?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Fenugreek (Trigonella foenum-graecum L.) is an antidiabetic plant. Its bioactive compound, trigonelline, is known to counter diabetes through insulin secretion, modulation of β cell regeneration and quick activity of glucose metabolism related enzymes. A pot experiment was conducted in the natural conditions of net house of the Department of Botany, Aligarh Muslim University, Aligarh (UP), India, to evaluate the effect of four concentrations of GA3 (0, 10−7 M, 10−6 M and 10−5 M), alone and in combination with phosphorus (40 kg P ha−1), on growth, biochemical and yield attributes of fenugreek. Compared to control, the combination of GA3 and phosphorus (P40 + 10−6 M GA3) significantly increased the activities of nitrate reductase (30.8%) and carbonic anhydrase (30.7%) enzymes; it also enhanced the seed yield (140.6%) and the content of total chlorophyll (28.5%) and carotenoids (26%). There was also significant increase (19.51%) in the content of seed trigonelline.
Public Interest Statement
Trigonella foenum-graecum L., commonly known as fenugreek, is an annual herb belonging to the family Leguminosae. It is used as an antidiabetic plant. It contains bioactive compounds as trigonelline, diosgenin, 4-hydroxyisoleucine (4-HI), galactomannan flavonoids, carotenoids, coumarins, saponins and lipids. Diosgenin is used by pharmaceutical and nutraceutical industries as a precursor for the production of steroidal drugs and hormones such as testosterone, progesterone and glucocorticoids. Diabetic patients are recommended to take fenugreek as a vegetable in diet. Being such an immense medicinal plant, there is a need to enhance the growth and active constituents of this plant. Gibberellic acid (GA3) is a plant growth hormone (C19H22O6) that regulates seed germination, stem elongation, leaf expansion, fruit development, flowering and stimulation of secondary metabolite production in plants. In the present study, the foliar application of GA3 in combination with soil applied phosphorus enhanced growth, biochemical attributes and yield of fenugreek. It significantly enhanced the trigonelline production as well.
1. Introduction
Diabetes mellitus is one of the most common diseases in the present day world with 150 million of diabetics worldwide and the number is unfortunately likely to increase to 300 million by 2025 (Shaw, Sicree, & Zimmet, Citation2010). For centuries, mankind has been using the medicinal plants to overcome numerous diseases and according to World Health Organization, 80% of the people in developing countries get through traditional medicines. Trigonella foenum-graecum L., commonly known as fenugreek, is one such medicinal plant that has been comprehensively used as a source of antidiabetic compounds, from its leaves, seeds and extracts in different model systems (Khalki, M’hamed, Bennis, Chait, & Sokar, Citation2010; Srinivasan, Citation2006). In India, fenugreek is mostly used in Unani and Ayurvedic therapeutic systems (Srinivasan, Citation2006). The important constituents of fenugreek include 4-hydroxyisoleucine (4-HI), trigonelline, galactomannan flavonoids, carotenoids, coumarins, proteins, saponins and lipids (Basch, Ulbricht, Kuo, Szapary, & Smith, Citation2003). Trigonelline controls the diabetes through operation of the mechanism of insulin secretion, modulation of β cell regeneration and stimulation of activity of glucose metabolism-related enzymes (Abd El-Mawla & Osman, Citation2011; Zhou, Chan, & Zhou, Citation2012). The seeds of fenugreek contain about 50% of fibre that is known to slow the rate of post-parandial glucose absorption, which may be responsible for the hypoglycaemic activity. The seed extract of fenugreek stimulates the secretion of insulin from pancreatic cells and inhibits the activities of α-amylase and sucrose (Amin, Abdul Ghani, & Suleiman, Citation1988). Fenugreek seeds have sapogenins which are known to lower serum cholesterol level in humans (Yadav, Moorthy, & Baquer, Citation2004, Citation2005).
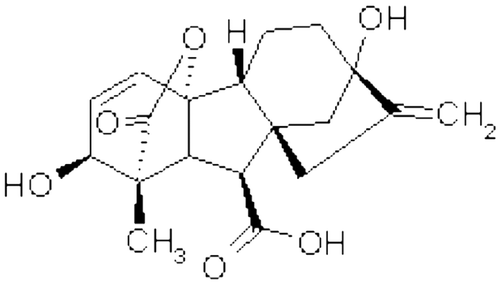
Gibberellins (GAs) constitute a group of tetracyclic diterpenoids, involved in plant growth and development. Gibberellic acid (GA3), (Figure ) a well known phytohormone, has numerous physiological effects on plants including seed germination, growth, stem elongation, leaf expansion, photosynthesis, flowering and cell expansion (Taiz & Zeiger, Citation2010; Yuan & Xu, Citation2001). Exogenous application of GA3 to plants causes the increase in the activities of many key enzymes like carbonic anhydrase (CA), nitrate reductase (NR) (Afroz, Mohammad, Hayat, & Siddiqui, Citation2005; Aftab et al. Citation2010) and ribulose-1,5-biphosphate carboxylase/oxygenase (RuBPCO) (Yuan & Xu, Citation2001).
Phosphorus (P) is the second growth-limiting nutrient after nitrogen (Moinuddin et al., Citation2014) and is an integral constituent of biological macromolecules such as that of nucleic acids, phospholipids and ATP. Consequently, plants cannot grow successfully without a reliable supply of this nutrient. There may be large quantities of phosphorus present in the soil but its amount, available to plants, is generally low because of the insoluble forms of soil phosphorus. For that reason, phosphate fertilizers are added to the soil to overcome the phosphorus deficiency. In fact, there is significant gap in the knowledge regarding phosphorus nutrition of crops in Indian cropping systems. We also need to fill the gaps in our knowledge about how crops respond to phosphorus in different situations and how can phosphorus efficiency be improved supplying adequate P for crop productivity.
The present study was conducted to investigate the effect of foliar application of GA3 alone and in combination with the soil applied P on the crop productivity, physiological and biochemical parameters, and trigonelline yield of T. foenum-graecum L.
2. Material and methods
2.1. Plant material, chemicals and growth conditions
Gibberellic acid (GA3) was purchased from Duchefa Biochemie B.V, The Netherlands (Product Number: G0907; CAS Number 77-06-5) and Trigonelline hydrochloride was brought from Sigma Aldrich (Product Number: T5509; CAS Number 6138-41-6). The seeds of T. foenum-graecum L. were purchased from National Seed Corporation Unit, Indian Agricultural Research Institute (IARI), New Delhi, India. Healthy seeds of T. foenum-graecum L. were initially surface sterilized with 80% ethyl alcohol for 5 min and then washed thoroughly with double distilled water before sowing. Each experimental pot carried 5 kg homogenous mixture of soil and farmyard manure (4:1). The soil samples were tested at Soil Testing Laboratory, IARI, New Delhi, India. The experimental soil was sandy loam with pH (1:2) 7.94, E.C (1:2) 0.57 mmhos/cm, and available N, P and K 98.45, 7.15 and 141.8 mg kg−1 soil, respectively.
2.2. Experimental design and pot culture
The pot experiment was conducted at the Botany Department, Aligarh Muslim University, Aligarh, India (27° 52′ N latitude, 78° 51′ E longitude, and 187.45 m altitude) in order to ascertain the changes mediated by GA3 and P, applied alone or in combination, on physiological and biochemical parameters as well as on growth, yield and quality attributes of T. foenum-graecum L. The experiment was conducted according to simple randomized design in the natural conditions of Net house using earthen pots (25 cm diameter × 25 cm height). Deionised water (DIW) was used as the control. Four levels of GA3, viz. 0 M (DIW), 10−7 M GA3, 10−6 M GA3 and 10−5 M GA3] were employed as foliar spray treatments. Two P levels, viz. 0 (P0) and 40 kg P ha−1 (P40), were applied to the soil in the form of KH2PO4. Four concentrations of GA3 and two levels of P were employed to constitute eight treatments [(I) Control (DIW), (II) 10−7 M GA3 (III) 10−6 M GA3 (IV) 10−5 M GA3 (V) 40 kg P ha−1 (P40) (VI) P40 + 10−7 GA3 (VII) P40 + 10−6 GA3 (VIII) P40 + 10−5 GA3]. In about 90% of the seeds, the germination took place within 5–6 days of sowing. After a month of germination, the number of seedlings was maintained to five per pot. Totally, 40 pots were employed for the experiment. Seeds were sown at a depth of 2 cm. Each treatment was replicated five times. Totally, six spray treatments were given at 7 days interval, using a hand sprayer, the first foliar spray treatment being applied at 30 days after sowing (DAS). The pots were watered and made weeds-free as and when required.
2.3. Determination of growth and yield attributes
Growth attributes of the T. foenum-graecum L. were determined at 60 and 90 DAS. At the respective sampling stage, five plants from each treatment (one plant from each replicate) were carefully harvested with the roots and washed with running tap water to remove adhering foreign particles. Water adhering to the roots was removed with blotting paper. Fresh weight of the clean and blot-dried plants was recorded using an electronic balance. Thereafter, the plants were oven-dried at 80°C for 24 h. Dry weight of plants was recorded subsequently. The shoot and root length of the plants was measured with the help of a metre scale. Number of leaves was counted and leaf area was measured by the graph paper method. Yield parameters were noted at the time of harvesting. From each pot, the plants were harvested above ground to measure the different yield attributes, viz. seed yield and total biomass per plant, harvest index, 1,000-seed weight, number of pods per plant, number of seeds per plant, number of seeds per pod and pod length. The harvest index was calculated as (seed yield/total biomass) × 100.
2.4. Determination of biochemical parameters
2.4.1. Total chlorophyll and carotenoids contents
Contents of total chlorophyll and carotenoids were estimated on fresh weight basis by the method of Mac Kinney (Citation1941) and Maclachlan and Zalik (Citation1963), respectively. Fresh tissue from interveinal leaf areas was grinded with mortar–pestle using 80% acetone. The optical density (OD) of the solution was recorded at 645 and 663 nm for chlorophyll estimation and at 480 and 510 nm for carotenoids estimation employing a spectrophotometer (Spectronic UV-1700, Shimadzu, Japan). Concentrations of chlorophyll and carotenoids were expressed as mg g−1 FW.
2.4.2. Determination of NR activity
NR activity was estimated by the intact tissue assay method developed by Jaworski (Citation1971), which is based on the reduction of nitrate to nitrite according to the following biochemical reaction:
The amount of nitrite () formed was determined spectrophotometrically. Interveinal area of fresh chopped leaves, weighing 0.2 g, was transferred to each of the plastic vials. Reaction mixture of each vial was comprised of 2.5 mL phosphate buffer (pH 7.5), 0.5 mL of potassium nitrate solution and 2.5 mL of 5% isopropanol solution. The vials, carrying the reaction mixture, were kept in an incubator maintained at 30°C for the manifestation of maximum enzyme activity. After the incubation period (2 h), 0.4 mL of the content was transferred to a test tube. To it, 0.3 mL each of 1% sulphanilamide and 0.02% N-(1-Naphthyl) ethylenediamine dihydrochloride (NED-HCl) was added. The test tubes were kept for 20 min at room temperature for maximum colour development. The OD of the content was recorded at 540 nm using the spectrophotometer. The NR activity was expressed as nanomoles of nitrite produced per gram fresh weight of the leaf tissue per hour (nM
g −1 FW h−1).
2.4.3. Determination of CA activity
CA activity was analysed in fresh leaves using the method as described by Dwivedi and Randhawa (Citation1974). Two hundred milligram (0.2 g) of fresh interveinal leaf tissue was transferred to Petri plate, followed by its incubation in 10 mL of 0.2 M cystein hydrochloride solution for 20 min at 4°C. Thereafter, 4 mL of 0.2 M sodium bicarbonate solution and 0.2 mL of 0.02% bromothymol blue was added to the homogenate. The reaction mixture was titrated against 0.05 N HCl using methyl red as indicator. The activity of CA was expressed as moles of CO2 produced per kg of fresh leaf tissue per second (mM CO2 kg−1 leaf FW s−1).
2.4.4. Estimation of N, P and K contents in leaves
Leaf samples from each treatment were digested for the estimation of leaf-N, -P and -K contents. The leaves were dried in a hot-air oven maintained at 80°C for 24 h. Dried leaves were powdered using mortar–pestle, followed by passing the powder through a 72 mesh. The sieved leaf powder was used for the estimation of N, P and K contents. One hundred mg of oven-dried leaf powder was carefully transferred to a digestion tube, to which 2 mL of concentrated sulphuric acid (analytical grade) was added subsequently. The content was heated for about two hours on a temperature-controlled digestion assembly maintained at 80°C; and then, the content was cooled at room temperature for about 15 min. To the cooled content, 0.5 mL of 30% hydrogen peroxide (H2O2) was added drop by drop. The addition of H2O2 was followed by gentle heating of the content and then cooling at room temperature. This step was repeated until the content of the tube turned colourless. The aliquot (peroxide-digested leaf material), thus prepared, was used to estimate the percentage of N, P and K contents in the leaves on dry weight basis.
2.4.4.1. Determination of leaf-N content
Leaf-N content was estimated according to method of Lindner (Citation1944) with slight modification by Novozamsky, Houba, van Eck, and van Vark (Citation1983). A 10 mL aliquot (peroxide digested leaf material) was poured into a 50 mL volumetric flask. To it, 2 mL of 2.5 N sodium hydroxide solution and 1 mL of 10% sodium silicate solution was added to neutralize the excess acid and prevent turbidity, respectively. A 5 mL aliquot of the peroxide digested material was poured into a 10 mL graduated test tube, followed by addition of 0.5 mL of Nessler’s reagent. The OD of the solution, thus obtained, was recorded at 525 nm using the spectrophotometer.
2.4.4.2. Determination of leaf-P content
The method of Fiske and Subba Row (Citation1925), with slight modification by Rorison, Spencer, and Gupta (Citation1993), was used to estimate the leaf-P content. A 5 mL aliquot of the peroxide-digested leaf material was poured into a 10 mL graduated test tube. To it, 1 mL of molybdic acid (2.5%) was added, followed by addition of 0.4 mL of 1-amino-2-naphthol-4-sulphonic acid. When the colour of the content turned blue, its volume was made up to 10 mL using double distilled water. The OD of the solution, thus obtained, was recorded at 620 nm using the spectrophotometer.
2.4.4.3. Determination of leaf-K content
Leaf-P content was determined using a flame photometer (Model, C150, AIMIL, India) with the help of emission spectra using specific filter. In the flame photometer, the solution (peroxide-digested leaf material) was discharged through an atomizer in the form of a fine mist into a chamber, where it was drawn into a flame. Combustion of the solution elements produced the light of a particular wavelength [k max for K = 767 nm (violet)]. The light, thus produced, was passed through an appropriate filter to impinge upon a photoelectric cell that subsequently activated a galvanometer (electric current detector).
2.5. Seed alkaloid content
To estimate total seed alkaloid content, 1 g of seed powder was ground with 80% methanol and a pinch of magnesium oxide (MgO), using mortar–pestle. The sample was incubated at 60°C for about 30 min; the homogenate was centrifuged, followed by collecting the supernatant. Methanol was evaporated completely and the residue was dried. Thereafter, the weight of the beaker containing the residue and that of the empty beaker was recorded.
Total seed alkaloid content (%) was calculated using following formula:
where WE is the Weight of empty beaker (g); WA is the Weight of porcelain dish after evaporation (g); and WR is the Weight of the seed powder (g).
2.6. Extraction and estimation of trigonelline by high performance liquid chromatography
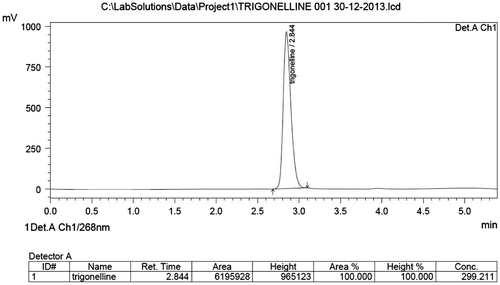
To extract seed trigonelline content of T. foenum-graecum L., the method of Zheng and Ashihara (Citation2004) was adopted with a slight modification. One gram of seed powder was grinded with 80% methanol with a pinch of magnesium oxide (MgO) using mortar–pestle. The sample was incubated at 60°C for about 30 min, the homogenate was centrifuged, followed by collecting the supernatant. Methanol was evaporated and the extract was dissolved in distilled water. The content was filtered using disposable syringe filter unit and stored thereafter in sealed vials for the determination of trigonelline by high performance liquid chromatography (HPLC). Seed trigonelline content was determined by isocratic HPLC equipment (Model, LC-20AD, Shimadzu Corporation Analytical & Measuring Instrument Division, Kyoto, Japan) with reverse phase C-18 (5 μm, 150 mm, 4.6 mm I.D,) column. Mobile phase consisted of methanol and water in the ratio of 50:50 (v/v) and the pH was adjusted to 5 using acetic acid. The elution was made in an isocratic mode at the flow rate of 1 mL min−1 and the detection was made at 268 nm by UV–vis detector. Calibration curve was made by using graded concentrations (0, 50, 100, 150 and 200 mg L−1) of standard trigonelline hydrochloride (Sigma Aldrich, USA), (Figure ) with the help of HPLC equipment.
2.7. Statistical data analysis
The data were analysed statistically using SPSS-17 statistical software (SPSS Inc., Chicago, IL, USA) according to randomized design. Means were compared applying the Fisher’s least significant difference (LSD) test at p < 0.05.
3. Results
Among different concentrations of gibberellic acid, 10−6 M GA3 proved to be the best for most of the parameters studied. GA3, applied in combination with phosphorus (i.e. P40 + 10−6 M GA3) proved to be the best treatment for almost all of the parameters studied. This treatment was followed by P40 + 10−7 M GA3. The spray treatment of DIW (control) resulted in the lowest values (Tables and ; Figures and ).
Table 1. Effect of foliar application of different concentrations of gibberellic acid and soil applied phosphorus on growth parameters of fenugreek (T. foenum-graecum L.)
Table 2. Effect of foliar application of different concentrations of gibberellic acid and soil applied phosphorus on yield parameters of fenugreek (T. foenum-graecum L.) recorded at 120 DAS
Figure 3. Effect of different concentrations of foliar sprays of GA3 (10−7 M, 10−6 M, 10−5 M) and soil applied phosphorus (40 kg P ha−1) on NR activity (A), CA activity (B), total chlorophyll content (C), and carotenoids content (D) of fenugreek (Trigonella foenum-graecum L.) studied at 60 and 90 DAS
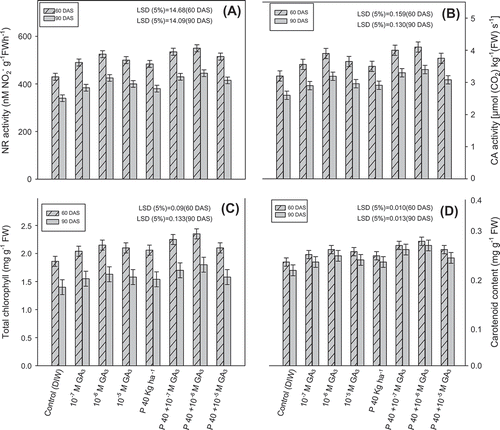
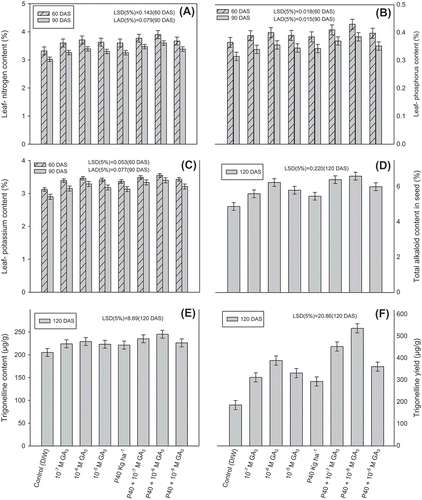
Figure 4. Effect of different concentrations of foliar sprays of GA3 (10−7 M, 10−6 M, 10−5 M) and soil applied phosphorus (40 kg P ha−1) on leaf-nitrogen content (A), leaf-phosphorus content (B), leaf-potassium content (C), total alkaloid content in seed (D) seed trigonelline content (E) trigonelline yield (F) of fenugreek (Trigonella foenum-graecum L.) studied at 60 and 90 days after sowing (DAS)
3.1. Growth attributes
Foliar application of GA3 progressively improved the growth attributes of fenugreek both at 60 and 90 DAS (Table ). The highest values of growth parameters were attained at 90 DAS with the combination treatment, i.e. P40 + 10−6 M GA3. In comparison to the control, P40 + 10−6 M GA3 increased the shoot length by 41 and 44%, root length by 31.33 and 45.19%, leaf number by 33.32 and 43.24%, leaf area by 33.26 and 32.43%, shoot fresh mass by 41.46 and 39.96%, root fresh mass by 37.65 and 39.95%, shoot dry mass by 46.73 and 42.45%, root dry mass by 36.59 and 40% at 60 and 90 DAS, respectively (Table ).
3.2. Physiological and biochemical characteristics
3.2.1. Photosynthetic pigments
Gibberellic acid treatment increased the total chlorophyll and carotenoids content in the treated plants significantly. Of all the GA3 concentrations applied alone, 10−6 M GA3 proved to be the best. However, among combinations, P40 + 10−6 M GA3 gave the best results. In comparison to the control, application of P40 + 10−6 M GA3 enhanced the total chlorophyll content by 26.34 and 28.57% and carotenoids content by 20 and 26% at 60 and 90 DAS, respectively.
3.2.2. NR activity
Treatment combination P40 + 10−6 M GA3 increased the NR activity to the highest extent, increasing the leaf NR activity by 27.91 and 30.88% at 60 and 90 DAS, respectively.
3.2.3. CA activity
In the present investigation, CA activity was found to be improved over the control as a result of phosphorus application along with 10−6 M GA3. In the present study, P40 + 10−6 M GA3 exhibited greater CA activity than those sprayed with deionized water (control) to the extent of 28.13 and 30.77% at 60 and 90 DAS, respectively.
3.2.4. Leaf-N, -P and -K contents
Leaf-N, -P and -K contents were also significantly enhanced by foliar application of GA3, with optimum combination treatment (P40 + 10−6 M GA3) proving the best. The application of 10−6 M GA3 in combination with soil-applied P increased the content of leaf-N by 17.47 and 19.20%, leaf-P by 17.80 and 22.22% and leaf-K by 13.78 and 17.24% at 60 and 90 DAS, respectively.
3.3. Yield attributes
Among all the treatments, the combined treatment, i.e. P40 + 10−6 M GA3, proved to be the best; it resulted in significant increase in the seed yield (140.66%), total alkaloid content (35.25%), biological yield (47.50%), harvest index (63.12%), 1,000 seed weight (26.98%) pod length (40.91%) and number of pods per plant (62.79%). Of the treatments, combination treatment P40 + 10−6 M GA3 resulted in the maximum increase in the content (19.51%) and yield (187.67%) of trigonelline as compared to the control.
4. Discussion
Very few studies have been carried out to investigate the effect of gibberellic acid (GA3) on fenugreek. Besides, no work has till date been cited exploring the effect of GA3 and P combination on fenugreek, which is an antidiabetic plant. Hence, it might be considered as the first report of its kind, revealing the combined effect of GA3 and P on growth and other physiological attributes, yield and bioactive constituents of fenugreek. Plant growth and development is known to be governed by several exogenous and endogenous factors, including the growth regulators (Taiz & Zeiger, Citation2010). In the present investigation, the significant increase in the shoot and root length, fresh and dry weight, number of leaves and leaf area might be ascribed to the well known effects of GA3 and P in plants. It is a recognized fact that GA3 promotes cell enlargement and cell division (Buchanan, Gruissem, & Jones, Citation2000; Taiz & Zeiger, Citation2010). The studies conducted by Srivastava and Srivastava (Citation2007) also support the fact that foliar application of GA3 could increase the plant height and leaf length in Catharanthus roseus. Increase in growth parameters in the plants treated with GA3 might be ascribed to the induction of inherent genetic potential of the plant by exogenous application of PGRs, which might cause increase in elongation of internodes as a result of cell division and cell wall extensibility (Khan et al., Citation2006; Taiz & Zeiger, Citation2010). In the present study, GA3 increased the leaf area of fenugreek; similar results were obtained regarding Saccharum officinarum (Dhaliwal, Malik, Gosal, & Dhaliwal, Citation1997) and several field grown plants (Zhou, MacKenzie, Madramootoo, & Smith, Citation1999) on account of GA3 application. Growth promotion by the exogenous application of GA3 is also reported in C. roseus (Srivastava & Srivastava, Citation2007) and Nigella sativa (Shah, Ahmad, & Samiullah, Citation2006). In view of the essential role of phosphorus in plant growth and development (Marschner, Citation2002), the increment in plant fresh and dry mass could be expected as a result of soil-applied phosphorus (Table ). In the present study, the interactive effect of P and GA3 resulted into the best values of growth parameters. The beneficial effects of phosphorus on growth of plants have been reported with regard to different medicinal plants (Chaudhary et al., Citation2009; Dutta & Bandyopadhyay, Citation2009; Naeem, Khan, & Mohammad, Citation2009; Naeem, Masroor, & Khan, Citation2009).
As per the present study, massive improvement (28.57 and 26%) was found in chlorophyll and carotenoids content when P and GA3 were applied together (Figure ). The increased contents of chlorophyll and carotenoids could, possibly, be attributed to the enhancement of ultrastructural morphogenesis of plastids by GA3 as suggested by Arteca (Citation1996). Our results are in agreement with other studies conducted by different workers in different plants like Zea mays (Sinha, Srinivatsa, & Tripathi, Citation1993), Glycine max (Zhao, Lin, Shi, & Chang, Citation1995) and Schefflera arboricola L. (Salehi Sardoei, Roein, Shahadadi, Sadeghi, & Mokhtari, Citation2014). The enhancement of the photosynthetic pigments due to P application has earlier been reported by Naeem and Khan (Citation2009) and Naeem, Khan, et al. (Citation2009) and Naeem, Masroor, et al. (Citation2009).
CA is one of the most abundant zinc containing proteins in plants. It catalyzes the reversible hydration of CO2 to carbonic acid, thereby increasing the availability of CO2 to RuBisCO in photosynthesis (Khan, Javed, & Samiullah, Citation2004). An increase in CA activity in this study could be ascribed to the ample accessibility of phosphorus at the site of its metabolism. A probable reason for the enhancement of CA activity by the foliar application of GA3 alone or in combination with soil-applied P might be the de nova synthesis of CA which might involve translation/transcription of respective genes (Okabe, Angela, Mikio, & Shigetoh, Citation1980). Furthermore, the enhanced activity of CA in the treated plants might have increased the CO2 fixation, which ultimately might be responsible for the increase in fresh and dry mass of treated plants (Table ).
NR is known to be a rate-limiting enzyme in nitrogen assimilation and its level depends on a variety of factors, either external or internal. The activity of this enzyme in plants gives a good approximation of the nitrogen eminence of the plant and is very often correlated with growth, yield and protein synthesis. One of the reasons of GA3- enhanced NR activity in this study might be the GA3- improved leaf nitrogen content that might have increased the concentration of nitrate in the leaves to be acted upon by NR (Figure ). Above all, P40 + 10−6 M GA3 treatment proved to be the best and increased the NR activity by 30.88% over the control. Phosphorus application has resulted in increased NR activity in other plants like Zea mays (de Magalhães et al., Citation1998) and Phaseolus vulgaris (Gniazdowska, Krawczak, Mikulska, & Rychter, Citation1999), which confirmed the enhanced assimilation of leaf content due to P application. Besides, enhancement in leaf NR activity with phosphorus application has also been reported in different medicinal and aromatic plants such as Cassia tora (Naeem & Khan, Citation2005), Cyamopsis tetragonoloba L. (Burman, Kumar Garg, & Kathju, Citation2007) and Senna occidentalis L. (Naeem & Khan, Citation2009).
Foliar application of GA3 alone and in combination with soil-applied P proved effective for N, P and K contents in fenugreek (Figure ). The concentration of leaf nutrients (N, P and K) was significantly greater in plants treated with P40 + 10−6 M GA3 as compared to the control. According to Al Wakeel, Hamed, and Dadoura (Citation1995) application of GA3 increased the root membrane permeability, which might ultimately facilitate the absorption, transport and utilization of mineral nutrient in plants as argued by Khan, Ansari, and Samiullah (Citation1998) in the case of Brassica juncea. Moreover, the plants treated with GA3 had more biomass as reflected from the shoot and root dry mass of the plants (Table ). The enhancement in plant biomass in the present investigation could presumably be as a result of increased uptake of nutrients, enhanced photosynthesis and improved translocation of photosynthates and other metabolites to the reproductive parts as suggested by Miniraj and Shanmugavelu (Citation1987) regarding Capsicum annum. As per Figure , the enhancement in leaf-N, -P and -K content could be due to phosphorus-encouraged absorption of additional amount of nutrients from the soil as noted by Graciano, Goya, Frangi, and Guiamet (Citation2006) with regard to Eucalyptus grandis. Our results in this regard are consistent with those of earlier studies on different medicinal crops (Khan, Samiullah, Afaq, & Afridi, Citation2000; Naeem & Khan, Citation2005, Citation2009; Naeem, Khan, et al. (Citation2009); Naeem, Masroor, et al. (Citation2009).
GA3 application improved the seed yield in fenugreek (Table ). Such an observation has been made in other plants also like Zea mays (Naghashzadeh, Citation2007) and Glycine max (Azizi, Moradii, Heidari, Khalili, & Feizian, Citation2012). The increase in seed yield due to GA3 might be accounted for its possible positive effects on 1,000 seed weight, pod set, increased CO2 fixation and increase in leaf area. Besides, the enhanced values of yield parameters with phosphorus might be because of its important role in root and shoot development, energy translocation and other metabolic processes of plants (Marschner, Citation2002). In fact, the adequate amount of phosphorus in the early plant growth causes rapid cell division and cell elongation in the meristematic regions, leading to enhanced development of seeds and increased seed yield (Spencer & Chan, Citation1991; Turk, Tawaha, & Samara, Citation2003). The increase in seed yield ultimately might cause the increase in the yield of trigonelline. Trigonelline (N-methylnicotinic acid) is derived from nicotinic acid and the reaction is catalyzed by S-adenosyl-L-methionine (SAM)-dependent nicotinate enzyme N-methyltransferase. The positive effect of GA3 on trigonelline content could be attributed to the improved plant growth and metabolism as observed in our study (Tables and ; Figures and ). Srivastava and Srivastava (Citation2007) claimed that GA3 enhanced the accumulation of secondary metabolites like steroids, terpenoid and anthocyanin production. In addition, GA3 improved the accumulation of steroidal alkaloids in Solanum aviculare (Subroto & Doran, Citation1994). In Catharanthus roseus, application of GA3 significantly enhanced the alkaloids content in stem, leaves and roots as compared to untreated plants (Srivastava & Srivastava, Citation2007).
5. Conclusion
The present investigation will help to find out the optimum concentration of gibberellic acid to enhance the growth aspects and productivity of fenugreek. The results concluded that application of 10−6 M GA3 in combination with phosphorus (40 kg P ha−1) was the best among all treatments for most of the parameters studied. Also, the combination of GA3 and P was favourably effective in production of the antidiabetic alkaloid trigonelline.
Cover image
Source: Author.
Additional information
Funding
Notes on contributors
Tariq Ahmad Dar
Our research group deals with Plant Physiology and Biochemistry with a thrust on the study “Effect of plant growth regulators, mineral nutrients, nanoparticles and marine polysaccharides on growth, physiology and secondary metabolite production of medicinal and aromatic plants”.
The present work shows that the combined application of gibberellic acid and phosphorus significantly enhanced the growth, physiological activities and trigonelline production in Trigonella foenum-graecum L. Further research is needed to find how GA3 acts at molecular level to increase the production of trigonelline in this plant.
References
- Abd El-Mawla, A. M. A. , & Osman, H. E. H. (2011). Elicitation of trigonelline and 4-hydroxyisoleucine with hypoglycemic activity in cell suspension cultures of Trigonella foenum graecum L. The Open Conference Proceedings Journal , 2 , 80–87.10.2174/2210289201102010080
- Afroz, S. , Mohammad, F. , Hayat, S. , & Siddiqui, M. H. (2005). Exogeneous application of gibberellic acid counteracts the ill effects of sodium chloride in mustard. Turkish Journal of Biology , 29 , 233–236.
- Aftab, T. , Khan, M. M. A. , Idrees, M. , Naeem, M. , Singh, M. , & Ram, M. (2010). Stimulation of crop productivity, photosynthesis and artemisinin production in Artemisia annua L. by triacontanol and gibberellic acid application. Journal of Plant Interactions , 5 , 273–281.10.1080/17429141003647137
- Al Wakeel, S. A. M. , Hamed, A. A. , & Dadoura, S. S. (1995). Interactive effects of water stress and gibberellic acid on mineral composition of fenugreek plant. Egyptian Journal of Physiology Science , 18 , 269–282.
- Amin, R. , Abdul Ghani, A. S. , & Suleiman, M. S. (1988). Effect of fenugreek and lupine seeds on the development of experimental diabetes in rats. Planta Medica , 54 , 286–290.
- Arteca, R. N. (1996). Plant growth substances: Principles and applications . New York, NY: Chapman and Hall.
- Azizi, K. , Moradii, J. , Heidari, S. , Khalili, A. , & Feizian, M. (2012). Effect of different concentrations of gibberellic acid on seed yield and yield components of soybean genotypes in summer intercropping. International Journal of AgriScience , 2 , 291–301.
- Basch, E. , Ulbricht, C. , Kuo, G. , Szapary, P. , & Smith, M. (2003). Therapeutic applications of fenugreek. Alternative Medicine Review , 8 , 20–27.
- Buchanan, B. B. , Gruissem, W. , & Jones, R. L. (2000). Biochemistry and molecular biology of plants . Rockville, MD: American Society of Plant Physiologists.
- Burman, U. , Kumar Garg, B. , & Kathju, S. (2007). Interactive effects of phosphorus, nitrogen, and thiourea on clusterbean (Cyamopsis tetragonoloba L.) under rainfed conditions of the Indian arid zone. Journal of Plant Nutrition and Soil Science , 170 , 803–810.10.1002/jpln.v170:6
- Chaudhary, M. I. , Adu-Gyamfi, J. J. , Saneoka, H. , Nguyen, N. T. , Suwa, R. , Kanai, S. , … Fujita, K. (2009). The effect of phosphorus deficiency on nutrient uptake, nitrogen fixation and photosynthetic rate in, mungbean and soybean. Acta Physiologiae Plantarum , 30 , 537–544.
- de Magalhães, J. V. , Alves, V. M. C. , de Novais, R. F. , Mosquim, P. R. , Magalhães, J. R. , Filho, A. F. C. , & Hubert, D. M. (1998). Nitrate uptake by corn under increasing periods of phosphorus starvation. Journal of Plant Nutrition , 21 , 1753–1763.10.1080/01904169809365520
- Dhaliwal, R. K. , Malik, C. P. , Gosal, S. S. , & Dhaliwal, L. S. (1997). Studies on hardening of micro propagated sugarcane (Saccharum officinarum L.) plantnet. II Leaf parameters and biochemical estimations. Annals of Biology (Ludhiana) , 13 , 15–20.
- Dutta, D. , & Bandyopadhyay, P. (2009). Performance of chickpea (Cicer arietinum L.) to application of phosphorus and bio-fertilizer in laterite soil. Archives of Agronomy and Soil Science , 55 , 147–155.10.1080/03650340802398864
- Dwivedi, R. S. , & Randhawa, N. S. (1974). Evaluation of a rapid test for the hidden hunger of zinc in plants. Plant Soil , 40 , 445–451.10.1007/BF00011531
- Fiske, C. H. , & Subba Row, Y. (1925). The colorimetric determination of phosphorus. The Journal of Biological Chemistry , 66 , 375–400.
- Gniazdowska, A. , Krawczak, A. , Mikulska, M. , & Rychter, A. M. (1999). Low phosphate nutrition alters bean plants ability to assimilate and translocate nitrate. Journal of Plant Nutrition , 22 , 551–563.10.1080/01904169909365651
- Graciano, C. , Goya, J. F. , Frangi, J. L. , & Guiamet, J. J. (2006). Fertilization with phosphorus increases soil nitrogen absorption in young plants of Eucalyptus grandis . Forest Ecology and Management , 236 , 202–210.10.1016/j.foreco.2006.09.005
- Jaworski, E. G. (1971). Nitrate reductase assay in intact plant tissues. Biochemical and Biophysical Research Communications , 43 , 1247–1279.
- Khalki, L. , M’hamed, S. B. , Bennis, M. , Chait, A. , & Sokar, Z. (2010). Evaluation of the developmental toxicity of the aqueous extract from Trigonella foenum-graecum (L.) in mice. Journal of Ethnopharmacology , 15 , 321–325.
- Khan, M. M. A. , Gautam, C. , Mohammad, F. , Siddiqui, M. H. , Naeem, M. , & Khan, M. N. (2006). Effect of gibberellic acid spray on performance of tomato. Turkish Journal of Biology , 30 , 11–16.
- Khan, M. M. A. , Samiullah , Afaq, S. H. , & Afridi, R. M. (2000). Response of black nightshade (Solanum nigrum L.) to phosphorus application. Journal of Agronomy and Crop Science , 184 , 157–163.10.1046/j.1439-037x.2000.00334.x
- Khan, N. A. , Ansari, H. R. , & Samiullah (1998). Effect of gibberellic acid spray during ontogeny of mustard on growth, nutrient uptake and yield characteristics. Journal of Agronomy and Crop Science , 181 , 61–63.10.1111/j.1439-037X.1998.tb00399.x
- Khan, N. A. , Javed, S. , & Samiullah (2004). Physiological role of carbonic anhydrase in CO2-fixation and carbon partitioning. Physiology and Molecular Biology of Plants , 10 , 153–166.
- Lindner, R. C. (1944). Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiology , 19 , 76–89.10.1104/pp.19.1.76
- Mac Kinney, G. (1941). Absorption of light by chlorophyll solutions. Journal of Biological Chemistry , 140 , 315–322.
- Maclachlan, S. , & Zalik, S. (1963). Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Canadian Journal of Botany , 41 , 1053–1062.10.1139/b63-088
- Marschner, H. (2002). Mineral nutrition of higher plants (2nd ed.). London: Elsevier Science.
- Miniraj, N. , & Shanmugavelu, K. G. (1987). Studies on the effect of triacontanol on growth, flowering, yield, quality and nutrient uptake in chillies. South Indian Hort. , 35 , 362–366.
- Moinuddin, Dar T. A. , Hussain, S. , Khan, M. M. A. , Hashmi, N. , Idrees, M. , Naeem, M. , & Ali, A. (2014). Use of N and P biofertilizers together with phosphorus fertilizer Improves growth and physiological attributes of chickpea. Global Journal of Agriculture and Agricultural Sciences , 2 , 168–174.
- Naeem, M. , & Khan, M. M. A. (2005). Growth, physiology and seed yield of Cassia tora as affected by phosphorus fertilization. JMAPS , 27 , 4–6.
- Naeem, M. , & Khan, M. M. A. (2009). Phosphorus ameliorates crop productivity, photosynthesis, nitrate reductase activity and nutrient accumulation in coffee senna (Senna occidentalis L.) under phosphorus-deficient soil. Journal of Plant Interactions , 4 , 145–153.10.1080/17429140802193178
- Naeem, M. , Khan, M. M. A. , & Mohammad, F. (2009). Augmenting photosynthesis, enzyme activities, nutrient content, yield and quality of senna sophera (Cassia sophera L.) by P fertilization. Indian Journal of Plant Physiology , 14 , 278–282.
- Naeem, M. , Masroor, M. M. A. , & Khan, A. (2009). Promotive effects of phosphorus on crop productivity, enzyme activities, anthraquinone and sennoside content in Cassia tora L.—A medicinal herb. Journal of Plant Interactions , 4 , 49–57.10.1080/17429140802323338
- Naghashzadeh, M. (2007). Investigation of the effect of gibberellic hormone on cropping factors of maize in Khorranabad (Dissertation). Islamic Azad University, Khoramabad.
- Novozamsky, I. , Houba, V. J. G. , van Eck, R. , & van Vark, W. (1983). A novel digestion technique for multi-element plant analysis. Communications in Soil Science and Plant Analysis , 14 , 239–248.10.1080/00103628309367359
- Okabe, K. , Angela, L. , Mikio, T. , & Shigetoh, M. (1980). Effects of carbonic anhydrase on ribulose 1,5-biphosphate carboxylase and oxygenase. FEBS Letters , 114 , 142–144.10.1016/0014-5793(80)80879-9
- Rorison, I. H. , Spencer, R. E. , & Gupta, P. L. (1993). Chemical analysis. In G. A. E. Hendry & J. P. Grime (Eds.), Methods in comparative plant ecology (pp. 156–161). New York, NY: Chapman and Hall.
- Salehi Sardoei, A. , Roein, A. , Shahadadi, F. , Sadeghi, T. , & Mokhtari, T. S. (2014). Endogenus gibberellic acid and benzyladenine effects on stem elongation and leaf in Schefflera arboricola L. plants. IJPAES , 4 , 431–437.
- Shah, S. H. , Ahmad, I. , & Samiullah . (2006). Effect of gibberellic acid spray on growth, nutrient uptake and yield attributes during various growth stages of black cumin (Nigella sativa). Asian Journal of Plant Sciences , 5 , 881–884.
- Shaw, J. E. , Sicree, R. A. , & Zimmet, P. Z. (2010). Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice , 87 , 4–14.10.1016/j.diabres.2009.10.007
- Sinha, S. K. , Srinivatsa, H. S. , & Tripathi, R. D. (1993). Influence of some growth regulators and cations on inhibition of chlorophyll biosynthesis by lead in Maize. Bulletin of Environmental Contamination and Toxicology , 51 , 241–246.
- Spencer, K. , & Chan, C. K. (1991). Critical phosphorus levels in sunflower plants. Australian Journal of Experimental Agriculture , 21 , 91–97.
- Srinivasan, K. (2006). Fenugreek (Trigonella foenum-graecum): A review of health beneficial physiological effects. Food Reviews International , 22 , 203–224.10.1080/87559120600586315
- Srivastava, N. K. , & Srivastava, A. K. (2007). Influence of gibberellic acid on 14CO2 metabolism, growth, and production of alkaloids in Catharanthus roseus . Photosynthetica , 45 , 156–160.10.1007/s11099-007-0026-0
- Subroto, M. A. , & Doran, P. M. (1994). Production of steroidal alkaloids by hairy roots of Solanum aviculare and the effect of gibberellic acid. Plant Cell, Tissue and Organ Culture , 38 , 93–102.10.1007/BF00033866
- Taiz, L. , & Zeiger, E. (2010). Plant physiology (5th ed.). Sunderland, MA: Sinauer Associates.
- Turk, M. A. , Tawaha, A. M. , & Samara, N. (2003). Effects of seeding rate and date and phosphorus application on growth and yield of narbon vetch (Vicia narbonensis). Agronomie , 23 , 355–358.10.1051/agro:2003009
- Yadav, U. C. S. , Moorthy, K. , & Baquer, N. Z. (2004). Effects of sodium orthovanadate and Trigonella foenum-graecum seeds on hepatic and renal lipogenic enzymes and lipid profile during alloxan diabetes. Journal of Biosciences , 29 , 81–91.10.1007/BF02702565
- Yadav, U. C. S. , Moorthy, K. , & Baquer, N. Z. (2005). Combined treatment of sodium orthovanadate and Momordica charantia fruit extract prevents alterations in lipid profile and lipogenic enzymes in alloxan diabetic rats. Molecular and Cellular Biochemistry , 268 , 111–120.10.1007/s11010-005-3703-y
- Yuan, L. , & Xu, D. Q. (2001). Stimulation effect of gibberellic acid short-term treatment on leaf photosynthesis related to the increase in RuBisCO content in broad bean and soybean. Photosynthesis Research , 68 , 39–47.10.1023/A:1011894912421
- Zhao, H. J. , Lin, X. W. , Shi, H. Z. , & Chang, S. M. (1995). The regulating effects of phenolic compounds on the physiological characteristic and yield of soybeans. Acta Agronomica Sinica , 21 , 351–355.
- Zheng, X. Q. , & Ashihara, H. (2004). Distribution, biosynthesis and function of purine and pyridine alkaloids in Coffea arabica seedlings. Plant Science , 166 , 807–813.10.1016/j.plantsci.2003.11.024
- Zhou, J. , Chan, L. , & Zhou, S. (2012). Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Current Medicinal Chemistry , 19 , 3523–3531.10.2174/092986712801323171
- Zhou, X. M. , MacKenzie, A. F. , Madramootoo, C. A. , & Smith, D. L. J. (1999). Effects of stem-injected plant growth regulators, with or without sucrose, on grain production, biomass and photosynthetic activity of field-grown corn plants. Journal of Agronomy and Crop Science , 183 , 103–110.10.1046/j.1439-037x.1999.00331.x