kadri et al., Citation2021; Pardo et al., Citation2003).
Aluminum alloys, such as Al7075 and Al2024, have garnered significant attention owing to their exceptional machinability and impressive strength-to-weight ratio. Al7075, which belongs to the 7XXX series of precipitation-hardening-enhanced aluminum alloys, has excellent mechanical properties. Owing to its robust mechanical characteristics, Al7075 has extensive applications in various fields, including the automotive sector. The aerospace industry also benefits from the use of this alloy in aircraft components and structures, where strength and weight considerations are of paramount importance (Al-Salihi et al., Citation2019; Babajanzade Roshan et al., Citation2013). Indeed, the Al2024 alloy, also called ‘duralumin’, is classified as a member of the 2XXX series of aluminum alloys, and it boasts exceptional machinability along with an impressive balance of toughness and strength. Owing to its favorable combination of machinability, toughness and strength, Al2024 is commonly used in various applications, particularly aerospace and aircraft manufacturing. It is useful in aircraft structural components, wing spars and fuselage frames, where the material must withstand mechanical and environmental stresses (Afzal et al., Citation2013; Sameezadeh et al., Citation2011). At the outset, metal matrix composites (MMCs) have gained tremendous importance owing to their specific properties and applications in various industries. It has been noted that metallic or nonmetallic compounds can be coated onto reinforcement surfaces to improve machine-driven qualities, increase wettability and prevent any unfavorable chemical interactions between the reinforcement and matrix at elevated temperatures. Additionally, it has been discovered that covering reinforcements significantly improve the mechanical properties of MMCs. In our previous work, a novel composite material was fabricated by incorporating Ni-coated Al2024 powder into an Al7075 alloy matrix using a stir casting process (Sambathkumar et al., Citation2017). This work emphasizes the mechanical characterization and microstructure analysis and their influence on the overall properties of hybrid composites.
Corrosion is a naturally occurring spontaneous process that causes significant harm and financial loss to a variety of industrial materials and operations. The industrial pickling agents of choice include phosphoric acid, sulfuric acid and hydrochloric acid, which are diluted mineral acids. Before applying any coating, they can be easily utilized as cleaning agents for industrial equipment to remove oxide layers (Tang et al., Citation2011). During the cleaning process, these mineral acids also cause the dissolution of metals along with the removal of impurities from their surface. In marine applications, sodium chloride solutions are widely employed as electrolytes for steel, aluminum and their alloys (Ashish et al., Citation2024). Because a protective oxide film forms on the metal surface, alloys and composites made of aluminum have good corrosion resistance in hostile environments. Nevertheless, Cl− ions frequently contribute to instability and potential passive film deterioration, which can result in metal breakup and pitting corrosion. The aluminum-based composites quickly polarized in the acid chloride solution when exposed to air. The development of soluble chlorinated aluminum hydroxide in the presence of oxygen was likely facilitated by the chlorides, impeding the formation and/or repassivation of the protective oxide (Loto, Citation2013).
The World Corrosion Organization (WCO) estimates that the annual cost of corrosion might reach $US2.4 trillion (Olajire, Citation2017), with the global economy bearing the brunt of the disease at 3.4% of GDP. According to the National Association of Corrosion Engineers (NACE), the cost of corrosion is estimated to be approximately $2.5 trillion USD, or 3.4% of the world’s gross domestic product (Hou et al., Citation2017). Therefore, along with other mechanical properties of hybrid composites, the corrosion resistance of MMCs is also a subject of study to compare their corrosion resistance to various corrosive environments. Several studies have reported the degradation behavior of various Al-matrix composites in different corrosive media (Huimin et al., Citation2023; Karthikraja et al., Citation2023). However, to the best of our knowledge, the corrosion behavior of the studied composite material has not been explored in any corrosive media. Hence, the present work is a continuation of exploring the corrosion behavior of hybrid composites, that is, Al7075 reinforced with Ni-coated Al2024 powder in 0.1 M HCl and 3.5% NaCl media, using electrochemical techniques.
Materials and methods
Material
The Al7075 and Al2024 compositions are listed in . The hybrid composite was fabricated by reinforcing the Al7075 matrix phase with Ni-coated duralumin (Ni coated on Al2024 powder) using the stir casting method. Al 7075 alloy used in this study is in the form of a rectangular bar, which is melted in the furnace. The Al 2024 is in the form of powder which is coated with Ni. This powder is mixed with molten Al 7075 alloy by using stir casting Method (Karthik et al., Citation2022).
Table 1. Composition of the matrix and dispersion used in the fabrication of the hybrid composite.
Preparation of test coupons
The working specimens for the corrosion study were prepared in the form of cylindrical rods with a length of 5 cm and molded with cold-setting resin with an exposed surface area of 1 cm2. The specimens were polished with emery papers of different grades, followed by a disc polisher. Finally, the dried polished specimens were immersed in a corrosive medium, and corrosion tests were performed.
Preparation of the corrosive solution
0.1 M HCl and 3.5% NaCl were used as corrosive media. A standard solution of 0.1 M HCl was used by diluting a known volume of 80% pure AR-grade HCl solution in distilled water. NaCl (3.5%) was prepared by dissolving 35 g of solid NaCl in 1 liter of distilled water. Further experiments were performed using these media at various temperatures (303–323 K).
Surface morphology study
Scanning electron microscopy (SEM, JEOL JSM-6380 L) and atomic force microscopy (AFM, 1B342 Innova model) were used to perform surface morphological studies. The polished specimen of the Al7075 hybrid composite was immersed in 0.1 M HCl and 3.5% NaCl for two hours. The cleaned specimens were washed, dried and subjected to SEM and AFM.
Electrochemical measurements
An electrochemical workstation (CH Instrument USA Model 604D series with beta software) was used to perform electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP) in three conventional electrode cell systems, with platinum as the auxiliary electrode, an Al7075 hybrid composite as the working electrode, and a calomel electrode (SCE) as a reference. The steady open circuit potential (OCP) was measured by dipping polished specimens in 0.1 M HCl and 3.5% NaCl in the temperature range from 303 K to 323 K. EIS measurements were carried out with a steady OCP distribution with an amplitude of 10 mV AC signal in the frequency range from 10 kHz to 0.01 Hz. The PDP plots were recorded by sweeping the potential in the range of −250 mV to +250 mV for OCP.”
Results and discussion
Tafel polarization (PDP) technique
The PDP curves for the corrosion of the Al7075 hybrid composite in 0.1 M HCl and 3.5% NaCl at three different temperatures are shown in , respectively. The anodic part of the curves indicates metal oxidation, and the cathodic part of the curve corresponds to metal reduction. The plots enabled us to obtain electrochemical constraints such as the corrosion potential (Ecorr), corrosion current density (icorr), cathodic (βc) and anodic (βa) Tafel slope values and these experimental results are recorded in . The obtained icorr values were further used to calculate the corrosion rate (CR) using EquationEquation (1)(1)
(1) (Shetty, Kumari, et al., Citation2020):
(1)
(1)
where 3270 is a proportionality constant in mmpy, EW is the equivalent weight and d is the density of the corroding material in g/cm3.
Figure 1. PDP plots for the corrosion of the Al7075 hybrid composite in (a) 0.1 M HCl and (b) 3.5% NaCl.
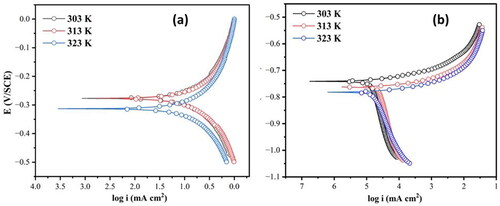
Table 2. Tafel polarization results for the corrosion of the Al7075 hybrid composite in 0.1 M HCl and 3.5% NaCl media at different temperatures.
Generally, in composite materials, the reinforcing phase plays a substantial role in the corrosion process. In the present case, the reinforced nickel-coated duralumin powder in Al7075 led to discontinuities throughout the matrix phase. This reinforcing phase initiated the formation of more reactive sites on the surface of the composite material. Corrosion may also be accelerated by galvanic contact between the reinforcement and the Al7075 matrix. Rapid penetration through the wide interfacial areas in hybrid composites can be caused by preferential corrosion along the particle-matrix interface. As a result, the corrosion rate of the hybrid composite was greater than that of the monolithic matrix (Bhatt et al., Citation2021; Suresh et al., Citation2018).
As shown in , the icorr and CR of the Al7075 hybrid composite in both media increased from 303 to 323 K. This may be due to an increase in temperature, leading to an increase in the conductance of the medium, which directly affects the oxidation of the metal. The PDP plots obtained for the Al7075 hybrid composite in 0.1 M HCl and 3.5% NaCl media showed a cathodic shift in the Ecorr values with increasing temperature. Because of the predominate oxygen reduction and hydrogen evolution processes on the composite surface, a cathodic shift was seen in both of the corrosive media. During the activation process, the cathodic polarization is accountable for the destabilization of the oxide film, leading to the deterioration of composite surface. This reaction involves alkalinization of the aluminum/solution boundary during oxygen reduction causing chemical dissolution of the oxide layer (Serdechnova et al., Citation2014). The anodic and cathodic slopes remained unaltered as the temperature of the medium did not affect the mechanism of the corrosion process. In the case of 3.5% NaCl, the anodic polarization curve was linear, suggesting that the anodic metal dissolution reaction was under activation control. The cathodic branches were not linear and showed an inflection point at a potential more negative than Ecorr, possibly owing to the deposition of corrosion products. In such cases, the corrosion rate was measured by extrapolating the linear portion of the anodic curve to the corrosion potential (Teenu et al., Citation2022). The icorr for the corrosion of the Al7075 hybrid composite is directly proportional to the corrosion rate on the order of 10−3 A cm−2 in 0.1 M HCl and that of 10−5 A cm−2 in 3.5% NaCl. This indicates that the corrosion rate of the Al7075 hybrid composite in an acidic medium was greater than that in a neutral medium. The greater corrosion rate of the hybrid composite in an acidic medium is due to the increased conduction of H+ ions, which can enhance the hydrogen evolution reaction and have a direct impact on the metal dissolution process (Robledo et al., Citation2022). In the case of the 3.5% NaCl medium, the corrosion rate is much lower than that of the 0.1 M HCl medium. This may have been due to the formation of a stable passivation film. Aluminum forms a protective oxide film that may remain stable when the pH is between 4 and 8.5 and hence shows more resistance to corrosion.
Electrochemical impedance spectroscopy (EIS)
EIS is considered a dependable method for analyzing the corrosion behavior at the interface between Al7075 hybrid composites and corrosive media. Al is oxidized to Al3+, and corrosion products accumulate at the interface when the Al7075 hybrid composite is exposed to an acidic medium. Moreover, there is significant adsorption of Cl in the solution, which makes the dense corrosion product film that forms on the surface of the hybrid composites susceptible to assault by Cl. As a result, the corrosion product film did not effectively shield the specimen materials.
show the Nyquist plots that were recorded once the Al7075 hybrid composites were stabilized in 0.1 M HCl and 3.5% NaCl solutions, respectively, and after reaching the open-circuit potential. Distinct equivalent circuits can be used to mimic the properties of the composites, as the Nyquist plots generated for the Al7075 hybrid composite in both media have somewhat different shapes. One capacitive loop operating at a higher frequency and one inductive loop operating in an intermediate frequency range constitute analogous circuits. The low-frequency range of the Nyquist curve highlights the relationship between the double-layer capacitance and charge transfer resistance, while the high-frequency area of the curve links the capacitive loops to the corrosion product layer on the Al7075 hybrid composite (Kenneth & Michael, Citation2011). The capacitive loop is due to the interfacial response, especially the aluminum oxidation response at the metal/oxide/electrolyte interface. This loop incorporates the development of Al+ particles at the metal/oxide interface and their movement through the oxide/arrangement interface, where they are oxidized to Al3+ (Charitha & Rao, Citation2017).
Figure 2. Nyquist plot for the corrosion of the Al7075 hybrid composite in (a) 0.1 M HCl and (b) 3.5% NaCl.
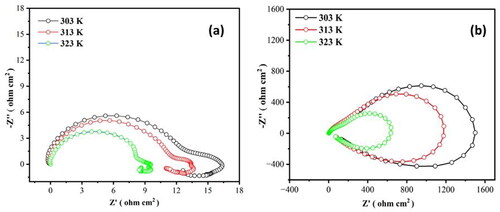
The formation of the inductive loop has frequently been ascribed to surface or bulk relaxation species in the oxide layer. The adsorption and consolidation of chloride particles on and into the oxide coating may be the cause of the inductive loop (Morad, Citation1999). As the temperature rises, the diameter of the capacitive loops in the Nyquist plot decreases. This suggests that as temperatures rise, the rate of corrosion in both systems will increase. Moreover, the inhomogeneity, composite surface roughness or reaction product deposition are responsible for the depressed nature of the capacitive loops (Brett et al., Citation1992).
The corresponding nine-element circuits illustrated in and five-element circuits in are used to analyze the EIS parameters for the composite corrosion at the metal/acid interface and 3.5% NaCl medium, respectively. The equivalent circuit includes the solution resistance Rs; charge transfer resistance Rt; inductive elements RL and L; constant phase element CPE (Q) parallel to capacitors C1 and C2; and resistors R1, R2, RL and Rt, which are in series. Rp is the polarization resistance, and Cdl is the capacitance at the double layer, which are computed from the following equations (Prabhu & Rao, Citation2017):
(2)
(2)
(3)
(3)
Figure 3. Equivalent circuit used to fit the experimental EIS data for the corrosion of Al7075 hybrid composite in (a) 0.1 M HCl and (b) 3.5% NaCl.
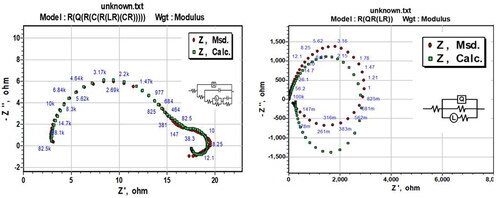
shows the CPE in parallel with the parallel resistors Rt and RL. Rp can be calculated from
(4)
(4)
The experimental results obtained for the corrosion of the Al7075 hybrid composite in (a) 0.1 M HCl and (b) 3.5% NaCl media are provided in .
Table 3. Results of EIS measurements for the corrosion behavior of Al7075 in 0.1 M HCl and 3.5% NaCl media at different temperatures.
The recorded values of the polarization resistance decreased as the temperature increased in both media, indicating an increase in the rate of corrosion of the hybrid composite with increasing temperature. This signifies a decrease in the resistance toward the corrosion process, and the results are consistent with the findings from the PDP technique. The frequency range over which the samples exhibit capacitive behavior is smaller in 0.1 H HCl than in 3.5% NaCl, as can be observed from the impedance spectra. The reduced protectiveness of the naturally produced oxide layer on the composite surface is indicated by the shorter frequency range observed (Bhatt et al., Citation2021). The capacitance of the electrical double layer increased with increasing temperature for both systems. This suggests thinning of the electrical double layer at the M+/solution interface due to the increase in temperature. The Cdl values (821–1464 µF cm−2) obtained for the hybrid composite in 0.1 M HCl were greater than those obtained for the hybrid composite in 3.5% NaCl (72.9–78.5 µF cm−2), suggesting a greater rate of corrosion of the hybrid composite in acidic media.
Effect of temperature
By evaluating the corrosion rate in the range of 303–323 K for both systems, the impact of temperature on the corrosion rate of the Al7075 hybrid composite was investigated. Using previously reported equations (Kumari et al., Citation2023), the activation energy (Ea), enthalpy (ΔH#) and entropy (ΔS#) of the activation parameters for the corrosion of the Al7075 hybrid composite were determined. display the Arrhenius plots (ln CR versus 1/T) at various temperatures in 0.1 M HCl and 3.5% NaCl media, respectively, whereas display the plots of ln (CR/T) versus 1/T for the corrosion of the Al7075 hybrid composite. records the corresponding results for both systems.
Figure 4. The Arrhenius plot (ln CR versus 1/T) for the Al7075 hybrid composite in 0.1 M HCl and 3.5% NaCl media.
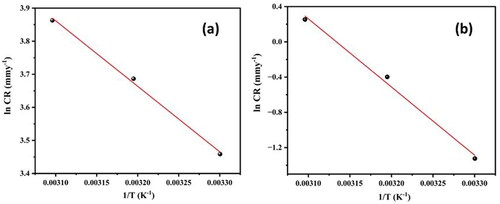
Figure 5. Plot of ln (CR/T) versus 1/T for the Al7075 hybrid composite in 0.1 M HCl and 3.5% NaCl media.
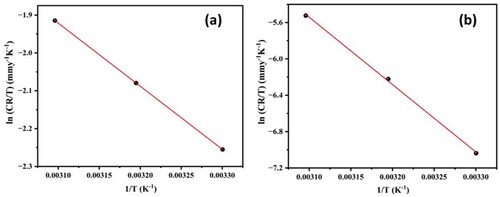
Table 4. The activation parameters for the Al7075 hybrid composite in both studied corrosive systems.
The Ea obtained in 0.1 M HCl was less than 20 kJ/mol, which is indicative of concentration polarization is the main reaction at the electrode–electrolyte interface. However, at 3.5% NaCl, the Ea values was greater than 20 kJ/mol. This phenomenon may be because the activation energy for the corrosion reaction is independent of concentration polarization and is mainly due to activation polarization (Oguzie, Citation2007). The higher Ea values for the hybrid composite in 3.5% NaCl indicate an increase in the energy barrier for the corrosion process, which is well correlated with the results of the PDP and EIS techniques (Shetty, Sunil, et al., Citation2020).
Mechanism of hybrid composite corrosion in HCl and NaCl medium
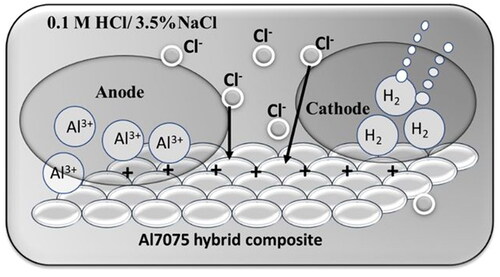
The aluminum and its alloys were covered with a passivating oxide coating. When exposed to an acidic environment, the amphoteric characteristics of the surface aluminum oxide coating allow it to easily disintegrate (Shetty & Shetty, Citation2015). The metal dissolves during the anodic process, resulting in the formation of metal ions. The metal oxide-free surface allows the metal ions to permeate into the acidic environment. The discharge of hydrogen ions during the cathodic process results in the release of hydrogen gas. In this study, the aluminum alloy matrix was composed of duralumin particulates as a reinforced phase, which led to discontinuities in the oxide film on the aluminum surface and enhanced corrosion. However, aluminum, with a lower typical electrode potential of 1.66 V, operates as an anode and is quickly oxidized. The reinforced phase behaves as a cathodic site, favoring the hydrogen evolution reaction.
When Al and its composites exposed to a neutral aqueous solution, the amorphous γ-alumina oxide film formed on its surface thickens and forms a crystalline layer of hydrated alumina. The following processes are typically involved in the breakdown of aluminum in an acidic medium (Sunitha et al., Citation2019):
The oxide layer on the metal surface will be less able to mend itself in the presence of HCl because Cl– ions are more likely to seep through the film. As a result, an intermediate soluble complex is formed, as indicated by the equation below:
(5)
(5)
As a result, this soluble complex promotes the dissolution of the aluminum ions from the lattice into the solution and causes the passive layer to thin locally. This allows the chloride ions to attack the passive layer further and causes severe pitting attack.
Below are the anodic and cathodic reactions under acidic conditions.
(6)
(6)
(7)
(7)
The overall reaction occurs during the composite corrosion is,
(8)
(8)
However, in a NaCl medium, the possible cathodic reactions are given below:
(9)
(9)
(10)
(10)
After reaching icorr and Ecorr, the current on the anodic side increases due to the aggressiveness of chloride ions.
(11)
(11)
(12)
(12)
Similarly, the presence of a neutral medium results in the formation of an aluminum oxide layer.
(13)
(13)
(14)
(14)
The pits on the specimen surface formed because of chloride ion diffusion, which created tiny channels that extended across the interface after they diffused through the tiny flaws. In such a scenario, the hydroxide protective layer cannot form a strong enough bond with the composite surface to prevent the matrix from dissolving and to provide aluminum with adequate corrosion resistance. Through an autocatalytic mechanism, Cl significantly improves the initiation and propagation of pits. Because of anodic dissociation and metal degradation, positive metallic cations are formed in the pits, which hastens the corrosion process (Kshama, Citation2015; Loto, Citation2013). A schematic of the corrosion mechanism is given in .
Surface morphology studies
Scanning electron microscopy (SEM) analysis
SEM investigations were carried out to differentiate between the surface morphology of the freshly polished Al7075 hybrid composite and that of the specimens immersed in the corresponding corrosive media. show SEM images of the freshly polished Al7075 hybrid composite and the composites immersed in 0.1 M HCl and 3.5% NaCl media, respectively. The surface morphology of the freshly polished Al7075 hybrid composite included a fine surface along with boundaries on its surface owing to the reinforcement of duralumin. The corroded specimen of the hybrid composite in 0.1 M HCl had a large number of pits at the boundaries, indicating the possibility of pitting corrosion. The corrosion started initially along the boundary region and then spread throughout the entire surface. Generally, the surface of the alloy is uniform and covered with an oxide layer, which acts as a resistance to the spread of corrosion. However, in the hybrid composite, owing to the reinforcement process, these oxide layers breakdown, which results in an increased rate of corrosion. However, in the presence of 3.5% NaCl, the surface of the hybrid composite was relatively smooth compared to that of 0.1 M HCl. This may be due to the passivation of the oxide film in the neutral medium.
Atomic force microscopy (AFM)
Atomic force microscopy (AFM), a form of scanning probe microscopy (SPM), is the most versatile and powerful microscopy technology for studying the topography of metal surfaces at the nanoscale. The difference in the surface morphology of the freshly polished Al7075 hybrid composite and corroded specimens was investigated using AFM, and the results are depicted in . In the absence of a corroding medium, the Al7075 hybrid composite exhibited smooth and relatively flat surfaces () with average surface roughness (Ra) and root-mean-square roughness (Rq) values of 26.7 nm and 33.9 nm, respectively. Compared with those of the plain composite surface, the AFM images of the hybrid composites immersed in 0.1 M HCl () and 3.5% NaCl () media showed increases in the average surface roughness (Ra) and root-mean-square roughness (Rq). The roughness of the composite surface in the corrosive medium evidently indicates better coarseness with highly damaged surfaces due to the vigorous action of acidic and NaCl media and the formation of corrosion products on the surface. The AFM image obtained for the composite surface in 0.1 M HCl showed a rough surface with a large number of peaks compared to the 3.5% NaCl medium. This is evident from the higher rate of composite corrosion in the acidic medium. This may be because of the aggressiveness of the corrosive medium. The presence of H+ ions at the cathode leads to more hydrogen evolution, which increases metal dissolution. The surface roughness values obtained for the composites in both media are listed in .
Figure 8. 3D images of (a) freshly polished AA hybrid composites, (b) immersed in 0.1 M HCl, (c) and immersed in 3.5% NaCl.
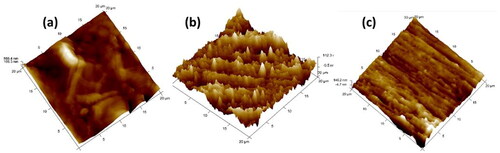
Table 5. AFM results obtained for the Al7075 hybrid composite.
Conclusions
A methodical study on the corrosion behavior of the Al7075 hybrid composite was carried out in 0.1 M HCl and 3.5% NaCl media, and the following observations were made.
The results revealed that the corrosion rate of the Al7075 hybrid composite was more intense in 0.1 M HCl than in 3.5% NaCl, as the hydrogen evolution reaction predominates in 0.1 M HCl.
The negative shift in the PDP plots with increasing temperature confirms the increase in the rate of the cathodic reaction, which in turn influences the dissolution of the hybrid composite surface.
The decrease in the polarization resistance with increasing temperature confirms the decrease in the resistance toward the charge transfer process.
The higher values of Ea in 3.5% NaCl compared to 0.1 M HCl confirm the increase in the energy barrier for the corrosion process for hybrid composites in 3.5% NaCl medium.
The surface roughness values also confirmed the increased rate of corrosion of the Al7075 hybrid composites in the 0.1 M HCl medium compared to the 3.5% NaCl medium.
The corrosion rate of the Al7075 hybrid composite increased with increasing temperature in both 0.1 M HCl and 3.5% NaCl media owing to an increase in the conductance of the medium.
Ethical approval
Not applicable.
Author contributions
Gowri Shankar MC, Srinivas D, Karthik BM: Composite preparation and its characterization. Preethi Kumari P: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Prasanna Nayak: Manuscript preparation, Experimental Investigation. Poornima Bhagavath: Data curation, Formal analysis, Methodology. Suma A Rao and Akshatha R Shetty: Methodology and reviewing and editing.
Disclosure statement
The authors declare no competing interests.
Data availability statement
The data that support the findings of the study are available from corresponding author, upon reasonable request.
Additional information
Funding
References
- Afzal, N., Shah, T., & Ahmad, R. (2013). Microstructural features and mechanical properties of artificially aged AA2024. Strength of Materials, 45(6), 684–692. https://doi.org/10.1007/s11223-013-9504-8
- Al-Salihi, H. A., Mahmood, A. A., & Alalkawi, H. J. (2019). Mechanical and wear behavior of AA7075 aluminium matrix composites reinforced by Al2O3 nanoparticles. Nanocomposites, 5(3), 67–73. https://doi.org/10.1080/20550324.2019.1637576
- Alsamuraee, A. M. A., Ameen, H. A., & Al-Rubaiey, S. I. J. (2011). Evaluation of the pitting corrosion for aluminum alloys 7020 in 3.5% NaCl solution with range of temperature (100–500)°C. American Journal of Scientific and Industrial Research, 2, 283–296. https://doi.org/10.5251/ajsir.2011.2.2.283.296
- Ashish, K., Virendra, P. S., Singh, R. C., Chaudhary, R., & Kumar, D. (2024). Enhancing microstructural, tribological and corrosion responses of Al–Zn–Mg–Cu alloy via nano-/micro-Al2O3 particulates. Journal of Materials Science, 59(17), 7235–7257. https://doi.org/10.1007/s10853-024-09638-w
- Babajanzade Roshan, S., Behboodi Jooibari, M., Teimouri, R., Asgharzadeh-Ahmadi, G., Falahati-Naghibi, M., & Sohrabpoor, H. (2013). Optimization of friction stir welding process of AA7075 aluminium alloy to achieve desirable mechanical properties using ANFIS models and simulated annealing algorithm. Journal of Minerals and Materials Characterization and Engineering, 69(5–8), 1803–1818. https://doi.org/10.1007/s00170-013-5131-6
- Bhatt, Y., Kumari, P. P., Sunil, D., Rao, S. A., Shetty, P., & Sneha, K. (2021). The impact of naphthalimide derivative on the mitigation of mild steel corrosion in sulfamic acid medium: experimental and theoretical insights. Chemical Papers, 75(8), 3831–3845. https://doi.org/10.1007/s11696-021-01608-9
- Brett, C. M. A., Brock, A. J., & Wood, G. C. (1992). On the electrochemical behaviour of aluminium in acidic chloride solution. Corrosion Science, 33(2), 203–210. https://doi.org/10.1016/0010-938X(92)90145-S
- Charitha, B. P., & Rao, P. (2017). "Environmentally benign green inhibitor to attenuate acid corrosion of 6061Aluminium-15%(v) SiC(P) Composite. Journal of Industrial and Engineering Chemistry, 58, 357–368. https://doi.org/10.1016/j.jiec.2017.09.049
- Hou, B., Li, X., Ma, X., Du, C., Zhang, D., Zheng, M., Xu, W., Lu, D., & Ma, F. (2017). The cost of corrosion in China. Npj Materials Degradation, 1:4. https://doi.org/10.1038/s41529-017-0005-2
- Huimin, H., Longtao, J., Zhenlong, C., Xue, W., Ming, Z., Bingzhuo, H., Runwei, Z., Du, S., Luo, T., & Mei, Y. (2023). Revealing corrosion behavior of B4C/pure Al composite at different interfaces between B4C particles and Al matrix in NaCl electrolyte. Journal of Materials Research and Technology, 27, 7213–7227. https://doi.org/10.1016/j.jmrt.2023.11.163
- Kadri, Y., Srasra, E., Bekri-Abbes, I., & Herrasti, P. (2021). Facile and eco-friendly synthesis of polyaniline/ZnO composites for corrosion protection of AA2024 aluminium alloy. Journal of Electroanalytical Chemistry, 893, 115335. https://doi.org/10.1016/j.jelechem.2021.115335
- Karthik, B. M., Sharma, S. S., Gowrishankar, M. C., Hegde, A., & Srinivas, D. (2022). Effect of weight of reinforcement and coating thickness on the hardness of stir cast Al7075-Nickel coated duralumin powder MMC. Journal of Applied Engineering Science, 20(3), 900–907. https://doi.org/10.5937/jaes0-34780
- Karthikraja, M., Ramanatha, K., Loganathan, K. T., & Selvaraj, S. May (2023). Corrosion behaviour of SiC and Al2O3 reinforced Al 7075 hybrid aluminium matrix composites by weight loss and electrochemical methods. Journal of the Indian Chemical Society, 100(5), 101002. https://doi.org/10.1016/j.jics.2023.101002
- Kenneth, K. A., & Michael, B. (2011). “Corrosion behavior of alumina reinforced aluminium (6063) metal matrix composites. Journal of Minerals and Materials Characterization and Engineering, 10, 1153–1165. https://doi.org/10.4236/jmmce.2011.1012088
- Kshama, S. S. (2015). Studies on some ionic liquids as corrosion inhibitors on 6061 Al–15 vol. pct. SiC(p) composites in acidic media, decertation. National Institute of Technology.
- Kumari, P. P., Anusha, G., Cheerlin Mishma, J. N., Rajeev, K. S., Aishwarya, S. S., & Gaonkar, S. L. (2023). New benzisoxazole derivative: A potential corrosion inhibitor for mild steel in 0.5 M hydrochloric acid medium -insights from electrochemical and density functional theory studies. Heliyon, 9(10), e210104. https://doi.org/10.1016/j.heliyon.2023.e21014
- Loto, R. T. (2013). Pitting corrosion evaluation of austenitic stainless-steel type 304 in acid chloride media. Journal of Materials and Environmental Science, 4(4), 448–459.
- Morad, M. S. (1999). Influence of propargyl alcohol on the corrosion of mild steel in H3PO4 solution. Materials Chemistry and Physics, 60(2), 188–195. https://doi.org/10.1016/S0254-0584(99)00090-5
- Oguzie, E. E. (2007). Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trisfasciata extract. Corrosion Science, 49(3), 1527–1539. https://doi.org/10.1016/j.corsci.2006.08.009
- Olajire, A. A. (2017). Corrosion inhibition of off shore oil and gas production facilities using organic compound inhibitors - a review. Journal of Molecular Liquids, 248, 775–808. https://doi.org/10.1016/J.Mol.Liq.2017.10.097
- Pardo, A., Merino, M. C., López, M. D., Viejo, F., Carboneras, M., & Merino, S. (2003). Influence of reinforcement grade and matrix composition on corrosion resistance of cast aluminium matrix composites (A3xx.x/SiCp) in a humid environment. Materials and Corrosion, 54(5), 311–317. https://doi.org/10.1002/maco.200390070
- Prabhu, D., & Rao, P. (2017). Corrosion behaviour of 6063 aluminium alloy in acidic and in alkaline media. Arabian Journal of Chemistry, 10(2), S2234–S2244. https://doi.org/10.1016/j.arabjc.2013.07.059
- Robledo, J. D. E., Shetty, P., Kumari, P. P., Shankar, M. C. G., & Kagatikar, S. (2022). Gravimetric, electrochemical and theoretical study on corrosion of AA6061/3wt% SiC/3wt% B4C hybrid composite in acid medium using EDTA. Tribology in Industry, 44(1), 73–86. https://doi.org/10.24874/ti.1052.02.21.05)
- Sambathkumar, M., Navaneethakrishnan, P., Ponappa, K., & Sasikumar, K. S. K. (2017). Mechanical and corrosion behavior of Al7075 (hybrid) metal matrix composites by two step stir casting process. Latin American Journal of Solids and Structures, 14(2), 243–255. https://doi.org/10.1590/1679-78253132
- Sameezadeh, M., Emamy, M., & Farhangi, H. (2011). Effects of particulate reinforcement and heat treatment on the hardness and wear properties of AA 2024-MoSi2 nanocomposites. Materials & Design, 32(4), 2157–2164. https://doi.org/10.1016/j.matdes.2010.11.037
- Serdechnova, M., Volovitch, P., Brisset, F., & Ogle, K. (2014). On the cathodic dissolution of Al and Al alloys. Electrochimica Acta, 124, 9–16. https://doi.org/10.1016/j.electacta.2013.09.145
- Shetty, D., Kumari, P. P., Rao, S. A., & Shetty, P. (2020). Anticorrosion behaviour of a hydrazide derivative on 6061 Al-15%(v) SiC(P) composite in acid medium: Experimental and theoretical calculations. Journal of Bio- and Tribo-Corrosion, 6(2), 59. https://doi.org/10.1007/s40735-020-00356-9
- Shetty, K. S., & Shetty, A. N. (2015). Studies on corrosion behavior of 6061 Al–15 vol. pct. SiC(p) composite in HCl medium by electrochemical techniques1. Surface Engineering and Applied Electrochemistry, 51(4), 374–381. https://doi.org/10.3103/S1068375515040134
- Shetty, P., Sunil, D., Preethi Kumari, P., & Nagalaxmi. (2020). Effect of cysteine as environmentally friendly inhibitor on AA6061-T6 corrosion in 0.5 M HCl: Electrochemical and surface studies. Surface Engineering and Applied Electrochemistry, 56(5), 624–634 https://doi.org/10.3103/S1068375520050087
- Sunitha, N., Manjunatha, K. G., Khan, S., & Sravanthi, M. (2019). Study of SiC/graphite particulates on the corrosion behavior of Al 6065 MMCs using tafel polarization and impedance. SN Applied Sciences, 1(9), 1024. | https://doi.org/10.1007/s42452-019-1063-6
- Suresh, S., Gowd, G. H., & Devakumar, M. L. S. (2018). Corrosion behaviour of Al 7075/Al2O3/SiC MMNCs by weight loss method. Journal of Bio Tribo Corrosion, 4, 4. https://doi.org/10.1007/s40735-018-0182-8
- Tang, S. W., Hu, J., & Zhao, X. H. (2011). Corrosion behavior of a cerium-based conversion coating on alumina borate whisker-reinforced AA6061 composite pre-treated by hydrogen fluoride. Corrosion Science, 53(8), 2636–2644. https://doi.org/10.1016/j.corsci.2011.04.026
- Teenu, S., Shetty, P., Kumari, P. P., & Sneha, K. (2022). 2-Aminobenzothiazole as an efficient corrosion inhibitor of AA6061-T6 in 0.5 M HCl medium: Electrochemical, surface morphological, and theoretical study. Journal of Applied Electrochemistry, 52(11), 1675–1689. https://doi.org/10.1007/s10800-022-01742-6