Abstract
The influence of plant growth promoting bacteria (PGPB) and cyanobacteria, alone and in combination, was investigated on micronutrient enrichment and yield in rice–wheat sequence, over a period of two years. Analysis of variance (ANOVA) in both crops indicated significant differences in soil dehydrogenase activity and micronutrient enrichment in grains (Fe, Zn in rice, and Cu, Mn in wheat). The combined inoculation of Anabaena oscillarioides CR3, Brevundimonas diminuta PR7, and Ochrobactrum anthropi PR10 (T6) significantly increased nitrogen, phosphorus, and potassium (NPK) content and improved rice yield by 21.2%, as compared to the application of recommended dose of NPK fertilizers (T2). The treatment T5 (Providencia sp. PR3 + B. diminuta PR7 + O. anthropi PR10) recorded an enhancement of 13–16% in Fe, Zn, Cu, and Mn concentrations, respectively, in rice grains. In wheat, Providencia sp. PW5 (T6) recorded the highest yield (5.23 Mg ha−1) and significantly higher enrichment of Fe and Cu (44–45%) in the grains. This study highlighted the promise of combinations of cyanobacteria/bacteria and their synergistic action in biofortification and providing savings of 40–60 kg N ha−1. Future focus needs to be towards integrating such promising environment-friendly and environmentally sustainable options in nutrient management strategies for this cropping sequence.
Public Interest Statement
The significance of the research work presented in the manuscript is that currently no microbial consortia have proved consistent in their performance for the rice–wheat cropping sequence. The present manuscript provides a critical evaluation of phototrophic–heterotrophic consortia in field level studies, over a period of two years, and their promise in sustaining soil health, improving soil fertility, and enhancing crop quality and productivity, besides providing savings of 20–40 kg N ha−1 season−1. The major findings also include the synergy in action of the developed consortia in terms of enrichment of micronutrients in grains in rice and wheat crops, over two consecutive years. Such consortia represent environment-friendly options for integration in breeding, nutrient management, and biofortification strategies for these staple crops.
Competing interests
The authors declare no competing interest.
1. Introduction
Micronutrients are gaining attention for their essential role in optimizing animal and human health, but the current trend of intensive cropping patterns is leading to decreasing crop yields and macro/micronutrient deficiencies in soil, grain/seed, and feedstock. This is a matter of serious concern in the global scenario (World Health Organization, Citation2011). The four most prominent micronutrient deficiencies of worldwide concern are those of iron, vitamin A, iodine, and zinc. Ideally, these essential nutrients should be obtained from a balanced diet, but a majority of people do not have access to a healthy diet, attributed to a variety of reasons, mainly socioeconomic and political. A deficiency of these elements over prolonged periods of time can lead to impaired metabolism and detrimental effects on physical growth and mental development (Singh, Citation2009).
Despite bumper harvests, the imbalanced use of macro- and micronutrient fertilizers, intensive farming, and the ineffective efforts towards recycling of crop residues in the past three decades have led to secondary and micronutrient deficiencies in the IGP (Indo-Gangetic Plains), especially where rice and wheat are grown as the major crops (Shukla, Sharma, & Tiwari, Citation2005; Singh, Citation2009; Singh, Manibhushan, Meena, & Upadhyaya, Citation2012; United States Agency for International Development, Citation2009). Rice and wheat are the major edible crops all over the world and by increasing their nutrient value, particularly in the edible parts; the problem of malnutrition can be solved to a great extent. The deficiency of zinc (Zn), iron (Fe), and manganese (Mn) in high yielding varieties of crops, particularly rice and wheat, are escalating the malnutrition problems in the developing countries (Bell & Del, Citation2008; Singh, Citation2009).
Worldwide, rice occupies almost 150 million ha and wheat is cultivated on almost 215 million ha. Research on biofortification of staple food crops (including rice, maize, wheat, cassava, pearl millet, beans, and sweet potato), primarily on iron, zinc, and vitamin A, has been supported by the HarvestPlus Challenge Program launched in 2004 (http://www.harvestplus.org/). Efforts are being undertaken for improving nutritional quality and yields through plant breeding and molecular marker-assisted strategies (Joshi, Chand, Arun, Singh, & Ortiz, Citation2007; Kamran, Kubota, Yang, Randhawa, & Spaner, Citation2014; Morgounov et al., Citation2007; Velu, Ortiz-Monasterio, Cakmak, Hao, & Singh, Citation2014; Zuo & Zhang, Citation2011). During the last couple of decades, a number of reports on the positive response to inoculation with plant growth promoting rhizobacteria (including cyanobacteria), accompanied by a significant increase in growth and yield of economically important cereal crops and improved soil fertility have been published (Adak et al., Citationin press; Jha et al., Citation2009; Mäder et al., Citation2011; Manjunath, Prasanna, Sharma, Nain, & Singh, Citation2011; Nain et al., Citation2010; Prasanna et al., Citation2013; Prasanna, Jaiswal et al., Citation2012; Rana et al., Citation2011; Thakuria et al., Citation2009). However, microbe-mediated biofortification has not been adopted as a strategy, except for a few scattered reports (Adak et al., Citationin press; Mäder et al., Citation2011; Prasanna et al., Citation2015; Prasanna, Joshi, Rana, Shivay, & Nain, Citation2012; Rana, Joshi, Prasanna, Shivay, & Nain, Citation2012; Rana, Saharan, Nain, Prasanna, & Shivay, Citation2012).
Plant growth promoting rhizobacteria (PGPR) are known to employ one or more direct and indirect mechanisms to improve plant growth and health, although the major mode of action of many PGPRs is facilitated through increasing the availability of nutrients in the rhizosphere (Glick, Citation1995). In the current scenario, cropping system mode of research is becoming extremely important. Therefore, the aim of the present study was to evaluate the performance of plant growth promoting bacterial and cyanobacterial inoculants (applied along with reduced doses of Nitrogen, phosphorus, and potassium (NPK)) in the rice–wheat cropping sequence, as environment-friendly options for enhancing crop productivity and micronutrient (Fe, Zn, Cu, and Mn) and macronutrient content (N, P, K).
2. Materials and methods
2.1. Site, experimental design, and management
The rice experiment was carried out during the summer rainy season (Kharif 2010, 2011 July–October). The wheat crop was sown in winter (Rabi) and harvesting undertaken in March–April 2010 and 2011. The research farm of the Indian Agricultural Research Institute, New Delhi (India) is located at latitude of 28°40′ N 77°12′ E and 228.6 meters above the mean sea level of Arabian Sea. The physicochemical and nutrient levels of the soil in the experimental field included pH and EC of soil which were 7.79 (1:2.5 soil and water ratio) and 0.4 mS cm−1, respectively. The other properties of soil measured before cultivation were as follows: Organic Carbon—0.53% (Walkley & Black, Citation1934), Available N—225 kg ha−1 (Subbiah & Asija, Citation1956), and Available P—13.45 kg ha−1 (Olsen, Cole, Watanabe, & Dean, Citation1954). The diethylene triamine penta acetate (DTPA)—extractable Fe, Zn, Cu, and Mn concentrations in the soil (mg kg−1) were 3.27, 0.84, 1.59, and 1.75, respectively. The meteorological data for the period are given as Supplementary Tables 1–3. The field experiments were laid out in a completely randomized block design (RBD) with three replications.
2.2. Plant growth promoting strains and their formulations
The selected bacterial strains: Providencia sp. PR3; Brevundimonas diminuta PR7; Ochrobactrum anthropi PR10; Bacillus pumilus PW1; Providencia sp. PW5; and Brevundimonas diminuta PW7, and the cyanobacterial strains: Anabaena laxa CR1; Anabaena azollae (Cyanobiont from Azolla sp.) CR2; A. oscillarioides CR3; A. laxa CW1; Calothrix crustacea CW2; and A. oscillarioides CW3, were tested for in vitro seed germination, and their plant growth promoting activities had been earlier evaluated under gnotobiotic and net house conditions in both rice and wheat crops (Nain et al., Citation2010; Prasanna et al., Citation2013; Prasanna, Joshi et al., Citation2012). These strains were inoculated alone and in combination in field experiments for two years (2010, 2011 for rice and 2009–2010, 2010–2011 for wheat).
The broth-based inocula of bacterial strains were prepared by growing the cultures in nutrient broth up to log phase under shaking conditions (150 rpm) at 28 ± 2°C. The selected cyanobacterial strains were grown in BG-11 medium and incubated under optimal conditions of light and temperature (27 ± 2°C and a light intensity of 52–55 μmol photon m2 s−1 and 16 h: 8 h Light: Dark cycles) for 14 d. Paddy straw compost was used as carrier material for the preparation of formulations. The physicochemical characteristics of the paddy straw compost used are: pH, 7.8; EC, 3.8 mS cm−1; Humus, 7.55%; C/N ratio, 15.66; Available P, 0.31%; C, 26%; N, 1.66%. The cyanobacterial strains were added at a rate of 20 μg chlorophyll g−1 carrier, whereas the population load of bacteria was ~ 1010 CFU g−1 carrier. The bacteria and cyanobacterial formulations were amended with 1% CMC (carboxymethyl cellulose, as a sticker), prior to the application on wheat seeds and rice seedlings. The rate of application was 500 g of formulation or carrier ha−1.
2.3. Agronomic practices and field layout
The varieties of rice and wheat used for the experiment were “Pusa Sugandh 5 (Pusa 2511)” and Pusa Gold (WR 544), respectively. The plot size was 4 × 3 m for each treatment. The recommended doses of chemical fertilizers for rice were: 120 kg ha−1 nitrogen (applied in two split doses of prilled urea) and the application of 60 kg ha−1 P2O5 and 60 kg ha−1 K2O, as single dose at the time of transplantation in the form of single super phosphate (SSP) and muriate of potash (MOP), respectively. Eight treatments were evaluated in rice crop, including selected combinations of plant growth-promoting (PGP) bacterial (PR3, PR7, and PR10) and cyanobacterial strains (CR1, CR2, and CR3). In wheat, the doses of recommended chemical fertilizers were: 120 kg ha−1 nitrogen (applied in two split doses of prilled urea) and the application of 60 kg ha−1 P2O5 and 60 kg ha−1 K2O, as a single dose at the time of sowing in the form of SSP and MOP, respectively. Three PGP bacterial strains (PW1, PW5, and PW7) and three cyanobacterial strains (CW1, CW2, and CW3), individually and in combination, with a total of nine treatments, were evaluated in wheat crop. The treatments and fertilizer doses were selected on the basis of our previous studies (Manjunath et al., Citation2011; Nain et al., Citation2010; Prasanna et al., Citation2013; Prasanna, Joshi et al., Citation2012; Rana et al., Citation2011). All the treatments receiving microbial inoculants are known to be nitrogen fixers, therefore the dose of N fertilizer applied was reduced (Manjunath et al., Citation2011; Nain et al., Citation2010; Rana et al., Citation2011). Hence, in rice, the treatments receiving bacterial and cyanobacterial inoculants were supplemented with two-third of the recommended dose of N along with full doses of P and K fertilizers, whereas in wheat crop, the microbial treatments were supplemented with half dose of N and full dose of P and K fertilizers. An absolute control treatment, only 500 g ha−1 carrier (paddy straw compost) was used, without any chemical fertilizers, as one of the treatments (T1). All experiments were conducted in the same fixed plots, in three replicates, as a RBD. The details of the treatments are given in Table .
Table 1. Details of treatments containing combinations of fertilizers, bacterial, and cyanobacterial strains
Seedlings of rice (25 d old) were transplanted in the first fortnight of July, whereas wheat seeds were sown in the first week of December in both the years, respectively. The rice and wheat plants were harvested after the grain maturation stage, generally after 120 and 140 d respectively.
2.4. Soil microbiological variables
Soil samples were taken by auger from each plot at harvest stage. All samples were taken from the area close to the root rhizosphere. A set of three to five soil cores (5 cm diameter, 0–15 cm depth) were taken from each replicate plot. The soil samples from each replicate were mixed and placed in polyethylene bags and transported to the laboratory. The soil samples were thoroughly mixed and sieved (2 mm mesh) and visible plant material was removed manually. The samples were stored at 4°C for microbiological analyses.
Microbial activity was expressed in terms of fluorescein diacetate (FDA) hydrolysis. The fluorescein released from FDA was measured at 490 nm using a fluorescein standard (Green, Stott, & Diack, Citation2006). The activity was expressed as μg fluorescein released g soil−1 h−1. Dehydrogenase activity was assayed using the method of Casida, Klein, and Santoro (Citation1964). The values were expressed as μg of triphenyl formazan released g soil−1 d−1.
2.5. Grain yield and harvest index
After harvest, the plants were threshed using the pullman thresher. Grains were cleaned by tractor drawn winnower and the yield of rice and wheat was recorded at 14% moisture. Straw yield was obtained by subtracting grain yield from the total biomass. Yield was expressed in Mg ha−1. Harvest index (HI) of cereal crops was calculated as the ratio of grain and total biomass yield (Grain yield/Total biomass).
2.6. Analyses of micronutrients and NPK contents in rice and wheat grains
Rice and wheat grains were ground and digested with di-acid mixture (nitric acid and perchloric acid). The digested samples were used for analysis of micronutrient concentrations (Fe, Zn, Cu, and Mn) using an Atomic Absorption Spectrophotometer at the most sensitive wavelengths for Fe (248.7 nm), Zn (213.7 nm), Cu (324.6 nm), and Mn (279.5 nm) (Lindsay & Norvell, Citation1978). Potassium content was analyzed with a Flame photometer and compared with standard values. Phosphorus content was measured by the method of Jackson (Jackson, Citation1967). The total nitrogen of the grain samples was estimated by Kjeldahl’s method and the percent nitrogen content in the samples was recorded using N-autoanalyzer (Jackson, Citation1967).
2.7. Statistical analysis
The data of the various parameters were analyzed in triplicates and subjected to ANOVA (Analysis of variance) using WINDOWSTAT 8.0 statistical package in accordance with the experimental design (complete RBD) to evaluate and quantify the source of variation. The treatment means were compared at 5% level of significance and the ranking of treatments denoted by letters using the SPSS-16 statistical package. Path coefficient analysis was undertaken using the procedure outlined by Singh and Chaudhry (Citation1979).
3. Results
3.1. Variability in soil and plant parameters
Analysis of variance (ANOVA) indicated significant variation among the microbial treatments and years, in terms of dehydrogenase activity in soil and Fe/Zn concentration in grains in rice crop (Table ). In wheat crop, significant variation among the microbial treatments and years was recorded in terms of dehydrogenase activity in soil and Cu/Mn concentration in grains. In particular, microbial inoculants were significantly different for Fe concentration in wheat, while years were significantly different for dehydrogenase activity in soil for the wheat crop. However, microbial inoculants × year interactions were not significant for both crops (Table ).
Table 2. Analysis of variance (ANOVA) for parameters, as influenced by the bacterial and cyanobacterial treatments in rice and wheat
3.2. Soil microbiological variables
At the time of harvesting of rice, higher activities of microbial parameters (FDA hydrolysis and dehydrogenase activity) were recorded in all the treatments that involved inoculation of cyanobacteria or bacterial strains or their combinations. In both years, significantly higher values of FDA hydrolase and dehydrogenase activity were observed in the treatment T6 (N90P60K60 + CR3 + PR7 + PR10) in rice crop (Figure (a) and (b); p < 0.05). Both these microbiological parameters exhibited a significant correlation with one another (r = 0.564, 0.523; p < 0.05). In wheat, the treatment T6, receiving only bacterial strain PW5, recorded significantly higher values of FDA hydrolysis and dehydrogenase activity in the first year and in the second year, the values were higher than the fertilizer controls (T2–T4) (Figure (a) and (b); p < 0.05).
Figure 1. Influence of inoculation of bacteria/cyanobacteria, individually and in combination on soil microbiological parameters at harvest stage of rice (2010, 2011). (a) FDA and (b) Dehydrogenase activity
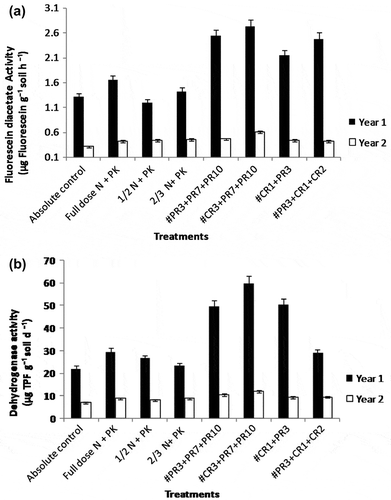
Figure 2. Effect of plant growth promoting bacterial and cyanobacterial inoculants, individually and in combination on soil microbiological parameters at harvest stage of wheat (2009–2010; 2010–2011). (a) FDA and (b) Dehydrogenase activity
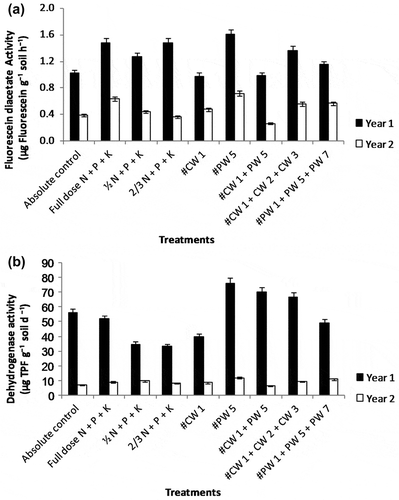
3.3. Grain yield and harvest index
Grain yield of rice ranged from 6.26 to 7.88 Mg ha−1. The significantly higher yield of rice was recorded in the treatment T6 (N90P60K60 + CR3 + PR7 + PR10) as compared to the fertilizer controls (T2–T4), (Table ; p < 0.05). Wheat grain yields ranged from 3.25 to 5.23 Mg ha−1. The treatment T6 (N60P60K60 + PW5) recorded highest values (Table ; p < 0.05).
Table 3. Effect of plant growth promoting bacterial and cyanobacterial strains individually and in combination on the HI and yield parameters of rice and wheat (mean of two year trials)
The highest values of HI (50.11%) of rice were recorded in the treatment T6 (N90P60K60 + CR3 + PR7 + PR10), however, no significant differences were found among all other treatments. In wheat (Table ), the treatment T6 (PW5) showed the highest HI (39.40%; p < 0.05), which were significantly higher than the control treatments.
3.4. NPK content in rice and wheat grains
The data illustrated in Table clearly indicate that the NPK content enhanced significantly in rice and wheat grains, in treatment T6 which were inoculated with specific bacterial and cyanobacterial strains, when applied with N90P60K60 (for rice) and N60P60K60 (for wheat) as compared to RDF. In rice grains, significantly higher N content (1.73%) was recorded in the treatment T6 (N90P60K60 + CR3 + PR7 + PR10). Phosphorus and potassium content was also increased in rice grain in the same treatment T6 (N90P60K60 + CR3 + PR7 + PR10). Higher values were recorded in the rice grains treated with CR3 + PR7 + PR10 along with N90P60K60 (T6) during both years (Table ; p < 0.05). The NPK content of rice was significantly higher in treatment T6 as compared to fertilizer’s controls (T1–T4). The N content in rice grains revealed a high correlation with P and K content (r = 0.740 and r = 0.667, respectively; p < 0.01).
Table 4. Effect of plant growth promoting strains of bacteria and cyanobacteria alone and in combination on nitrogen (N), phosphorus (P), and potassium (K) content in rice and wheat grains (mean of two year trials)
The wheat plants treated with Providencia sp. PW5 along with N60P60K60 were found to have higher N (2.29%) and K (1.28%) content in grains. Phosphorus content (1.15%) was enhanced in the plots treated with bacterial strains PW1, PW5, and PW7 (T9) (Table ; p < 0.05).
3.5. Micronutrient (Fe, Zn, Cu, and Mn) concentration in rice and wheat grains
The grains of rice and wheat were analyzed for micronutrients and details are given in Tables and . The micronutrient concentrations of Fe, (176.25 mg kg−1), Zn (36.62 mg kg−1), Cu (6.07 mg kg−1), and Mn (25.93 mg kg−1) were significantly higher in the grains harvested from plots treated with N90P60K60 + PR3 + PR7 + PR10 (T5), but Mn concentration was significantly at par with treatment T8. Correlation analyses of the two years data showed that in rice grains, all the micronutrients tested in the present investigation were significantly correlated with one another and with N, P, and K content of grains (r = 0.50–0.80 at p < 0.01; p < 0.05%).
Table 5. Effect of PGP microbial inoculants, individually and in combination on micronutrient concentration (mg kg−1) in rice grains (mean of two year trials)
Table 6. Effect of PGP microbial inoculants, individually and in combination on micronutrient concentration (mg kg−1) in wheat grains (mean of two year trials)
During the cycle of two years of the wheat experiment, a higher concentration of Fe (233.85 mg kg−1), Cu (67.16 mg kg−1), and Mn (37.88 mg kg−1) was measured in grains treated with N60P60K60 + PW5 (T6). In wheat crop, the highest Cu concentration was recorded in the treatments N60P60K60 + PW5 (T6) and N60P60K60 + PW5 + CW1 (T7). Mn concentration was significantly higher in treatment T6 N60P60K60 + PW5-T6 (37.88 mg kg−1) and N60P60K60 + PW1 + PW5 + PW7-T9 (41.15 mg kg−1). The two years data of wheat revealed that Fe concentration were significantly correlated with Cu (r = 0.666; p < 0.01) and Mn (r = 0.734; p < 0.01). Additionally, a significant correlation (r = 0.565; p < 0.01) was also found between Cu and Mn concentrations.
3.6. Path coefficient analyses
In rice crop, path coefficient analyses revealed that very few traits had a positive direct effect on grain yield of rice. Grain P content and Mn concentration had the highest direct effect on grain yield. Grain P also showed a high positive correlation with yield. The Cu, Zn, and Mn concentrations in grains had the highest direct effect and also exhibited a high concentration with the grain Fe concentration. A similar trend was recorded in wheat crop and Cu concentration had the highest direct effect and positive correlation with grain yield. The Cu and Mn concentrations in grains had the highest direct effect and exhibited a high correlation with the grain Fe concentration.
A comparative assessment of the influence of microbial inoculation vis-a-vis fertilizer controls treatments in terms of different variables revealed that T6 (N60P60K60 + PW5) and T9 (N60P60K60 + PW1 + PW5 + PW7) were the top ranked treatments for wheat crop in both years. In case of rice crop, T5 (N90P60K60 + PR3 + PR7 + PR10) and T6 (N90P60K60 + CR3 + PR7 + PR10) appeared the most number of times among the top three ranks for all the variables tested.
4. Discussion
Micro-organisms represent key resources in sustainable agriculture as they play a very important role in the biogeochemical cycling of C, N, P, and other macro/micronutrients (Caldwell, Citation2005). The influence of microbes has been most commonly attributed to the production of plant hormones such as auxins, gibberellins, and cytokinins, or through the supply of biologically fixed nitrogen, or increase in the mineral concentration, as a result of enhancement in root growth and development, leading to more efficient mobilization of nutrients. PGPR can also regulate the proliferation of deleterious microbes through the production of antibiotics, lytic enzymes, hydrogen cyanide, and siderophores or through competition for nutrients and space (Antoun & Kloepper, Citation2001).
Rice (Oryza sativa L.) and wheat (Triticum aestivum) represent the most prominent food crops and the staple diet for almost half of the world’s population. The rice–wheat cropping system is the mainstay of the IGP (Indo-Gangetic Plains), and intensive agricultural practices have led to depleting fertility and crop productivity. Biofortification of crops or their products have been suggested as cost-effective options to improve the nutritional quality and reduce malnutrition, particularly in developing countries (Ortiz-Monasterio et al., Citation2007; Zhu et al., Citation2012). Apart from breeding programs worldwide (Bouis & Welch, Citation2010; Kamran et al., Citation2014; Morgounov et al., Citation2007), including the Harvest Plus, biofortification of crops with micronutrients can also be achieved through microbial inoculants, organic practices, and proper soil management practices (Adak et al., Citationin press; Pooniya, Shivay, Rana, Nain, & Prasanna, Citation2012; Prasanna et al., Citation2015; Rana, Joshi et al., Citation2012; Rana, Saharan et al., Citation2012). Despite the increasing emphasis on identifying suitable organic inputs in agriculture, no efforts to understand microbe-mediated biofortification in cropping sequence mode have been made. Hence, the present study was undertaken to address this issue, in one of the widely employed cropping sequence-rice, followed by wheat, over a period of two years to evaluate the influence of plant growth promoting rhizobacteria/cyanobacteria on yield and biofortification.
The inoculation of selected PGP bacterial and cyanobacterial strains led to higher grain yields, harvest indices and content of N, P, K, Fe, Zn, Cu, and Mn in the grains in a field study with rice and wheat, over a period of two years. A significant increase of 21.2% and 16.3% in the grain yield of rice and wheat crop, respectively, over the fertilizer control (Full dose of NPK; T2), was recorded with microbial treatments. In rice crop, the treatments T5 (N90P60K60 + PR3 + PR7 + PR10) and T6 (N90P60K60 + CR3 + PR7 + PR10) performed better. In wheat crop, the treatment T6 (N60P60K60 + PW5) was found promising in terms of grain yield. Application of PGPR, singly or as mixture is known to augment grain yield, ranging from 29 to 36% in wheat (Mäder et al., Citation2011; Rosas et al., Citation2009), 20–27% in rice (Adak et al., Citationin press; Jha et al., Citation2009) or two–four folds enhancement in Phaseolus vulgaris (Zafar et al., Citation2011) or 15–20% through the use of cyanobacterial inoculants in maize (Prasanna et al., Citation2015). Furthermore, under poor fertility conditions, PGPR along with AMF are known to improve plant growth and grain yield of rice, wheat, and other crops most efficiently (Jat & Ahlawat, Citation2006; Secilia & Bagyaraj, Citation1994). Eleiwa, Eman, Hamed, and Shehata (Citation2012) reported a similar trend by inoculation with individual cultures of Azospirillum brasilense, Azotobacter chroococcum , or Bacillus polymyxa. HI is a quality index for measuring the yield potential of the crop. In the present investigation, the treatments involving bacterial/cyanobacterial inoculation recorded a higher HI (30.20–42.86%) in both years. This is reflective of the efficiency of the micro-organisms in promoting plant growth, biomass and thereby yields due to effective nutrient mobilization from soil to plant. Our earlier publications (Adak et al., Citationin press; Manjunath et al., Citation2011; Nain et al., Citation2010; Prasanna et al., Citation2013, Citation2015; Prasanna, Jaiswal et al., Citation2012; Prasanna, Joshi et al., Citation2012; Rana, Joshi et al., Citation2012; Rana et al., Citation2011; Rana, Saharan et al., Citation2012) have illustrated a similar trend. It can therefore be surmised that the PGP traits of the micro-organisms used and their colonization in the rhizosphere lead to the enhanced yields and nutrient quality of grains.
FDA hydrolysis is an important enzymatic activity of ubiquitous free and membrane-bound hydrolytic enzymes, such as lipase, protease, and esterase enzymes produced by soil microbes, which is directly proportional to the total population of microbial cells (Green et al., Citation2006). Dehydrogenase activity is also known to increase markedly with an increase in the population of active viable/living cells (Casida et al., Citation1964). An increase in activity was recorded in the selected microbial treatments (as compared to the control treatments in both rice and wheat). These findings are supported by our earlier investigations (Rana, Joshi et al., Citation2012; Rana, Saharan et al., Citation2012) and observations of Li et al. (Citation2009). The pronounced increase in viable cells, can lead to greater activity and mobilization of micro- and macro-nutrients, and enhanced root length or volume. PGPR are known to promote root elongation and surface area, which helps in better adaptation, and mobilization of nutrients from the soil to the plant (Antoun & Kloepper, Citation2001; Glick, Citation1995; Rosas et al., Citation2009).
Iron is among the third most limiting nutrient for plant growth, primarily due to the low solubility of the oxidized ferric form in aerobic environments (Samaranayake, Peiris, & Dssanayake, Citation2012; Zuo & Zhang, Citation2011). The World Health Organization (WHO) has estimated that nearly 3.7 billion people are iron deficient, with 2 billion, among these being anemic. The daily recommended iron intake for human beings ranges between 8 and 18 mg d−1 depending upon age and gender, with recommended dose of 30 mg d−1 for pregnant women (Institute of Medicine report, Citation2001). Rice is also considerably deficient in iron (Bouis & Welch, Citation2010). Low molecular weight organic acids (LMWOAs), such as DMA (deoxymugenoic acid), have been implicated in the acquisition of several nutrients, especially P and Zn in rice (Rose et al., Citation2013), with increased exudation being correlated with nutrient deficiency conditions. The role of this ligand in increasing Fe and Zn content in rice is known, however, lines overexpressing this gene have not been identified (Bashir, Nozoye, Ishimaru, Nakanishi, & Nishizawa, Citation2013). It was proposed that regulating rice genes involved in the synthesis of subcellular Fe transporters may prove beneficial in developing plants with increased efficiency. Our results clearly established that the Fe concentration in rice and wheat grains showed a significant increase in the bacteria/cyanobacteria inoculated treatments (T5, T6, T8 in rice and T5, T6, T7, T9 in wheat) in both years. Fe concentration in rice grains increased from 3.93 to 13.63%, over the fertilizer control (T2), in wheat grains by 44.62% after inoculation with Providencia sp. PW5 over the fertilizer control (T2). One of the properties of PGPRs relevant in this context is the production of siderophores which sequester iron from the soil and make it available to the plants (Schwyn & Neilands, Citation1987). The production of siderophores by the bacterial strains has been reported in our earlier studies (Rana, Joshi et al., Citation2012; Rana et al., Citation2011; Rana, Saharan et al., Citation2012), which can be responsible for the higher iron concentration in the root zone of the plants, leading to increased root activity and higher uptake of Fe in the plants, including translocation into the grains. Mishra et al. (Citation2011) also reported that the iron concentration doubled in lentil seeds, when grown in association with Pseudomonas species. de Santiago, Quintero, Avilés, and Delgado (Citation2011) found a 1.5-fold—enhancement in the Fe concentration in the aerial parts of wheat when treated with a siderophore producing strain Trichoderma asperellum. Sharma, Shankhdhar, and Shankhdhar (Citation2013) found that the translocation efficiency of iron from roots to grains was twofold higher in the treatments involving the inoculation of PGP bacterial strains—P. putida, P. fluorescens, and Azospirillum lipoferum.
Zinc (Zn) deficiency, similar to iron, is known to affect billions of people, weakening the immune system, and hampering growth and development. A positive, frequently close, relationship between the grain concentrations of protein, Zn, and Fe has been found in plant species such as wheat (Morgounov et al., Citation2007). Zn deficiency is ranked as the fifth most important risk factor responsible for illness and death in the developing world (WHO, Citation2011). In our study, Zn concentration was found to be higher (40.57 mg kg−1) in the wheat grains from plants treated with N60P60K60 + PW1 + PW5 + PW7. Moreover, the zinc concentration in the wheat grains increased from 0.70% to 7.26%, over the control (N120P60K60), by the end of the two year cycle. In rice grains, Zn and Mn concentrations improved (13.56% and 16.04%) in the treatment N90P60K60 + PR3 + PR7 + PR10. Similarly, Mäder et al. (Citation2011) found a substantial increase in Zn and Mn concentrations through the use of natural Arbuscular Mycorrhizal Fungi (AMF) consortium as well as with combined inoculation of two Pseudomonas strains. Tariq, Hameed, Malik, and Hafeez (Citation2007) demonstrated the efficiency of a commercial mixed PGPR consortium (containing Pseudomonas sp. and other strains of PGPR) acting as Zn solubilizer and increasing Zn concentration up to 157%. Pooniya et al. (Citation2012) recorded higher grain yield (3.79 tonnes ha−1) as well as total content of N, P, K and Zn concentrations in rice and also soil biological properties, through combined application of summer green manuring residues (Sesbania aculeata) and 2.0% Zn-enriched urea (ZEU as ZnSO4·H2O). In an earlier study, cyanobacterial inoculants brought about an enhancement in the mobilization of Zn to flag leaf in maize hybrids, without any negative effects on plant vigor and yields (Prasanna et al., Citation2015).
Nitrogen (N) nutrition status of plant can have a positive impact on the content and translocation of Fe and Zn in plants. The PGPRs used in this study are capable of fixing atmospheric nitrogen (Prasanna, Jaiswal et al., Citation2012; Prasanna, Joshi et al., Citation2012; Rana et al., Citation2011). During both the years, higher values of N, P, and K content in rice and wheat grains were recorded, as compared to control. Increased nutrient content in wheat with the application of PGPRs was reported earlier by Roesti et al. (Citation2006). Mutualistic root micro-organisms such as AMF and PGPR possess a high potential to improve the plant nutrient content, especially when they are applied in combination (Artursson, Finlay, & Jansson, Citation2006). A number of reports on this aspect are available in wheat and other crops, with respect to increased nutrient content (Roesti et al., Citation2006; Rosas et al., Citation2009; Selvakumar, Kundu, Gupta, Shouche, & Gupta, Citation2008). Adak et al. (Citationin press) observed 13–46% enhancement of iron and 15–41% enhancement of zinc in rice grains through the use of cyanobacterial inoculants, under different modes of rice cultivation. Several cyanobacteria, including unicellular marine forms possess the ability to sequester Fe or Zn using metallothioneins (Barnett et al., Citation2012), and exhibit several systems for homeostasis. Mäder et al. (Citation2011) also found that the mineral content (P, K, Cu, Fe, Zn, and Mn) in wheat grains was higher with single inoculation of AMF and PGPR or their combination. In our study, N content in wheat grain exhibited a positive correlation (r = 0.505; p < 0.01) with the Fe concentration. The N content was strongly correlated with other macronutrients P (r = 0.74; p < 0.01), K (r = 0.667; p < 0.01) and micronutrients Fe (r = 0.870; p < 0.01), Zn (r = 0.742; p < 0.01), Cu (r = 0.76; p < 0.01) and Mn (r = 0.741; p < 0.01) concentrations of rice grains. The P-solubilization ability of micro-organisms is one of the most important traits associated with plant P nutrition (Chen et al., Citation2006). Rice grain concentrations of Fe (r = 0.785%; p < 0.01), Zn (r = 0.793; p < 0.01), Cu (r = 0.68; p < 0.01), and Mn (r = 0.492; p < 0.05) correlated positively with P contents. In wheat, P content was positively correlated only to Fe (r = 0.559; p < 0.01) and Cu (r = 0.616; p < 0.01). Several bacterial species in the plant rhizosphere are capable of increasing availability of phosphorus to plants, either by mineralization of organic phosphate, or by solubilization of inorganic phosphate by production of acids (Rodrı́guez & Fraga, Citation1999). The strains used in this study possess P-solubilization activity (Rana et al., Citation2011) and interestingly, N content in rice was positively correlated with P (r = 0.740; p < 0.01) and K (r = 0.667; p < 0.01).
Plants are selective in the types of bacterial associations they develop and promote, and recruit those that are beneficial for their growth. Such interactions not only influence growth and development of plants, by altering nutrient uptake dynamics and susceptibility to pathogens, but also help in developing a “core rhizosphere microbiome” (Hirsch & Mauchline, Citation2012). Our study illustrates that the cyanobacteria-bacteria combinations used in the present field experiments for enhancing the yield of rice and wheat, and micronutrient concentrations in grains may represent members of the beneficial microbiome for these two crops. The increased mobilization of nutrients in the soil facilitates uptake by plants and enhances root growth and plant biomass and yield.
This is the first report from a rice–wheat cropping sequence based study, in which biofortification of Zn, Fe, Mn, and Cu with combined inoculation of bacterial and cyanobacterial strains has been recorded. Such synergistic microbial combinations can provide yield benefits and 40–60 kg N ha−1 savings (applied as urea), besides biofortification of grains in an environment-friendly manner. The N savings can be equivalent to 924–1,386 USD, based on conservative estimates of area under rice and wheat crops in India and China.
Our study represents a novel report interrelating microbial activity, micro/macro nutrient dynamics in plant and yield characteristics. In both years of the rice experiment, a positive correlation (r = 0.515–0.565; p < 0.01) was observed between FDA hydrolysis and dehydrogenase activity with micro/macro nutrient content of the grains; signifying the role of microbes in mobilizing nutrients, through their metabolic activities. This emphasizes the need for their inclusion as sustainable inputs in integrated nutrient management practices for this important cropping sequence; thereby, supplementing the efforts of plant breeders and biotechnologists in the development of transgenics or deploying marker-assisted breeding technologies for improving quality traits in these crops.
Supplementary_material.doc
Download MS Word (163.5 KB)Acknowledgements
We are grateful to Dr. Firoz Hossain, Division of Genetics, IARI and Dr. Amrit Kumar Paul, IASRI, New Delhi for their help in statistical analyses and assistance in the interpretation of the results. We are also thankful to the Division of Microbiology and Division of Agronomy, IARI, New Delhi for providing the necessary facilities for undertaking this study.
Supplementary material
Supplementary material for this article can be accessed here http://dx.doi.org./10.1080/23311932.2015.1037379.
Additional information
Funding
Notes on contributors
Lata Nain
The author’s group at the ICAR-Indian Agricultural Research Institute, New Delhi has been making significant contributions towards developing and evaluating microbial consortia as plant growth promoting and biofortifying agents in the rice–wheat cropping system. In the current scenario of depleting soil fertility and environment pollution due to the continuous use of chemical fertilizers in agriculture, green/organic options such as biofertilizers can be significant as inputs in integrated nutrient management practices in developing countries. The research investigations were initiated with the isolation of bacterial and cyanobacterial strains from the rhizosphere of rice and wheat and characterization in laboratory studies for their biochemical and plant growth promoting attributes. Further, the development of carrier-based formulations, their optimization for use as seed coating or seedling dip or soil application was undertaken, and systematic investigations in pot experiments followed by field trials for several consecutive years have proved their promise.
References
- Adak, A. , Prasanna, R. , Babu, S. , Bidyarani, N. , Verma, S. , Pal, M. , … Nain, L. (in press). Micronutrient enrichment mediated by plant-microbe interactions and rice cultivation practices. Journal of Plant Nutrition .
- Antoun, H. , & Kloepper, J. W. (2001). Plant growth promoting rhizobacteria. In S. Brenner & J. H. Miller (Eds.), Encyclopedia of genetics (pp. 1477–1480). New York, NY: Academic Press.10.1006/rwgn.2001.1636
- Artursson, V. , Finlay, R. D. , & Jansson, J. K. (2006). Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environmental Microbiology , 8 , 1–10.10.1111/EMI.2006.8.issue-1
- Barnett, J. P. , Millard, A. , Ksibe, A. Z. , Scanlan, D. J. , Schmid, R. , & Blindaue, C. A. (2012). Mining genomes of marine cyanobacteria for elements of zinc homeostasis. Frontiers in Microbiology , 3 , 142–147.
- Bashir, K. , Nozoye, T. , Ishimaru, Y. , Nakanishi, H. , & Nishizawa, N. K. (2013). Exploiting new tools for iron bio-fortification of rice. Biotechnology Advances , 31 , 1624–1633.10.1016/j.biotechadv.2013.08.012
- Bell, R. W. , & Dell, B. (2008). Micronutrients for sustainable food, feed, fibre and bioenergy production . Paris: International Fertilizer Industry Association (IFA).
- Bouis, H. E. , & Welch, R. M. (2010). Biofortification-A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Science , 50 , 20–32.
- Caldwell, B. A. (2005). Enzyme activities as a component of soil biodiversity: A review. Pedobiologia , 49 , 637–644.10.1016/j.pedobi.2005.06.003
- Casida, L. E. J. , Klein, D. A. , & Santoro, T. (1964). Soil dehydrogenase activity. Soil Science , 98 , 371–376.10.1097/00010694-196412000-00004
- Chen, Y. P. , Rekha, P. D. , Arun, A. B. , Shen, F. T. , Lai, W. A. , & Young, C. C. (2006). Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Applied Soil Ecology , 34 , 33–41.10.1016/j.apsoil.2005.12.002
- de Santiago, A. , Quintero, J. M. , Avilés, M. , & Delgado, A. (2011). Effect of Trichoderma asperellum strain T34 on iron, copper, manganese, and zinc uptake by wheat grown on a calcareous medium. Plant and Soil , 342 , 97–104.10.1007/s11104-010-0670-1
- Eleiwa, M. E. , Eman, R. , Hamed, E. R. , & Shehata, H. S. (2012). The role of biofertilizers and/or some micronutrients on wheat plant (Triticum aestivum L.) growth in newly reclaimed soil. Journal of Medicinal Plants Research , 6 , 3359–3369.
- Glick, B. R. (1995). The enhancement of plant growth by free-living bacteria. Canadian Journal of Microbiology , 41 , 109–117.10.1139/m95-015
- Green, V. S. , Stott, D. E. , & Diack, M. (2006). Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biology and Biochemistry , 38 , 693–701.10.1016/j.soilbio.2005.06.020
- Hirsch, P. R. , & Mauchline, T. H. (2012). Who’s who in the plant root microbiome? Nature Biotechnology , 30 , 961–962.10.1038/nbt.2387
- Institute of Medicine (IOM) Report . (2001). Dietary reference intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (797 p.). Washington, DC: The National Academies Press.
- Jackson, M. L. (1967). Soil chemical analysis . New Delhi: Prentice Hall.
- Jat, R. S. , & Ahlawat, I. P. S. (2006). Direct and residual effect of vermicompost, biofertilizers and phosphorus on soil nutrient dynamics and productivity of chickpea-fodder maize sequence. Journal of Sustainable Agriculture , 28 , 41–54.10.1300/J064v28n01_05
- Jha, B. , Thakur, M. C. , Gontia, I. , Albrecht, V. , Stoffels, M. , Schmid, M. , & Hartmann, A. (2009). Isolation, partial identification and application of diazotrophic rhizobacteria from traditional Indian rice cultivars. European Journal of Soil Biology , 45 , 62–72.10.1016/j.ejsobi.2008.06.007
- Joshi, A. K. , Chand, R. , Arun, B. , Singh, R. P. , & Ortiz, R. (2007). Breeding crops for reduced-tillage management in the intensive, rice–wheat systems of South Asia. Euphytica , 153 , 135–151.
- Kamran, A. , Kubota, H. , Yang, R. C. , Randhawa, H. S. , & Spaner, D. (2014). Relative performance of Canadian spring wheat cultivars under organic and conventional field conditions. Euphytica , 196 , 13–24.10.1007/s10681-013-1010-3
- Li, T. Y. , Rouland, C. , Benedetti, M. , Li, F. , Pando, A. , Lavelle, P. , & Dai, J. (2009). Microbial biomass, enzyme and mineralization activity in relation to soil organic C, N and P turnover influenced by acid metal stress. Soil Biology and Biochemistry , 41 , 969–977.10.1016/j.soilbio.2009.01.021
- Lindsay, W. I. , & Norvell, W. A. (1978). Development of DTPA soil test for zinc, iron manganese and copper. Soil Science Society of America Journal , 42 , 421–448.10.2136/sssaj1978.03615995004200030009x
- Mäder, P. , Kaiser, F. , Adholeya, A. , Singh, R. , Uppal, H. S. , Sharma, A. K. , … Fried, P. M. (2011). Inoculation of root microorganisms for sustainable wheat–rice and wheat black gram rotations in India. Soil Biology and Biochemistry , 43 , 609–619.10.1016/j.soilbio.2010.11.031
- Manjunath, M. , Prasanna, R. , Sharma, P. , Nain, L. , & Singh, R. (2011). Developing PGPR consortia using novel genera- Providencia and Alcaligenes along with cyanobacteria for wheat. Archives of Agronomy and Soil Science , 57 , 873–887.10.1080/03650340.2010.499902
- Mishra, P. K. , Bisht, S. C. , Ruwari, P. , Joshi, G. K. , Singh, G. , Bisht, J. K. , & Bhatt, J. C. (2011). Bioassociative effect of cold tolerant Pseudomonas spp. and Rhizobium leguminosarum-PR1 on iron acquisition, nutrient uptake and growth of lentil (Lens culinaris L.). European Journal of Soil Biology , 47 , 35–43.10.1016/j.ejsobi.2010.11.005
- Morgounov, A. , Gómez-Becerra, H. F. , Abugalieva, A. , Dzhunusova, M. , Yessimbekova, M. , Muminjanov, H. , & Zelenskiy, Y. (2007). Iron and zinc grain density in common wheat grown in Central Asia. Euphytica , 155 , 193–203.10.1007/s10681-006-9321-2
- Nain, L. , Rana, A. , Joshi, M. , Jadhav, S. D. , Kumar, D. , Shivay, Y. S. , … Prasanna, R. (2010). Evaluation of synergistic effects of bacterial and cyanobacterial strains as biofertilizers for wheat. Plant and Soil , 331 , 217–230.10.1007/s11104-009-0247-z
- Olsen, S. R. , Cole, C. V. , Watanabe, F. S. , & Dean, L. A. (1954). Estimation of available phosphorus in soil by extraction with sodium carbonate . Washington, DC: USDA.
- Ortiz-Monasterio, J. I. , Palacios-Rojas, N. , Meng, E. , Pixley, K. , Trethowan, R. , & Peἥa, R. J. (2007). Enhancing the mineral and vitamin content of wheat and maize through plant breeding. Journal of Cereal Science , 46 , 293–307.
- Pooniya, V. , Shivay, Y. S. , Rana, A. , Nain, L. , & Prasanna, R. (2012). Enhancing soil nutrient dynamics and productivity of Basmati rice through residue incorporation and zinc fertilization. European Journal of Agronomy , 41 , 28–37.10.1016/j.eja.2012.03.004
- Prasanna, R. , Babu, S. , Rana, A. , Kabi, S. R. , Chaudhary, V. , Gupta, V. , … Pal, R. K. (2013). Evaluating the establishment and agronomic proficiency of cyanobacterial consortia as organic options in wheat–rice cropping sequence. Experimental Agriculture , 49 , 416–434.10.1017/S001447971200107X
- Prasanna, R. , Bidyarani, N. , Babu, S. , Hossain, F. , Shivay, Y. S. , & Nain, L. (2015). Cyanobacterial inoculation elicits plant defense response and enhanced Zn mobilization in maize hybrids. Cogent Food & Agriculture , 1 , 998507.
- Prasanna, R. , Jaiswal, P. , Jadhav, S. D. , Joshi, M. , Nain, L. , Rana, A. , & Shivay, Y. S. (2012). Evaluating the potential of rhizo-cyanobacteria as inoculants for rice and wheat. Journal of Agricultural Technology , 8 , 157–171.
- Prasanna, R. , Joshi, M. , Rana, A. , Shivay, Y. S. , & Nain, L. (2012). Influence of co-inoculation of bacteria- cyanobacteria on crop yield and C- N sequestration in soil under rice crop. World Journal of Microbiology and Biotechnology , 28 , 1223–1235.10.1007/s11274-011-0926-9
- Rana, A. , Joshi, M. , Prasanna, R. , Shivay, Y. S. , & Nain, L. (2012). Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. European Journal of Soil Biology , 50 , 118–126.10.1016/j.ejsobi.2012.01.005
- Rana, A. , Saharan, B. , Joshi, M. , Prasanna, R. , Kumar, K. , & Nain, L. (2011). Identification of multi trait PGPR isolates and evaluating their potential as inoculants for wheat. Annals of Microbiology , 61 , 893–900.10.1007/s13213-011-0211-z
- Rana, A. , Saharan, B. , Nain, L. , Prasanna, R. , & Shivay, Y. S. (2012). Enhancing micronutrient uptake and yield of wheat through bacterial PGPR consortia. Soil Science and Plant Nutrition , 58 , 573–582.10.1080/00380768.2012.716750
- Rodrı́guez, H. , & Fraga, R. (1999). Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances , 17 , 319–339.10.1016/S0734-9750(99)00014-2
- Roesti, D. , Gaur, R. , Johri, B. N. , Imfeld, G. , Sharma, S. , Kawaljeet, K. , & Aragno, M. (2006). Plant growth stage, fertiliser management and bio-inoculation of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria affect the rhizobacterial community structure in rain-fed wheat fields. Soil Biology and Biochemistry , 38 , 1111–1120.10.1016/j.soilbio.2005.09.010
- Rosas, S. B. , Avanzini, G. , Carlier, E. , Pasluosta, C. , Pastor, N. , & Rovera, M. (2009). Root colonization and growth promotion of wheat and maize by Pseudomonas aurantiaca SR1. Soil Biology and Biochemistry , 41 , 1802–1806.10.1016/j.soilbio.2008.10.009
- Rose, T. J. , Impa, S. M. , Rose, M. T. , Pariasca- Tanaka, J. , Mori, A. , Heuer, S. , … Wissuwa, M. (2013). Enhancing phosphorus and zinc acquisition efficiency in rice: A critical review of root traits and their potential utility in rice breeding. Annals of Botany , 112 , 331–345.10.1093/aob/mcs217
- Samaranayake, P. , Peiris, B. D. , & Dssanayake, S. (2012). Effect of excessive ferrous (Fe2+) on growth and iron content in rice (Oryza sativa). International Journal of Agriculture and Biology , 14 , 296–298.
- Schwyn, B. , & Neilands, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry , 160 , 47–56.10.1016/0003-2697(87)90612-9
- Secilia, J. , & Bagyaraj, D. J. (1994). Evaluation and first-year field testing of efficient vesicular arbuscular mycorrhizal fungi for inoculation of wetland rice seedlings. World Journal of Microbiology and Biotechnology , 10 , 381–384.10.1007/BF00144455
- Selvakumar, G. , Kundu, S. , Gupta, A. D. , Shouche, Y. S. , & Gupta, H. S. (2008). Isolation and characterization of nonrhizobial plant growth promoting bacteria from nodules of Kudzu (Pueraria thunbergiana) and their effect on wheat seedling growth. Current Microbiology , 56 , 134–139.10.1007/s00284-007-9062-z
- Sharma, A. , Shankhdhar, D. , & Shankhdhar, S. C. (2013). Enhancing grain iron content of rice by the application of plant growth promoting rhizobacteria. Plant Soil and Environment , 59 , 89–94.
- Shukla, A. K. , Sharma, S. K. , & Tiwari, R. (2005). Nutrient depletion in the rice-wheat cropping system of the Indo-Gangetic plains. Better Crops , 89 , 28–31.
- Singh, A. K. , Manibhushan, M. , Meena, M. K. , & Upadhyaya, A. (2012). Effect of sulphur and zinc on rice performance and nutrient dynamics in plants and soil of Indo Gangetic plains. Journal of Agricultural Sciences , 4 , 162–170.
- Singh, M. V. (2009). Micronutrient nutrient problems in soils of India and improvement for human health and animal health. Indian Journal of Fertilisers , 5 , 19–26.
- Singh, R. K. , & Chaudhry, B. D. (1979). Biometrical methods in quantitative genetic analysis . Ludhiana: Kalyani Publishers.
- Subbiah, B. V. , & Asija, G. L. (1956). A rapid procedure for assessment of available nitrogen in rice soils. Current Science , 25 , 259–260.
- Tariq, M. , Hameed, S. , Malik, K. A. , & Hafeez, F. Y. (2007). Plant root associated bacteria for zinc mobilization in rice. Pakistan Journal of Botany , 39 , 245–253.
- Thakuria, D. , Talukdar, N. C. , Goswami, C. , Hazarika, S. , Kalita, M. C. , & Bending, G. D. (2009). Evaluation of rice–legume–rice cropping system on grain yield, nutrient uptake, nitrogen fixation, and chemical, physical, and biological properties of soil. Biology and Fertility of Soils , 45 , 237–251.10.1007/s00374-008-0320-4
- United States Agency for International Development . (2009). Revitalizing the rice‐wheat cropping systems of the Indo‐gangetic plains: Adaptation and adoption of resource‐conserving technologies in India, Bangladesh, and Nepal . Retrieved from http://pdf.usaid.gov/pdf_docs/PDACP630.pdf
- Velu, G. , Ortiz-Monasterio, I. , Cakmak, I. , Hao, Y. , & Singh, R. P. (2014). Biofortification strategies to increase grain zinc and iron concentrations in wheat. Journal of Cereal Science , 59 , 365–372.10.1016/j.jcs.2013.09.001
- Walkley, A. J. , & Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science , 37 , 29–38.10.1097/00010694-193401000-00003
- World Health Organization . (2011). Micronutrient deficiencies: Iron deficiency anemia . Retrieved from http://www.who.int/nutrition/topics/ida/en/index.html
- Zafar, M. , Abbasi, M. K. , Rahim, N. , Khaliq, A. , Shaheen, A. , Jamil, M. , & Shahid, M. (2011). Influence of integrated phosphorus supply and plant growth promoting rhizobacteria on growth, nodulation, yield and nutrient uptake in Phaseolus vulgaris . African Journal of Biotechnology , 10 , 16793–16807.
- Zhu, C. , Naqvi, S. , Gomez-Galera, S. , Pelacho, M. , Capell, T. , & Christou, P. (2012). Transgenic strategies for the nutritional enhancement of plants. Trends in Plant Science , 12 , 548–555.
- Zuo, Y. , & Zhang, F. (2011). Soil and crop management strategies to prevent iron deficiency in crops. Plant and Soil , 339 , 83–95.10.1007/s11104-010-0566-0
