?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was conducted to investigate the effect of storage temperatures on the shelf life of yellowfin tuna (YFT) loins by studying of microbial, chemical and organoleptic changes. Shelf life of YFT was determined through changes in total aerobic bacterial plate counts (APC), total volatile basic nitrogen (TVB-N) trimethylamine (TMA), organoleptic properties and histamine development during storage at 0, 4 and 7°C. Based on TVB-N value indices, YFT maintained an acceptable shelf life for 21, 17 and 12 days at 0, 4 and 7°C, respectively. Nevertheless, YFT was rejected earlier by the sensory panellists than their TVB-N value indicated. Histamine development was found to be lower than the European Union safety level for 100 mg/kg fish during storage at 0 and 7°C for 21 and 17 days, respectively. Aerobic bacteria initially dominated the micro-flora on YFT; however, as storage time increased, aerobic bacteria became dominant at cold storage, but the numbers exceeded the International Commission on Microbiological Specifications for Foods (ICMSF) limit of 107 cfu/g in storage at 7°C after 17 days. Therefore, it can be concluded that the storage of fresh YFT in below 4°C has good enough to the shelf life of products (two weeks).
Public Interest Statement
The yellofin tuna fish has a two week shelf life under domestic refrigeration conditions (< 4°C) without exceeding the values of chemical, microbiological standards.
1. Introduction
Yellowfin tuna (YFT) belongs to the family Scombridae and they are pelagic large size marine fish. YFT fisheries are of crucial importance to Sri Lanka due to their economic importance of fresh, frozen and sashimi trade, and are very popular and preferable among Sri Lankans. Further, YFT is one of the largest earners of foreign exchange in the fisheries sector. The annual commercial catch for YFT, in the Sri Lanka in 2011 and 2012, was 44,320 t and 42,780 t, respectively (MOFAR, Citation2013). The main YFT exports are fresh and frozen tuna (especially sashimi-grade tuna), and high prices are offered in the highly competitive and demanding sashimi markets in Japan. Chilled YFT, however, has problems regarding freshness due to increasing histamine and others with time or heavy metals. The Rapid Alert System for Food and Feed (RASFF) in the European Union (EU) rejected 10 cases of YFT from Sri Lanka due to the above-mentioned problem during the period of 2010–2013 (RASFF, Citation2010–2013).
The principle components of the YFT muscles are water, fat, protein, minerals and vitamin compounds. The protein content of YFT is around 15–20% and its composition varies with the time period. Fresh seafood has its own unique microflora and is characterized by a relatively short shelf life, and its spoilage caused by gram-negative aerobic bacteria such as Pseudomonas sp. and Shewanella sp. (Donna et al., Citation2007).
The crucial aim of fish quality control according to Domingo (Citation2007) is to find some methods by defining suitability or excellence of the fish for processing operation and it embraces essential composition, degree of contamination which disagreeable materials, nutritive values, degree of spoilage, storage, sale and presentation to the consumers. Thus, the freshness of the fish as a food is the most important attribute when assessing the quality, but loss of fish freshness is a complex combination of microbiological, chemical and physical degradation, and those are associated with fish quality during handling and storage (Eliseo et al., Citation2002). Therefore, they are included in the food group that should be considered the most important in terms of conservation from production to consumption. For this reason, they should be consumed soon after fishing or should be conserved by processing methods. Different methods are universally applied to estimate freshness and quality of different fish species, and most were based on the concept of bacterial action.
Biochemical methods based on production of trimethylamine (TMA), total volatile basic nitrogen (TVB-N) and biogenic amines have also commonly used to evaluate fish quality. Histamine is biogenic amines naturally present in various living organisms, and responsible for many physiological and pathophysiological functions. The high histamine level is most frequently present in the fish and fishery products especially in family Scombroidae fish and their accumulation is related to bacterial spoilage (Donna et al., Citation2007). Thus, mainly histamine, but also other biogenic amines, has been used as a marker to evaluate fish freshness. Histamine or scombroid poisoning is caused mainly by the consumption of the fish, fish products and fermented foods that are processed under poor hygiene and/or stored at elevated temperatures. This allows the growth of histamine-producing bacteria that may result in dangerous histamine levels (> 500 ppm). The symptoms of scombroid poisoning are urticaria, flushing, itching, vomiting, diarrhoea and nausea. Histamine poisoning is increasing internationally in countries including the USA, Great Britain, Japan and Taiwan (Maycock & Benford, Citation2007).
This study is therefore designed to determine the shelf life at cold storage of the YFT using some chemical, microbiological and organoleptic characters as indices, to established the limit of acceptability of the YFT and to determine if the length of storage as effects on the rate of deterioration.
2. Materials and methods
2.1. Preparation of the fish samples and storage conditions
Fish used for this study was taken from the five fish export factories in Sri Lanka. The samples go through their processing line, and are finally vacuum sealed and packed using the same procedures they followed for exporting fish. In total, 140 fish (28 fillets from each company) of an average weight of each fillet 250 g was selected for the study; it was received on 1 June 2013 and transported to Analytical Chemistry Laboratory, NARA with a temperature <4°C maintained during the transportation. On arriving at the laboratory, yellowfin tuna fillet temperature was below 3°C, and they were randomly divided into three lots of nine fish fillets for different treatments. The remaining fillets were immediately evaluated in the laboratory using chemical, microbiological and sensory techniques described later. Samples were stored at 7, 4 and 0°C. At 4 and 7°C, the samples were stored in the laboratory refrigerators, and for 0°C, the samples were packed in ice and again stored in the laboratory’s refrigerator. The temperatures in the sample storage were monitored using a data logger. Samples from each treatment were taken in the day 3, 5, 7, 9, 11, 15, 17, 19 and 21, and were used for analyses.
2.2. Temperature measurements
The temperature of each treatment was recorded morning and evening for each day, by temperature data loggers (Gemini Tinytag data loggers, West Sussex, UK) placed in the middle layer of yellowfin tuna fish fillets. Those loggers were used to measure temperature on fillet during experiment.
2.3. pH measurement
Sample pH was measured according to the method of laboratory manual of analytical methods and procedures for fish products (Marine Fisheries Research Department, Citation1992) by homogenizing 5 g of samples in 20 mL distilled water for 1 min at room temperature. pH was monitored using a digital pH metre (Hanna pH 211, USA).
2.4. Determination of histamine
Histamine was determined by reverse-phase HPLC in fish extract with derivation by O-phtalaldehyde (OPA) using the internal developed and validated method based on a training manual of Southeast Asian Fisheries Development Centre, 2006. The samples were prepared by blending 10 g of fish flesh in 20 mL of 10% trichloroacetic acid solution and 20 mL of distilled water for 30 s. Make up the final volume to 100 mL with distilled water and then filtered through a Whatman No. 1 filter paper after stand the 10 min. The 10 mL of filtered sample passes through the Amberlite CG-50 resin, and the fluorophore standards and samples were prepared by mixing 5 mL of column chromatography elute with 0.1% O-Phthaldialdehyde solution. For each injected batch, a set of five standards with a concentration of 0.5, 1.0, 1.5, 2.0 and 3 mg/mL of histamine were run at the beginning of injected samples, as well as a recovery sample. Duplicates of each sample were also analysed, and the concentration of histamine (mg/kg) was calculated by the following equation:
The high-performance liquid chromatography (HPLC) model Shimadzu, SIL 20A equipped with a quaternary pump and an online degasser model LC 20 AD and injection valve with a loop capacity of 20 μL was used. The detector used to be a programmable fluorescence detector RF10AXL with a 350 nm excitation, 450 nm emissions. The histamine compound was determined on reverse-phase ODS Hypersil (150 × 4.6 mm), C18 column. The separations were achieved with the mobile phase of 0.2 M NaCl: Methanol (20: 80, v/v, pH adjusted to 3.1) using a flow rate of 0.5 mL/min.
2.5. Determination of total volatile basic nitrogen (TVB-N) and trymethyl amines (TMA)
TVB-N and TMA were determined based on an adaptation of the current official European steam-distillation method (EU/EC, Citation1995; Malle & Poumeyrol, Citation1989). The method is based on the extraction of TVB using alkaline solution and the titration of the recovered ammonia is as follows: the fish was homogenized with a laboratory blender for 1 min and 100 g of fish was weighed and placed into the beaker. Then 200 mL of 7.5% trichloroacetic acid (TCA) was added and the extract was homogenized for 2 min. After that, the mixture was filtered using Whatman No. 2 filter paper to obtain a clear solution for analysis. Then, 25 mL of fish extract was placed in the distillation flask in VELP mark apparatus (model UDK-6, Milan, Italy). Then 30 mL of 10% NaOH solution was added, and the apparatus was immediately sealed and the end of the steam distillate was collected in a flask containing 25 mL of 4% boric acid and few drops of mixed indicator (methyl red/methylene blue 2:1). The steam distillation procedure was continued until 5 min and distillate had been collected. The obtained basic solution was titrated against 0.025 N H2SO4 to the endpoint that was indicated by colour change from a green to pink colour. Same procedure was followed to determine TMA after adding 10 mL of formaldehyde into 25 mL of fish extract.
2.6. Microbiological analysis
Aerobic plate count (APC) was followed in the Sri Lanka standard Institute (SLS) 516: PART 1:1991. Ten grams of the sample were weighed aseptically into a sterile stomacher bag, and 90 mL of diluents (maximum recovery diluents-Oxoid-UK) was added and blended in a stomacher blender for 1–2 min to make up the 10−1 dilution. Four dilutions were used starting from the 10−2 dilution for plating. One mL of 10−2 dilution was transferred to each of the two sterile petri plates using sterile pipettes. The same procedure was repeated with the other dilutions. About 15 mL of standard plate count agar (Oxoid) medium at 45 ± 0.5°C was poured into each petri plate, and mixed with the inoculums and allowed to solidify. Plates were incubated at 30°C for 72 h. After the incubation period, bacterial count was taken.
2.7. Sensory evaluation
Six semi-trained panellists were asked to evaluate the fish stored at three different temperatures. The panellists evaluated the fish using the Quantitative Descriptive Analysis (QDA) terms that are listed in Table according to the assessment scheme of the MATIS, Iceland with slight modifications. The intensity of the descriptive terms was rated using hedonic scales and each panellist will evaluate the samples in one session, each day of the sensory evaluation.
Table 1. The sensory assessment scheme for YFT loin
2.8. Quality control
Quality assurance procedures included the use of analytical grade reagents, method blanks, quality control samples and matrix spikes. The suitability of the analytical method was evaluated in terms of their respective limits of detection (LOD = mean blank + 3S), precision and recoveries using spiked samples. Standard quality control materials T2742QC & T25118QC from FAPAS (Food and Environment Research Agency, Sand Hutton, York, UK) were used for the quality control of the histamine analysis and TVB-N, while microbiological analysis was performed in the ISO 17025 accredited laboratory.
2.9. Statistical analysis
Data were analysed using the Statistical Package for Social Sciences (IBM SPPS Amos-21) Statistical Package. Descriptive statistics of means, standard deviation, one-way and two-way ANOVA, regression analysis, and correlation coefficient (Pearson’s) were applied in analysing the results. A significance level of 5% was used.
3. Results and discussion
The quality control materials and spiked samples were routinely analysed to perform the method validation procedure. The results of quality control samples were maintained within the range; histamine assign value = 30.3 mg/kg, received value = 26.3 ± 1.5, recovery = 88%, n = 6 and TVB-N assign value = 67.7 mg/100 g fish, received value = 65.9 ± 3.2, recovery = 97%, n = 6 (Figure ).
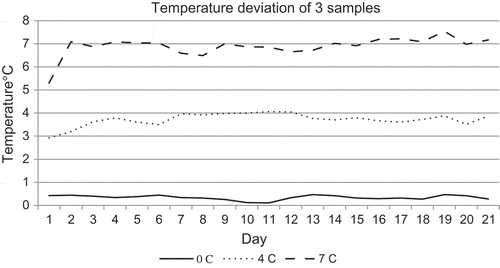
Even though the initial temperatures were maintained below 4°C at the receiving point, it has been changed with the storage trail purposes to 0, 4 and 7°C. Some time was taken to reach the high temperature condition (7°C), but thereafter the condition was maintained in required level.
3.1. pH evaluation
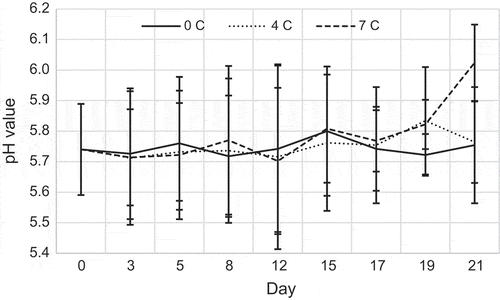
The changes in pH of three different storage temperatures are shown in Figure . The initial pH values of fresh samples were measured to be at 5.74 ± 0.15. This may be caused by high glycogen and it appears that the breakdown of glycogen to lactic acid changes the pH of fish after capture, especially in YFT. Figure revealed that 5.75 ± 0.19, 5.76 ± 0.13 and 6.02 ± 0.12 are the end pH values of storage temperatures 0, 4 and 7°C, respectively. The increase in pH values in the fish is mainly caused by the enzymatic degradation (Nikheel & Asif, Citation2013). The pH of the fish just after catching is reported between 6.0 and 6.5, in general, but the pH value is acceptable up to pH 6.8 and the pH of spoiled fish is above 7.0. However, some fish species might have alkaline meat just after catching. Therefore, the pH value should not be used as the only indicator for fish freshness. During the later postmortem changes, the pH value will be more or less constant or slightly increase due to the formation of basic compounds (Huss, Citation1988; Love, Citation1980; Shewan, Citation1977). Even though the changes in pH are generally rather small, they have great technological importance. The postmortem pH is, according to Huss (Citation1988), the most significant factor influencing the texture of the meat and the degree of “gaping”, i.e. the rupture of the connective tissue. One of the reasons for this is that even minor changes in pH drastically affect the properties of the connective tissue.
3.2. Histamine analysis
Generally, histamine production showed different patterns at the three different storage temperatures, which indicates that storage temperature may have an effect on histamine production (Figure ) and also fluctuations with the days were observed in all the storage temperatures. Histamine in YFT stored at 0°C was detected on day 0 with a level of 11 ± 3 mg/kg. However, a slow increase in the amount of histamine was observed throughout the storage period of 0 and 7°C, reaching a value of 21 ± 15 and 59 ± 44 mg/kg on day 21, which was below EU/EC standard. Histamine production was strongly suppressed at 0°C and no increase in the histamine concentration was observed up to day 8 and a reduction in the amount of histamine was observed at the end of the storage on day 15. Histamine formation occurred very quickly in YFT stored at 7°C and it was reached up to 136 ± 25 mg/kg in 21 days, but gradual increase was observed after day 5.
Figure 3. Changes in content of histamine in YFT during storage period (each point is the mean value of five determinations)
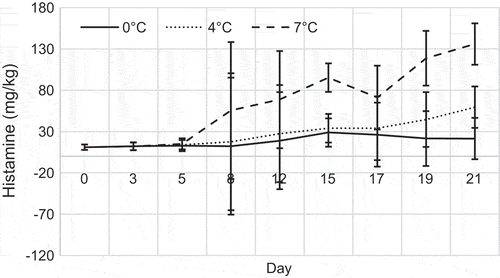
Histamine production is controlled when the temperature is maintained around 0°C or below 4°C. According to our results, the temperature of abuse is the most important factor in the histamine formation. However, the results showed that even at 4°C, has trended to reach the problematic level of histamine can be produced by the time. On the other hand, this study has shown that YFT subjected to storage at 7°C for a comparatively short period of time was yielded high level of histamine. As a result of this study, production of histamine showed different patterns at different temperatures; it can be concluded that the temperature has a strong effect of histamine formation. Researchers found that histamine production depends on histamine-forming bacteria, and histamine decomposition bacteria as well (Nejib, Moza, Ismail, Ann, & Mohammad, Citation2005). However, histamine-producing bacteria are mesophelic and at temperature 0 to −20°C, microorganisms are in a slowed down growth and cannot multiply and assault fish muscle (Hanane, Smail, & Mohamed, Citation2012). That is why, proper icing and cooling of fish can control the histamine production.
3.3. TVB-N and TMA analysis
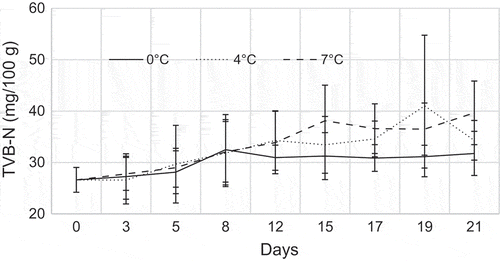
The initial TVB-N contents of YFT loins were 26.63 ± 2.41 mg/100 g which means that the fish is not of good quality and not very fresh fish (Figure ). The TVB-N content of the fish after 21 days were 31.77 ± 4.29, 34.32 ± 3.87 and 39.67 ± 6.18 mg/100 g fish for 0, 4 and 7°C, respectively.
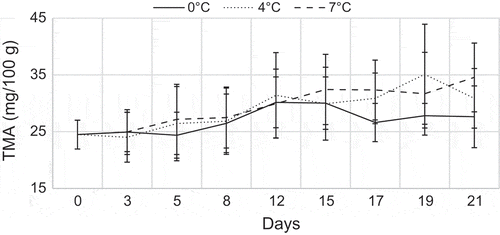
Total volatile basic nitrogen is a commonly used chemical method to determine spoilage of fish and especially TMA are associated with seafood spoilage (Nikheel & Asif, Citation2013, Yesim, Esmeray, Bahar, & Fatih, Citation2011). The spoilage is caused by micro-organisms; it is often detected with a fishy odour and this is due to the decomposition of trimethylamine oxide (TMAO) which is naturally present in the living tissue of many marine fish species. TMA can be used as a spoilage indicator since it appears after three or four days of storage (Figure ). The TVB-N values increased, according to time of storage temperature. (European-Commission, Citation1995; Gulsun, Esmeray, Serhat, & Fatih, Citation2009) reported that the quality classification of fish and fish products regarding TVB-N values would be “high quality” up to 25 mg/100 g, “good quality” up to 30 mg/100 g, “limit of acceptability” up to 35 mg/100 g and “spoilt” above 35 mg/100 g. In the present study, the initial TVB-N value 26.63 mg/100 g for YFT means that the fish does not belong to “high quality” category even at the initial stage. In the present study, since the TVB-N level showed fluctuations during the storage period, TVB-N could not provide a good index of common sole quality. Similar results were obtained for some fish species like common Sole (Solea solea), red mullet (Mullus barbatus) and gold band goatfish (Upeneus moluccensis) during storage in ice (Gulsun et al., Citation2009; Yesim et al., Citation2011).
3.4. APC determination
The APC number during storage of YFT at three different temperatures as 0, 4 and 7°C has been compared and is shown in Figure . The APC showed significant differences (p < 0.05) in relation to the storage time as well as the temperature of storage. At the time of storage, APC values in 7°C showed higher growth than the APC values in 0 and 4°C. On the other hand, even though the initial count of APC is same for the different temperatures, readings have increased with the duration of the storage trial.
Figure 6. Log values of APC for YFT stored at three different temperatures (each point is the mean value of five determinations)
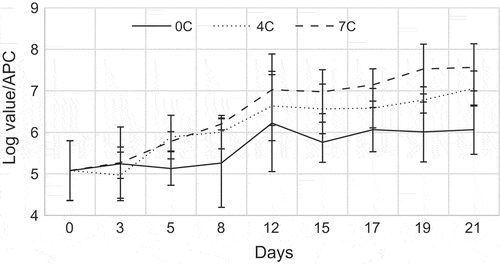
Bacteria can be found more or less in all fish, especially those that have been contaminated post catch (Widiastuti, Putro, Fardiaz, Trilakshani, & Inaoka, Citation2012). According to Kim et al. (Citation2002), the growth of histamine producing bacteria can be controlled with cold temperature (0–4°C). Changes in the compositions of micro-flora during cold storage agreed with the findings from some tropical country such as the Oman (Nejib et al., Citation2005).
3.5. Sensory evaluation
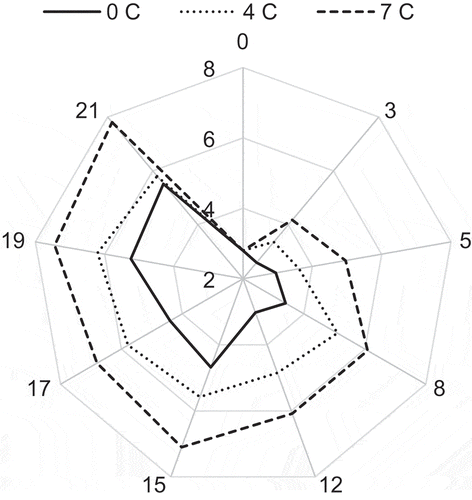
At atemperature (0°C), sensory evaluation of YFT indicates that the keeping time is higher than 14 days. Comparable times have been reported within 6 days for 4°C and 3 days for 7°C (Figure ). Upon storage, fish has developed rancid smell; in addition, unpleasant odour was developed with volatile base and bacterial growth (Hanane et al., Citation2012). The panellists rejected the fish very early even before exceeding the accepted values of the microbial and chemical values. Sensory quality decreased throughout storage at both temperatures, but the decrease was faster at 7 > 4 > 0°C. There were significant correlations between the level of histamine and sensory scores during the storage.
4. Conclusions
From the present study, it can be concluded that there is a variation between three different temperatures of YFT in regard to microbiological, chemical and sensory analysis during storage. The trial showed that high levels of histamine and APC are produced within a short period of time at 7°C and that the levels are much lower when the samples are stored at either 0 or 4°C. Therefore, the fish should be stored < 4°C, to prevent the formation of histamine during the shelf life of 14 days. The initial high level of TVBN value showed that the raw materials were not in the best quality, as well as sensory panellist accepted the 14 days of shelf life only for 0°C.
Cover image
Source: Author.
Acknowledgement
We would like to thank Ceylon Fresh Seafood (Pvt.) Ltd., Tropic Frozen Foods (Pvt.) Ltd, Jay Sea Food Processing (Pvt.) Ltd., Mrs. P.H. Ginigaddarage and Mrs. J.M. Chandrika Quality Control Laboratory, NARA for their constant assistance and contribution in sample collection and analysis during this project.
Additional information
Funding
Notes on contributors
B.K.K.K. Jinadasa
B.K.K.K. Jinadasa received the BSc (sp), MSc degrees and postgraduate diploma from the University of Ruhuna, University of Sri Jayewardenapura, Sri Lanka and United Nation University, Iceland, respectively. During 2005-up to date, he stayed in National Aquatic Resources Research and Development Agency (NARA), Sri Lanka as a senior scientist and technical manager of Analytical chemistry laboratory.
References
- Domingo, J. L. (2007). Omega-3 fatty acids and the benefits of fish consumption: Is all that glitters gold? Environment International , 33 , 993–998.10.1016/j.envint.2007.05.001
- Donna, M. , Henry, A. A. , Laurie, H. M. C. , Kathryn, R. M. , Michael, M. , Mineshi, S. , & Alan, H. S. (2007). Methylmercury exposure and health effects in humans: A worldwide concern. Ambio , 36 , 1–11.
- Eliseo, G. , Inmaculada, M. , Pieter, V. V. , Pieter, B. , Jorge, G. A. , Jeremy, D. K. , … Franse, J. K. (2002). Mercury, fish oils and the rsik of myocardial infraction. The New England Journal of Medicine , 347 , 1747–1754.
- EU/EC . (1995). 95/149 EC. Fixing the total volatile base nitrogen (TVB-N) limit values for certain categories of fishery products and specifying the analysis methods to be used. Official Journal of the European Communities , 84–87.
- European-Commission . (1995). Commission Regulation (EC), No 95/149 of 8th March 1995, fixing the total volatile basic nitrogen (TVB-N) limit values for certain categories of fishery products and specifying the analysis methods to be used. Official Journal of European Union , 97 , 84–87.
- Gulsun, O. , Esmeray, K. , Serhat, O. , & Fatih, O. (2009). Sensory, microbiological and chemical assessment of the freshness of red mullet (Mullus barbatus) and goldband goatfish (Upeneus moluccensis) during storage in ice. Food chemistry , 114 , 505–510.
- Hanane, O. , Smail, A.-M. S. , & Mohamed, E.-A. A.-A. (2012). Lipid oxidation and histamine production in Atlantic Mackerel (Scomber scombrus) versus time and mode of conservation. Journal of Life Sciences , 6 , 713–720.
- Huss, H. H. (1988). Fresh fish, quality and quality changes (FAO Fisheries Series No. 29). Rome: FAO.
- Kim, S. H. , Price, R. J. , Morrissey, M. T. , Filed, K. G. , Wei, C. I. , & An, H. (2002). Histamine production by Morganella morganii in mackerel, albacore, mahi-mahi and salmon at various storage temperature. Journal of Food Science , 64 , 340–343.
- Love, R. M. (1980). Biological factors affecting processing and utilization. In J. J. Connel (Ed.), Advances in fish science and technology . Farnham: Fishing New Books.
- Malle, P. , & Poumeyrol, M. (1989). A new chemical criterion for the quality control of fish; trimethyl amine/total volatile basic nitrogen (%). Journal of Food Protection , 52 , 419–423.
- Marine Fisheries Research Department . (1992). Laboratory manual on analytical methods and procedures for fish and fish products (2nd ed., pp. A3.1–A3.2). Singapore: Marine fisheries research department, Southeast Asian Fisheries Development Center.
- Maycock, B. J. , & Benford, D. J. (2007). Risk assessment of dietary exposure to methylmercury in fish in the UK. Human & Experimental Toxicology , 26 , 185–190.10.1177/0960327107070565
- Ministry of Fisheries and Aquatic Resources (MOFAR), fisheries statistics, Sri Lanka . (2013). Retrieved 10 April, 2014, from http://www.fisheries.gov.lk/doc_files/130625062842.pdf
- Nejib, G. , Moza, A. A. B. , Ismail, M. A. B. , Ann, M. , & Mohammad, S. R. (2005). The effect of storage temperature on histamine production and the freshness of yellowfin tuna (Thunnus albacares). Food Research International , 38 , 215–222.
- Nikheel, R. , & Asif, P. (2013). Biochemical and sensory quality changes of fish cutlets, made from Pangasius fish (Pangasianodon hypophthalmus), during storage in refrigerated display unit at −15 to −18°C. International Journal of Food, Agriculture and Veterinary Sciences , 3 , 1–8.
- Rapid alert System for Food and Feed (RASFF) . (2010–2013). Annual reports 2006–2013. European Union . Belgium: DG Sanco.
- Shewan, J. M. (1977). The bacteriology of fresh and spoiling fish and the biochemical changes induced by bacterial action. In Proceedings of the Conference on Handling, Processing and Marketing of Tropical Fish (pp. 51–66). Tropical Products Institute, London.
- Widiastuti, I. , Putro, S. , Fardiaz, D. , Trilakshani, W. , & Inaoka, T. (2012). Changes in freshness of steak and loin tuna (Thunnus albacares) during 15 days cold storage. Journal of Fisheries and Aquatic Science , 8 , 367–377.
- Yesim, O. , Esmeray, K. B. , Bahar, T. , & Fatih, O. (2011). Changes in biochemical, sensory and microbiological quality indices of Common Sole (Solea solea) from the Mediterranean sea, during ice storage. Turkish Journal of Fisheries and Aquatic Sciences , 11 , 243–251.