Abstract
Polycyclic aromatic hydrocarbons (PAHs) are widespread pollutants and can negatively affect plants. Fluorene is a prevalent PAH in the contaminated environment. In this study, the effects of higher concentrations of fluorene in soil on rate of seed germination, growth, and the physiological parameters of wheat, sunflower, and alfalfa were studied. The results showed that the higher concentration of fluorene decreased rate of seed germination and seedlings growth of plants. Wheat showed the highest resistance at seed germination and seedlings growth phases, and sunflower was the most sensitive species at both stages. Therefore, it was concluded that higher resistance at seed germination could be followed by the higher resistance of seedlings. Fluorene toxicity also induced oxidative stress in plants as shown by MDA accumulation in the plants. There was a significant correlation between the lower activity of CAT and MDA accumulation in the studied plants. Therefore, CAT could be an important enzyme involved in detoxification of ROS and plants resistance to fluorene toxicity. Depending on plant species and fluorene concentration, photosynthetic pigments content was differently affected.
Keywords:
Public Interest Statement
Environmental contamination is an important problem worldwide. Polycyclic aromatic hydrocarbons (PAHs) are widespread organic pollutants and have negative effects on living organisms including plants. Fluorene is abundant PAHs in many contaminated environments and is toxic for living organisms. Hence, in this research, the physiological effect of this compound on wheat, alfalfa, and sunflower (as tree important crop) was studied in order to evaluation of plants resistance mechanisms to fluorene toxicity. Fluorene could negatively affect plants in both seed germination and seedling growth phases, but the effects severity is different between plants. This finding could be used for selection of plants for cultivation in contaminated regions in order to increase plants production specially crops.
1. Introduction
During the last decades, environmental contamination has become one of the major problems worldwide. Polycyclic aromatic hydrocarbons (PAHs) are a class of diverse organic pollutants (Chauhan, Fazlurrahman, Oakeshott, & Jain, Citation2008; Jajoo et al., Citation2014) comprising at least two condensed benzene rings (Li et al., Citation2014; Watts, Ballestero, & Gardner, Citation2006) and several hundred of these compounds that exist in the environment (Chauhan et al., Citation2008; Wilcke, Citation2000). PAHs can be released to the environment by natural processes such as volcanic erosion (Chauhan et al., Citation2008; Jan et al., Citation2014) and anthropogenic activities such as the incomplete combustion of fossil fuels (Li et al., Citation2014; Wilcke, Citation2000). PAHs generally are the hydrophobic (Parrish et al., Citation2006) and are regarded as persistence compounds in the environment (Jan et al., Citation2014; Parrish et al., Citation2006; Wei, Song, Tian, & Liu, Citation2014). The bioaccumulation potential, toxicity for living organisms, and stability in the environment are higher for PAHs with a high-molecular weight (Chauhan et al., Citation2008). PAHs have adverse impacts on living organisms, and their carcinogenic and genotoxic potentials are well known (Khan, Aijun, Zhang, Hu, & Zhu, Citation2008; Su & Zhu, Citation2008). US Environmental Protection Agency (US-EPA) has recommended 16 PAHs as priority pollutants for monitoring in the environment (Gao & Zhu, Citation2004; Jan et al., Citation2014; Li et al., Citation2014).
Fluorene is one of the low-molecular weight PAHs (Khan et al., Citation2008) comprising three benzene rings (Chauhan et al., Citation2008; Khodadoust, Bagchi, Suidan, Brenner, & Sellers, Citation2000; Song, Jing, Fleischmann, & Wilke, Citation2002) and it is one of the 16 PAHs listed by EPA as priority pollutants (Mastral et al., Citation2003). Fluorene is widely used for the synthesis of pigments, dyes, polymers, and drugs (Han & Wang, Citation2009) and it is a toxic compound for plants (Somtrakoon, Chaimeungkoon, Phalaphol, & Chouychai, Citation2012). This compound shows relatively higher water solubility when compared to many other PAHs (Eom, Rast, Veber, & Vasseur, Citation2007) and it is one of the most abundant PAHs in the contaminated soils (Watts et al., Citation2006; Wilcke, Citation2000), sediments (Nishigima, Weber, & B00EDcego, Citation2001), and plants (Eom et al., Citation2007; Watts et al., Citation2006; Watts, Ballestero, & Gardner, Citation2008).
Plants can uptake PAHs from the contaminated water (Lee, Lee, Lee, & Kim, Citation2008; Watts et al., Citation2006) and soils (Gao & Zhu, Citation2004; Meudec, Dussauze, Deslandes, & Poupart, Citation2006). PAHs’ uptake and its effect on plants depend on some parameters such as initial concentrations and chemical properties of PAHs (Singh & Jain, Citation2003; Watts et al., Citation2006), physico-chemical properties of soil (Khan et al., Citation2008; Mohan, Kisa, Ohkuma, Kanaly, & Shimizu, Citation2006), and plant species (Khan et al., Citation2008; Lee et al., Citation2008). Plants can uptake PAHs by roots from soil (Gao & Zhu, Citation2004; Khan et al., Citation2008; Meudec et al., Citation2006; Sun, Liu, Jin, & Gao, Citation2014) or by shoots from atmosphere (Khan et al., Citation2008; Lee et al., Citation2008; Watts et al., Citation2006). PAHs have harmful effects on plants (Wilcke, Citation2000), leading to morphological, cytological, genetical, and metabolic disorders in the plants (Kvesitadze, Khatisashvili, Sadunishvili, & Ramsden, Citation2006). Inhibition and reduction of seed germination (Huang, El-Alawi, Penrose, Glick, & Greenberg, Citation2004; Reynoso-Cuevas, Gallegos-MartíDnez, Cruz-Sosa, & Gutiérrez-Rojas, Citation2008; Tomar & Jajoo, Citation2014), induction of oxidative stress in the plants (Liu et al., Citation2008; Pašková, Hilscherová, Feldmannová, & Bláha, Citation2006), and disruption in photosynthetic apparatus function (Huang et al., Citation2004; Liu et al., Citation2008; Tomar & Jajoo, Citation2013) are some known effects of PAHs on the plants. While the negative effects of PAHs on the plants are well known, the physiological aspects of those effects on plants, and the mechanisms of plants responses to PAHs contamination and toxicity have rarely been studied. Accordingly, in this research, the effects of the higher concentrations of fluorene on the rate of seed germination and seedling growth of wheat, sunflower, and alfalfa were studied. Moreover, the evaluation of the biochemical and physiological responses of plants to fluorene toxicity was another aim of this study.
2. Materials and methods
2.1. Experimental design
Experiment was conducted as a pot culture of plants using a completely randomized design with four replications for each treatment.
2.2. Soil preparation and treatment
Soil was collected from an agricultural district in the northwest of Iran (Moghan, Ardabil province), far from roads, urban settlements, and industries in order to minimize the initial concentration of PAHs in the soils. The soil texture was clay loam containing 58.2% clay, 35.6% silt, and 6.2% sand. The pH, electrical conductivity, cation-exchange capacity, and organic material content of soil were 6.2, 195 μs m−1, 112 meq 100 g−1, and 3.1%, respectively (Jaiswal, Citation2004). Soils were air-dried at lab conditions, passed through <2 mm sieve, and autoclaved for 2 h at 121°C in order to eliminate micro-organisms which could be affective in the dissipation of PAHs (Parrish et al., Citation2006). For determination of initial PAHs concentrations in collected soil, PAHs were extracted based on a method used by Mueller and Shann (Citation2006) and analyzed using a GC (Varian GC CP-3800). No PAHs including fluorene were detected in the soils collected from agricultural fields. For preparation of fluorene contaminated soil, a particular concentration (50 and 100 mg kg−1) of fluorene was dissolved in acetone and added to the soils at a rate of 20 ml of solution per kilogram of soil (Smith, Flowers, Duncan, & Alder, Citation2006). Only 20 ml of acetone per kilogram without any fluorene was added to the soil used for the cultivation of the control plants. Then, soils were thoroughly homogenized using paddle several times, left in dark for three days, and shaken daily to ensure uniform distribution of the fluorene and evaporation of acetone. Finally, soils were divided into 1 kg pots and used for the culture of plants.
2.3. Plant culture
Seeds of wheat (Triticum aestivum L.), alfalfa (Medicago sativa L.), and sunflower (Helianthus annus L.) were supplied by Seed and Plant Improvement Institute of Iran (SPII, Karaj, Iran) and stored at 4°C until cultivation. An appropriate number of plants seeds (10, 50, and 10 seeds of wheat, alfalfa, and sunflower, respectively) were selected based on their vitality and uniformity. The seed surface was disinfected using 1% (v/v) sodium-hypochlorite solution, rinsed by sufficient distilled water, and immediately sown in plastic pots containing control and fluorene-treated soil. The pots were transferred to the controlled condition in a greenhouse at Tabriz university with 25–30°C temperature range, 16/8 (light/dark) photoperiod, and the relative humidity of 50–60%. The water content of the pots was adjusted to 100% field capacity every three days using sterile distilled water until the harvesting of plants. Seed germination was monitored over one week and the number of germinated seeds in each pot was recorded. The rate of seed germination was calculated as the percentage of germinated seeds to the total number of seeds for each species.
2.4. Harvesting of plants and assays
The experiment was conducted for 14 days. Biochemical and physiological assays were performed using fresh samples before plants harvesting. After the estimation of shoot height and root length, the harvested plants were divided into the roots and shoots. Samples were completely washed with water, immediately dried on the towel paper, and transferred to 70°C after determination of the fresh weight. The dry weight of samples was measured after 72 h.
2.5. Estimation of photosynthetic pigments content
Photosynthetic pigments content (chlorophyll a, b, total chlorophyll, and total carotenoids) was measured according to Hartmut (Citation1987). Briefly, 0.5 g of fresh leaf samples was homogenized with 6 ml of in >99.5% acetone using a mortar and pestle on ice bath. Homogenates were filtered using a number 42 Whatman filter paper; and for the determination of pigments contents, the absorbance of extracts was recorded at 645, 663, and 470 nm (Analytic Jena, Specol 200).
2.6. Estimation of malondialdehyde content (MDA)
Lipid peroxidation was evaluated by measuring malondialdehyde (MDA) content in fresh shoot material according to Boominathan and Doran (Citation2002). The samples were homogenized with 0.1% (W/V) trichloroacetic acid (TCA, Merck, Germany) and centrifuged at 10,000 g for 5 min. Then, 0.5 ml of supernatants were mixed with 4 ml of 20% TCA containing 0.5% of 2-thiobarbituric acid (Merck, Germany) and heated for 30 min in hot water at 95°C. The mixtures were immediately transferred to ice bath and then centrifuged at 10,000 g for 15 min. Finally, the absorbance of supernatants recorded in 532 nm and MDA concentration was calculated according to standard curve prepared by using 3,1,1,3-tetraethoxy propane (0–100 nM) and expressed as μg g−1 FW.
2.7. Total soluble protein content estimation and enzyme assays
The shoot samples (2 g) were homogenized in 15 ml of 0.1 M sodium phosphate (Na2HPO4/NaH2PO4, pH 7) buffer solution on the ice bath using mortar and pestle. Homogenates were centrifuged at 10,000 g for 10 min at 4°C and supernatants were used for soluble protein content estimation and enzyme assays. Soluble protein content was determined according to a method described by Bradford (Citation1976).
The activity of peroxidase (POD, EC1.11.1.7) and catalase (CAT, EC 1.11.1.6) was assayed spectrophotometrically according to the methods of Obinger, Maj, Nicholls, and Loewen (Citation1997) and Chance and Maehly (Citation1955), respectively. Briefly, for the estimation of POD activity, the increase in absorbance at 470 nm during the polymerization of guaiacol to tetraguaiacol was recorded for 3 min and POD-specific activity calculated using the extinction coefficient of 26.6 mM−1 cm−1 for guaiacol. The reaction mixture contained 10 mM of potassium phosphate buffer (pH 7), 5 mM of H2O2, 4 mM of guaiacol, and 25 μM of enzyme extract. One unite of POD activity was considered as the enzyme amount capable of oxidizing 1 μM guaiacol to tetraguaiacol per minute. The activity of CAT was measured at 240 nm by following the decomposition of H2O2 for 3 min. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7), 10 mM H2O2, and 50 μM of enzyme extract. CAT-specific activity was calculated using the extinction coefficient of 27 M−1 cm−1 for H2O2 and one unite of CAT activity was considered as the amount of enzyme necessary for the reduction of 1 μM H2O2 per minute.
2.8. Statistical analysis
Analysis of variance was conducted with Tukey–Kramer multiple comparisons test using Sigma stat 3.2 software and reading was considered significant when p < 0.05. Microsoft excel 2007 software was used for the preparation of figures.
3. Results
3.1. Seed germination rate
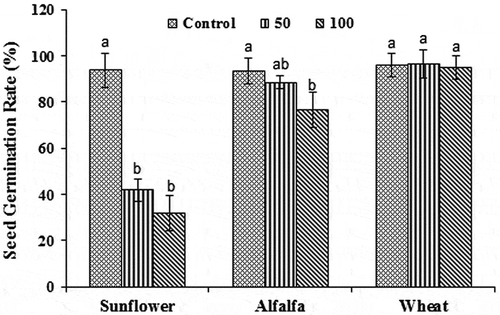
Seed germination rate of wheat plants was not notably affected by fuorene. In the alfalfa plant, the rate of seed germination was significantly decreased (−18%) by 100 mg kg−1 of fluorene (p < 0.05), but both 50 and 100 mg kg−1 of fluorene led to considerable reduction in the seed germination rate of sunflower plants (Figure ). The inhibition in the rate of seed germination in sunflower plant by 50 and 100 mg kg−1 of fluorene was 55 and 66%, respectively. The effect of 50 and 100 mg kg−1 of fluorene on the seed germination rates of all studied species was not significantly different (p < 0.05).
Figure 1. The effects of different fluorene concentrations in soil (Control, 50 and 100 mg kg−1) on the seed germination rates of wheat, alfalfa, and sunflower plants
3.2. Growth parameters
Fluorene negatively affected the growth parameters of all studied species. Shoots’ height of all species was significantly decreased by 100 mg kg−1 of fluorene, but the effect of 50 mg kg−1 of fluorene was only significant in sunflower plants (p < 0.05) (Table ). Similar to seed germination, sunflower and wheat plants were showed the highest and lowest reduction in shoot height, respectively. In comparison with the control, the shoot height of wheat, alfalfa, and sunflower were decreased 15, 17, and 56% by 100 mg kg−1 of fluorene, respectively
Table 1. The effects of different fluorene concentrations in soil (0, 500 and 100 mg kg−1) on the growth parameters of wheat, alfalfa, and sunflower plants
Root and shoot fresh weight of all studied species was significantly reduced by 50 and 100 mg kg−1 of fluorene (p < 0.05) (Table ). However, the difference between fluorene treatments was only significant in sunflower plants (p < 0.05). Plants treatment with 100 mg kg−1 of fluorene led to 18, 47, and 83% reduction in the shoot dry weight of wheat, alfalfa, and sunflower in comparison with the control, respectively. These values for the root fresh weight were 17, 18, and 90%, respectively. Therefore, similar to the rate of seed germination and shoot height, the highest and lowest decrease in both shoot and root fresh weight was observed in sunflower and wheat plants, respectively.
Both fluorene treatments significantly affected the root and shoot dry weight of all studied species (p < 0.05) (Table ). Similar to other growth parameters, wheat plants showed the lowest reductions in both root and shoot dry weight (20 and 50%, respectively). However, the highest decrease in the shoot dry weight was observed in alfalfa plants (90% in both treatments of fluorene). Therefore, sunflower was the most sensitive plant according to results of root dry weight. Shoot/root dry weight ratio in wheat plants decreased up to 38%; in alfalfa and sunflower plant, the ratios were increased up to 1.29 and 1.68 times, respectively. Shoot/root fresh weight ratio in wheat plants was slightly decreased, but in sunflower plant the ratio increased up to 1.79 times.
3.3. Enzymes activity
CAT activity in the shoots of all studied species was higher than that in the roots of the control and the treated plants. The activity of CAT in the shoots of wheat was considerably higher than that in sunflower and alfalfa plants. CAT activity in the roots and shoots of all species were significantly decreased by 100 mg kg-1 of fluorene (p < 0.05). The decrease rate in CAT activity in the shoots of wheat, alfalfa, and sunflower plants was 58.9, 76.5, and 52%, respectively. These values for CAT activity in the roots were 36, 45, and 40%, respectively (Table ). Therefore, the CAT activity in the shoots was more sensitive to fluorene toxicity in comparison to the roots. The effect of 50 mg kg−1 of fluorene on CAT activity was only significant in the shoots of alfalfa and the roots of wheat plants (p < 0.05).
Table 2. The effects of different fluorene concentrations in soil on catalase (μM H2O2 min−1 mg−1 protein) and peroxidase (μM guaiacol min−1 mg−1 protein) activity in the shoots and roots of wheat, alfalfa, and sunflower plants
POD activity in the shoots of wheat plants was significantly stimulated by 50 and 100 mg kg−1 of fluorene (54 and 94%, respectively), but it was significantly decreased in the roots (58 and 64%, respectively) (p < 0.05) (Table ). An exactly opposite result was observed in alfalfa plants. POD activity was slightly increased in the roots (13%) and significantly decreased in the shoots (39%). Furthermore, in wheat and alfalfa, fluorene differently affected POD activity in the roots and shoot, and treatment of sunflower plants with 100 mg kg−1 of fluorene caused significant reduction in POD activity in the roots (21%) and shoots (54%) (p < 0.05) (Table ). Therefore, the effects of fluorene on POD activity depended on plant species as well as plant organs.
3.4. Total soluble protein and MDA content
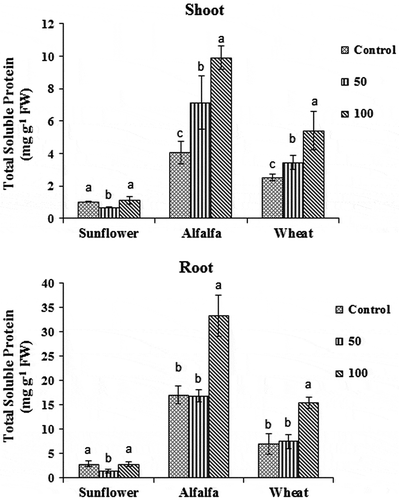
In all studied species, the total soluble protein content in the roots of control and treated plants was higher than that in the shoots. Both fluorene treatments caused significant increase in soluble protein accumulation in the shoots of alfalfa and wheat plants (Figure ). However, only with 100 mg kg−1 of fluorene, treated plant roots showed significantly higher accumulation of soluble protein (p < 0.05) (Figure ). Total soluble protein content in the roots and shoots of sunflower plants treated with 50 mg kg−1 of fluorene was decreased significantly, but there was no significant difference between the control plants and plants treated with 100 mg kg−1 of fluorene (p < 0.05).
Figure 2. The effects of different fluorene concentrations in soil (Control, 50 and 100 mg kg−1) on total soluble protein content in the shoots and roots of wheat, alfalfa, and sunflower plants
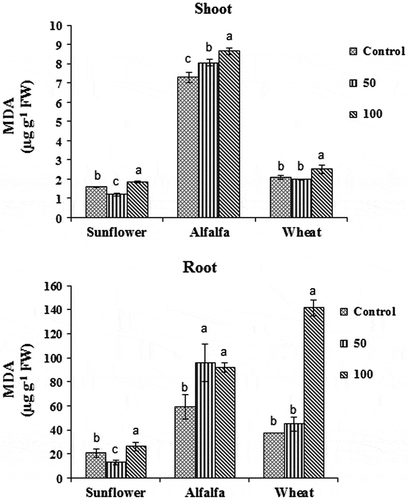
MDA content in the roots of the control and the treated plants was higher than that in the shoots of all studied species. Both fluorene treatments caused significantly higher MDA accumulation in the shoots and roots of alfalfa plants (p < 0.05). A significantly higher MDA content was recorded in the shoots and roots of wheat plants treated with 100 mg kg−1 of fluorene in comparison with the control and 50 mg kg−1 of fluorene plants treated (p < 0.05). The effect of fluorene on MDA content in sunflower was not similar to wheat and alfalfa plants. While, MDA content was significantly reduced in plants treated with 50 mg kg−1 of fluorene, 100 mg kg−1 of fluorene caused significantly higher MDA accumulation in the roots and shoots (p < 0.05) (Figure ).
Figure 3. The effects of different fluorene concentrations in soil (Control, 50 and 100 mg kg−1) on malondialdehyde (MDA) content in the shoots and roots of wheat, alfalfa, and sunflower plants
3.5. Photosynthetic pigments content
The effect of fluorene treatments on photosynthetic pigments content was not similar in the studied species (Table ). In all species, carotenoids content did not show significant changes in plants treated with fluorene. In wheat and alfalfa, chlorophyll a, chlorophyll b, and total chlorophyll contents significantly decreased in plants treated with 50 mg kg−1 of fluorene (p < 0.05). While, chlorophyll a, chlorophyll b, and total chlorophyll contents in alfalfa plants treated with 100 mg kg−1 of fluorene were significantly lower than those in control plants, but in wheat, the amounts significantly increased to those levels in the control plants (p < 0.05). In sunflower plants, both treatments of fluorene increased chlorophyll pigments content, but it was found that only the effect of 100 mg kg−1 of fluorene was significant (p < 0.05).
Table 3. The effects of different fluorene concentrations in soil on photosynthetic pigments content of wheat, alfalfa, and sunflower plants
4. Discussion
Fluorene significantly reduced the seed germination rates of alfalfa and sunflower plants. Inhibition and reduction of seed germination by PAHs had previously been reported in plants such as Lepidium sativum (Maila & Cloete, Citation2002) and Corn (Somtrakoon & Chuoychai, Citation2013). However, Smith et al. (Citation2006) showed that PAHs had no effects on seven plant species. In present study, the rate of seed germination of wheat was not affected by fluorene; however, the negative effects of phenanthrene (Wei et al., Citation2014) and fluoranthene (Tomar & Jajoo, Citation2014) on wheat seeds have been reported previously. Therefore, the effects of different PAHs compounds on the seed germination of plants can be completely different. Destruction of seed embryo by hydrocarbons can be an important reason for the prevention of seed germination (Reynoso-Cuevas et al., Citation2008).
In all studied species, growth parameters were significantly decreased by the higher concentrations of fluorene in soil, but the reduction rate was not similar in different plant species. Generally, sunflower and wheat plants were the most sensitive and most resistant species to fluorene contamination, respectively. The negative effects of PAHs contamination on the growth of different plant species have previously been reported in the literature (Alkio, Tabuchi, Wang, & Colón-Carmona, Citation2005; Muratova, Kapitonova, Chernyshova, & Turkovskaya, Citation2009; Smith et al., Citation2006). However, plants sensitivity to PAHs contamination can be different in species, even those belonging to the same family (Reynoso-Cuevas et al., Citation2008; Smith et al., Citation2006). The results of our study completely confirmed this finding.
According to the results of this study, in both seed germination and seedlings growth phase, sunflower, alfalfa, and wheat plants showed the lowest and highest resistance to the fluorene toxicity, respectively, with alfalfa being intermediate. Therefore, it can be concluded that there is a link between the higher sensitivity of seeds germination rate and seedlings growth reduction in hydrocarbon-contaminated soil. Such a link has been reported by Reynoso-Cuevas et al. (Citation2008), but Smith et al. (Citation2006) have shown that PAHs effects on seeds germination and the subsequent growth of seedlings can be completely different.
The higher concentrations of fluorene led to MDA accumulation in plants, but the accumulation rate was higher in roots, in comparison to the shoots. MDA accumulation due to PAHs toxicities in plants has been previously reported in Arabidopsis thaliana (Liu et al., Citation2008). MDA content is the biological marker for ROS accumulation and lipid peroxidation (Gunes, Pilbeam, & Inal, Citation2009), and it is usually used for the illustration of plants sensitivity to oxidative stress (Debiane et al., Citation2008). Thus, the results of this study clearly showed that fluorene could induce oxidative stress in plants. The lowest MDA content was recorded in sunflower plants treated by fluorene, but the highest reduction in seedling growth was also observed in this plant. Therefore, it can be calculated that MDA accumulation due to oxidative stress could not be a reliable marker for evaluation of the negative effect of fluorene on all plants species.
Except in sunflower, the total soluble protein content in plants treated with 100 mg g−1 of fluorene was significantly increased. Higher protein content could be an adaptive mechanism for plants resistance to PAHs, probably for the replacement of damaged proteins or the higher production of antioxidant enzymes. Hence, higher reduction in sunflower growth could be due to their low capability for protein production under fluorene toxicity.
In all studied species, higher concentrations of fluorene led to significantly lower CAT activity in the roots and shoots. POD activity was differently affected by fluorene, depending on species and plant organs. No significant changes in CAT activity in A. thaliana (Liu et al., Citation2008) and higher POD activity in alfalfa plants (Muratova et al., Citation2009) treated by PAHs have been previously reported in the literature. Induction of oxidative stress in plants is a known effect of PAHs (Liu et al., Citation2008; Pašková et al., Citation2006), and CAT and POD are important enzymes involved in the ROS detoxification and plants resistance to oxidative stress (Liang, Hu, Yang, & Yu, Citation2003). The results of this study clearly indicated that fluorene contamination led to MDA accumulation in plants, and there was a significant correlation (p ≤ 0.05) between the lower activity of CAT and MDA accumulation in plants root (r 2 = 0.48) and shoot (r 2 = 0.76). Therefore, MDA accumulation in the studied plants was a result of lower CAT activity and it could be concluded that CAT was the important antioxidant enzyme involved in ROS detoxifying and plants resistance to fluorene toxicity. Furthermore, there was no significant correlation between POD activity and MDA accumulation in the shoots (r 2 = 0.0016) and roots (r 2 = 0.0546) of plants treated with fluorene (p ≤ 0.05). Therefore, POD was not involved in ROS detoxifying and plants resistance to fluorene toxicity.
Photosynthetic pigments content in the studied species was changed differently depending on plant species. While, in sunflower, chlorophyll a and chlorophyll b content was increased significantly by fluorene treatment, but this plant was the most sensitive species to fluorene toxicity. Chlorophyll pigment content was decreased or unchanged in wheat plants treated by fluorene; however, this plant showed the highest resistance to fluorene toxicity. Accordingly, changes in photosynthetic pigments content cannot be regarded as an appropriate marker for the evaluation of plants sensitivity or resistance to PAHs contamination. However, Huang et al. (Citation2004) has reported that changes in photosynthetic pigment contents in response to PAHs contamination are related to species sensitivity. The lower photosynthetic pigment content in Arabidopsis thaliana plants treated by phenanthrene has been previously reported (Liu et al., Citation2008).
5. Conclusion
According to the results of this study, we can conclude that similar to other PAHs, fluorene was found to have a negative effect on the rate of seed germination and seedling growth of plants. Wheat was the most resistance plant in seed germination and seedling growth phases, and sunflower showed highest sensitivity in both phases. Thus, there was a link between better seed germination and the subsequent seedlings growth under PAHs contamination. Fluorene could induce oxidative stress and MDA accumulation in the plants, and there was a significant correlation between the lower activity of CAT and MDA accumulation in the plants. Therefore, CAT was an important enzyme in the detoxification of ROS in plants grown under PAHs contamination. Fluorene effects on photosynthetic pigment content were not similar in the studied plant as its effects are known to depend on plant species, the initial concentration of fluorene, and the pigment type.
Acknowledgments
The authors are thankful to Dr Hasan Jalali for careful reading of the manuscript.
Additional information
Funding
Notes on contributors
Seyed Yahya Salehi-Lisar
Seyed Yahya Salehi-Lisar is an academic member in department of plant sciences, University of Tabriz, Tabriz, Iran. Our group conducted some researches about PAHs uptake and accumulation by plants as well as plant physiological responses to PAHs toxicity. We submitted a manuscript on International Journal of Phytoremediation and one another manuscript on EurAsian Journal of Bioscience. This manuscript is third manuscript prepared using the data obtained from a project conducted in University of Tabriz.
References
- Alkio, M. , Tabuchi, T. M. , Wang, X. , & Colón-Carmona, A. (2005). Stress responses to polycyclic aromatic hydrocarbons in Arabidopsis include growth inhibition and hypersensitive response-like symptoms. Journal of Experimental Botany , 421 , 2983–2994. doi:10.1093/jxb/eri295
- Boominathan, R. , & Doran, P. M. (2002). Ni induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertoloni . New Phytologist , 156 , 202–205. doi:10.1046/j.1469-8137.2002.00506.x
- Bradford, M. M. (1976). A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry , 72 , 248–254. doi:10.1016/0003-2697(76)90527-3
- Chance, B. , & Maehly, A. C. (1955). Assay of catalases and peroxidases: Methods in enzymology . New York, NY: Academic Press.
- Chauhan, A. , Fazlurrahman , Oakeshott, J. G. & Jain, R. K. (2008). Bacterial metabolism of polycyclic aromatic hydrocarbons: Strategies for bioremediation. Indian Journal of Microbiology , 48 , 95–113. doi:10.1007/s12088-008-0010-9
- Debiane, D. , Garcon, G. , Verdin, A. , Fontaine, J. , Durand, R. , Grandmougin-Ferjani, A. , & Sahraoui, L. H. A. (2008). In vitro evaluation of the oxidative stress and genotoxic potentials of anthracene on mycorrhizal chicory roots. Environmental and Experimental Botany , 64 , 120–127. doi:10.1016/j.envexpbot.2008.04.003
- Eom, I. C. , Rast, C. , Veber, A. M. , & Vasseur, P. (2007). Ecotoxicity of a polycyclic aromatic hydrocarbon (PAH) contaminated soil. Ecotoxicology and Environmental Safety , 67 , 190–205. doi:10.1016/j.ecoenv.2006.12.020
- Gao, Y. , & Zhu, L. (2004). Plant uptake, accumulation and translocation of phenanthrene and pyrene in soils. Chemosphere , 55 , 1169–1178. doi:10.1016/j.chemosphere.2004.01.037
- Gunes, A. , Pilbeam, D. , & Inal, A. (2009). Effect of arsenic-phosphorus interaction on arsenic induced oxidative stress in chickpea plants. Plant and Soil , 314 , 211–220. doi:10.1007/s11104-008-9719-9
- Han, Y. , & Wang, Z. (2009). Solubility of fluorene in benzene, chloroform, acetone, 1-propanol, isobutanol, and methylbenzene from (283 to 323) K. Journal of Chemical & Engineering Data , 54 , 148–149. doi:10.1021/je800676s
- Hartmut, K. L. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In R. D. Lester Packer (Ed.), Methods in enzymology (pp. 350–382). New York, NY: Academic Press.
- Huang, X. D. , El-Alawi, Y. , Penrose, D. M. , Glick, B. R. , & Greenberg, B. M. (2004). Responses of three grass species to creosote during phytoremediation. Environmental Pollution , 130 , 453–463. doi:10.1016/j.envpol.2003.12.018
- Jaiswal, P. C. (2004). Soil, plant and water analysis . New Delhi: Kalyani Publishers.
- Jajoo, A. , Mekala, N. R. , Tomar, R. S. , Grieco, M. G. , Tikkanen, M. , & Aro, E. (2014). Inhibitory effects of polycyclic aromatic hydrocarbons (PAHs) on photosynthetic performance are not related to their aromaticity. Journal of Photochemistry and Photobiology B: Biology , 137 , 151–155. doi:10.1371/journal.pone.0068142
- Jan, F. , Khan, S. , Ishaq, M. , Naeem, M. , Ahmad, I. , & Hussain, S. (2014). Brick kiln exhaust as a source of polycyclic aromatic hydrocarbons (PAHs) in the surrounding soil and plants: A case study from the city of Peshawar. Pakistan. Arabian Journal of Geosciences , 7 , 13–19. doi:10.1007/s12517-013-0901-x
- Khan, S. , Aijun, L. , Zhang, S. , Hu, Q. , & Zhu, Y. (2008). Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. Journal of Hazardous Materials , 152 , 506–515. doi:10.1016/j.jhazmat.2007.07.014
- Khodadoust, A. P. , Bagchi, R. , Suidan, M. T. , Brenner, R. C. , & Sellers, N. G. (2000). Removal of PAHs from highly contaminated soils found at prior manufactured gas operations. Journal of Hazardous Materials , 80 , 159–174. doi:10.1016/S0304-3894(00)00286-7
- Kvesitadze, G. , Khatisashvili, G. , Sadunishvili, T. , & Ramsden, J. J. (2006). Biochemical mechanisms of detoxification in higher plants . Berlin: Springer.
- Lee, S. H. , Lee, W. S. , Lee, C. H. , & Kim, J. G. (2008). Degradation of phenanthrene and pyrene in rhizosphere of grasses and legumes. Journal of Hazardous Materials , 153 , 892–898. doi:10.1016/j.jhazmat.2007.09.041
- Li, F. , Zeng, X. , Yang, J. , Khou, K. , Zan, Q. , Lei, A. , & Tam, N. F. (2014). Contamination of polycyclic aromatic hydrocarbons (PAHs) in surface sediments and plants mangrove swamps in Shenzhen, China. Marine Pollution Bulletin , 85 , 590–596. doi:10.1016/j.marpolbul.2014.02.025
- Liang, Y. , Hu, F. , Yang, M. , & Yu, J. (2003). Antioxidative defenses and water deficit-induced oxidative damage in rice (Oryza sativa L.) growing on non-flooded paddy soils with ground mulching. Plant and Soil , 257 , 407–416. doi:10.1023/A:1027313902195
- Liu, H. , Weisman, D. , Yuan-bei, Y. , Cui, B. , Huang, Y. , Colón-Carmona, A. , & Wang, Z. (2008). An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana . Plant Science , 176 , 375–382. doi:10.1016/j.plantsci.2008.12.002
- Maila, M. P. , & Cloete, T. E. (2002). Germination of Lepidium sativum as a method to evaluate polycyclic aromatic hydrocarbons (PAHs) removal from contaminated soil. International Biodeterioration & Biodegradation , 50 , 107–113. doi:10.1016/S0964-8305(02)00059-8
- Mastral, A. M. , Callón, M. S. , López, J. M. , Murillo, R. , García, T. , & Navarro, M. V. (2003). Critical review on atmospheric PAH. Assessment of reported data in the Mediterranean basin. Fuel Processing Technology , 80 , 183–193. doi:10.1016/S0378-3820(02)00249-7
- Meudec, A. , Dussauze, J. , Deslandes, E. , & Poupart, N. (2006). Evidence for bioaccumulation of PAHs within internal shoot tissues by a halophytic plant artificially exposed to petroleum sediments. Chemosphere , 65 , 474–481. doi:10.1016/j.chemosphere.2006.01.058
- Mohan, S. V. , Kisa, T. , Ohkuma, T. , Kanaly, R. A. , & Shimizu, Y. (2006). Bioremediation technologies for treatment of PAH-contaminated soil and strategies to enhance process efficiency. Reviews in Environmental Science and Bio/Technology , 5 , 347–374. doi:10.1007/s11157-006-0004-1
- Mueller, K. E. , & Shann, J. R. (2006). PAH dissipation in spiked soil: Impacts of bioavailability, microbial activity, and trees. Chemosphere , 64 , 1006–1014. doi:10.1016/j.chemosphere.2005.12.051
- Muratova, A. Y. , Kapitonova, V. V. , Chernyshova, M. P. , & Turkovskaya, O. V. (2009). Enzymatic activity of alfalfa in a phenanthrene-contaminated environment. World Academy of Science, Engineering and Technology , 58 , 569–574. Retrieved from http://www.waset.org
- Nishigima, F. N. , Weber, R. R. , & B00EDcego, M. C. (2001). Aliphatic and aromatic hydrocarbons in sediments of Santos and Cananéia, SP, Brazil. Marine Pollution Bulletin , 42 , 1064–1072. doi:10.1016/S0025-326X(01)00072-8
- Obinger, C. , Maj, M. , Nicholls, P. , & Loewen, P. (1997). Activity, peroxide compound formation, and hemed synthesis in Escherichia coli HPII catalase. Archives of Biochemistry and Biophysics , 342 , 58–67. doi:10.1006/abbi.1997.9988
- Parrish, Z. D. , White, J. C. , Isleyen, M. , Gent, M. P. N. , Iannucci-Berger, W. , Eitzer, B. D. , & Mattina, M. I. (2006). Accumulation of weathered polycyclic aromatic hydrocarbons (PAHs) by plant and earthworm species. Chemosphere , 64 , 609–618. doi:10.1016/j.chemosphere.2005.11.003
- Pašková, V. , Hilscherová, K. , Feldmannová,, M. , Bláha, L. (2006). Toxic effects and oxidative stress in higher plants exposed to polycyclic aromatic hydrocarbons and their n-heterocyclic derivatives. Environmental Toxicology and Chemistry , 25 , 3238–3245. doi:10.1897/06-162R.1
- Reynoso-Cuevas, L. , Gallegos-Martı′neznez, M. E. , Cruz-Sosa, F. , & Gutie′rrez-Rojas, M. (2008). In vitro evaluation of germination and growth of five plant species on medium supplemented with hydrocarbons associated with contaminated soils. Bioresource Technology , 99 , 6379–6385. doi:10.1016/j.biortech.2007.11.074
- Singh, O. V. , & Jain, R. K. (2003). Phytoremediation of toxic aromatic pollutants from soil. Applied Microbiology and Biotechnology , 63 , 128–135. doi:10.1007/s00253-003-1425-1
- Smith, M. J. , Flowers, T. H. , Duncan, H. J. , & Alder, J. (2006). Effects of polycyclic aromatic hydrocarbons on germination and subsequent growth of grasses and legumes in freshly contaminated soil and soil with aged PAHs residues. Environmental Pollution , 141 , 519–525. doi:10.1016/j.envpol.2005.08.061
- Somtrakoon, K. , Chaimeungkoon, C. , Phalaphol, D. , & Chouychai, W. (2012). Co-phytotoxicity of fluorene and fluoranthene in phenanthrene and anthracene contaminated soil to seedling growth of poaceae and leguminosiae plant. Journal of Agricultural Research and Extension , 29 (1), 1–8. Retrieved from http://www.cabdirect.org
- Somtrakoon, K. , & Chuoychai, W. (2013). Phytotoxicity of single and combined polycyclic aromatic hydrocarbons towards economic crops. Russian Journal of Plant Physiology , 60 , 139–148. doi:10.1134/S1021443712060155
- Song, Y. F. , Jing, X. , Fleischmann, S. , & Wilke, B. M. (2002). Comparative study of extraction methods for the determination of PAHs from contaminated soils and sediments. Chemosphere , 48 , 993–1001. doi:10.1016/S0045-6535(02)00180-7
- Su, Y. H. , & Zhu, Y. G. (2008). Uptake of selected PAHs from contaminated soils by rice seedlings (Oryza sativa) and influence of rhizosphere on PAH distribution. Environmental Pollution , 155 , 359–365. doi:10.1016/j.envpol.2007.11.008
- Sun, K. , Liu, J. , Jin, L. , & Gao, Y. (2014). Utilizing pyrene-degrading endophytic bacteria to reduce the risk of plant pyrene contamination. Plant and Soil , 374 , 251–262. doi:10.1007/s11104-013-1875-x
- Tomar, R. P. , & Jajoo, A. (2014). Fluoranthene, a polycyclic aromatic hydrocarbon, inhibits light as well as dark reactions of photosynthesis in wheat (Triticum aestivum). Ecotoxicology and Environmental Safety , 109 , 110–115. doi:10.1016/j.ecoenv.2014.08.009
- Tomar, R. S. , & Jajoo, A. (2013). A quick investigation of the detrimental effects of environmental pollutant polycyclic aromatic hydrocarbon fluoranthene on the photosynthetic efficiency of wheat (Triticum aestivum). Ecotoxicology , 22 , 1313–1318. doi:10.1007/s10646-013-1118-1
- Watts, A. W. , Ballestero, T. P. , & Gardner, K. H. (2006). Uptake of polycyclic aromatic hydrocarbons (PAHs) in salt marsh plants Spartina alterniflora grown in contaminated sediments. Chemosphere , 62 , 1253–1260. doi:10.1016/j.chemosphere.2005.07.006
- Watts, W. A. , Ballestero, P. T. , & Gardner, H. K. (2008). Soil and atmospheric inputs to PAH concentrations in salt marsh plants. Water, Air, and Soil Pollution , 189 , 253–263. doi:10.1007/s11270-007-9572-0
- Wei, H. , Song, S. , Tian, H. , & Liu, T. (2014). Effects of phenanthrene on seed germination and some physiological activities of wheat seedling. Comptes Rendus Biologies , 337 , 95–100. doi:10.1016/j.crvi.2013.11.005
- Wilcke, W. (2000). Polycyclic aromatic hydrocarbons (PAHs) in soil—A review. Journal of Plant Nutrition and Soil Science , 163 , 229–248. doi:10.1002/1522-2624(200006)163:3<229
