Abstract
An experiment was conducted to assess the effect of Rhizobium sp., waste tea leaves, eggshell powder, and composted cow dung manure on the root-knot nematode, Meloidogyne incognita, on lentil in Botany department AMU, Aligarh, India. When used alone, composted cow dung was better in reducing galling and nematode multiplication and improving lentil growth followed by eggshell powder, Rhizobium sp., and waste tea leaves. Significant result in the integrated management of M. incognita was obtained when Rhizobium sp. was used in combination with cow dung and eggshell powder (with or without waste tea leaves). Combined application of root nodule bacterium and organic wastes like waste tea leaves, eggshell, and cow dung may be suggested to the farmers/growers or related persons who are having great enthusiasm to establish a lentil production business. Application of these organic materials along with the root nodule bacteria may be helpful to foster soil ecosystem which has been a hot topic in the present scenario.
Public Interest Statement
Lentil, Lens culinaris recognized as valuable pulse crop being grown across the world including India. This crop is used in various ways globally in several forms especially to the vegetarian person. Lentils also contain dietary fiber, folate, vitamin B1, and minerals. Red (or pink) lentils contain a lower concentration of fiber than green lentils. Present findings will indeed disseminate the paramount informations among the non-specialist readers especially the farmers/growers and business entrepreneurs who are getting hurdles in lentil cultivation across the world, especially in India. In this way, growers and related persons may be given assistance by deploying the methodology and doses of the different treatments and results obtained from this research. This will promote the organic production of pulses globally.
Competing interest
The authors declare no competing interest.
1. Introduction
Lentil (Lens culinaris Medic.) vern. masoor belongs to family Leguminosae and is recognized as a valuable pulse crop. Lentil is grown as winter crop and is cultivated in all parts of the India either as a separate or mixed crop. Unripe and green pods of lentil are used as vegetables. Moreover, the dried plants are used as fodder. India ranks second after Canada in lentil production producing 1,134,000 metric tons (FAO, Citation2013). About a quarter of the worldwide production of lentils is from India, most of which is consumed in the domestic market.
There are several fungal and nematodal diseases that have been found to occur on lentil in India and they are responsible for low production and uncertainty in the yield of lentil (Tiyagi, Khan, & Alam, Citation2001). Moreover, Meloidogyne incognita causes root-knot disease and about 16% yield losses (Ali, Citation1997) and is reported as top 10 plant parasitic nematodes in the world (Jones et al., Citation2013). M. incognita has been most devastating species on lentil causing drastic reductions in the yield (Singh, Hisamuddin, & Azam, Citation2010; Wani, Citation2006; Wani & Bhat, Citation2011).
A noteworthy number of bacterial species from the rhizosphere are known for improving plant growth (del Amor, Serrano-Martínez, Fortea, Legua, & Núñez-Delicado, Citation2008; Hayat, Ali, Amara, Khalid, & Ahmed, Citation2010; Kloepper, Lifshitz, & Zablotowicz, Citation1989). Symbiotic nitrogen fixation is reported to be found in legumes and some non-legume plants forming root nodules (Callaham, Deltredici, & Torrey, Citation1978). However, micro-organisms present in the soil play a key role in drawing atmospheric nitrogen such as Rhizobiacea (Jordan, Citation1984) and Frankia (Lechevalier & Lechevalier, Citation1989). Legumes stimulate rhizobia much more than other rhizosphere microorganisms (Nutman, Citation1965). Rhizobial nodulation is a complex symbiotic process between host plant and rhizobia. The plant provides an energy sources and ecological niche for the bacteria in return bacteria provides a source of fixed nitrogen for the plant (Vance & Johnson, Citation1981). Integrated application of Rhizobium, in the presence of P-enriched compost may be a suitable approach for improving growth, yield, and nodulation in lentil (Iqbal et al., Citation2012). Besides, many species of rhizobacteria have been found to act as bioagents playing a crucial role against various phytopathogens (Hayat et al., Citation2010). Plant parasitic nematode alters the establishment of nodules on or around the roots of legumes (Huang, Citation1987). As a result of nematode infection, the nodulation and nitrogen fixation has been reported to be suppressed (Hussaini & Seshadri, Citation1975) or stimulated (Hussey & Barker, Citation1976) or remain unaffected (Taha & Raski, Citation1969). The presence of rhizobia in the rhizosphere may also protect the host roots from damage caused by pathogens (Siddiqui & Husain, Citation1992; Siddiqui & Mahmood, Citation1995). Rhizobium spp. can reduce the hatching and causes significant mortality of Meloidogyne javanica (Siddiqui, Zareen, Shaukat, & Zaki, Citation2001). Application of Rhizobium along with other pseudomonads such as Pseudomonas putida causes maximum reduction in M. javanica galling and multiplication on lentil (Siddiqui, Baghel, & Akhtar, Citation2007). Mixture of Rhizobium leguminosorum, Bacillus thuringiensis, and Pseudomonas fluorescence exhibits surprising results by reducing the root-knot population and galls per root system (Ashoub & Amara, Citation2010).
Urban environment generates large quantity of organic wastes. Disposal of organic waste can pose problems and interest in the composting and recycling of these materials is increasing day by day. The benefits of organic amendment and mulches in improving crop performance are well known (McSorley & Gallaher, Citation1996). Organic amendments supply nutrients to the plants and improve the yields (McSorley & Gallaher, Citation1997). They are also supposed to be nutrient source for crop production (Abubakar, Adamu, & Manga, Citation2004).
Moreover, soil amendments could provide additional benefits for plant growth (Oka, Citation2010). Soil amendments with various materials including chitin or incorporation of other waste materials may also hold potential for nematode control (Chen, Abasi, & Zuckerman, Citation1999; Muller & Gooch, Citation1982; Stirling, Citation1991). Remarkable reductions in nematode populations have been achieved with significant increase in the yield (Abubakar & Majeed, Citation2000). Current scenario is supposed to be nurtured with organic agriculture which has received great impetus in many countries including India (Willer & Julia, Citation2014). Therefore, in the present study we chose to evaluate the impact of one root nodule bacterium, Rhizobium sp. and three different organically approved soil amendments on lentil. Emphasis was given on locally produced manures such as eggshell powder, waste tea leaves, and composted cow dung alone and in combination for the management of M. incognita on lentil.
2. Materials and methods
The root-knot nematode (RKN), M. incognita (Kofoid and White) Chitwood was selected as a test species and lentil (L. culinaris Medic.) cv. K-275 as the test plant. Root nodule bacterium Rhizobium sp., eggshell powder, waste tea leaves, and composted cow dung were used alone and in combination for the management of RKN.
2.1. Raising and maintenance of test plant
Seeds of test plant were surface sterilized with 0.01% mercuric chloride for two minutes and washed thrice in sterilized water. Five seeds were sown in each earthen pots and thinning was done after germination to maintain one plant per pot. One week after germination seedling were inoculated with pathogen, bacterial suspension, waste materials, and composted cow dung as shown in Tables and . Inoculated plants were kept on a glass house bench at 25–27°C. Pots were arranged in randomized block design and each treatment was replicated five times. Pots were watered periodically and experiment was terminated 60 days after inoculation.
Table 1. Effects of Rhizobium sp. waste tea leaves, eggshell powder, and cow dung on the growth of lentil (five replicates)
Table 2. Effects of Rhizobium sp. waste tea leaves, eggshell powder, and cow dung on the reproduction of M. incognita and growth of lentil (five replicates)
2.2. Preparation of nematode inoculum
A survey was conducted on the brinjal growing field in Aligarh to collect the RKN inoculum. The infected brinjal roots after observation were brought to the laboratory for further processing. Large numbers of egg masses were handpicked with the help of sterilized forceps from heavily infected brinjal roots on which pure culture of M. incognita was maintained. These egg masses were washed in distilled water and placed in 10-cm diam. course sieves mounted with crossed layered tissue paper. The sieve was placed in a Petri plate containing water. The water level was adjusted to touch lower portion of sieve having egg masses, only. The hatched larvae were collected from Petri plates after every 24 h and transferred to a beaker. Fresh water was added to Petri plates. Water suspension of second stage juvenile of M. incognita was thoroughly stirred for making homogenous distribution of nematodes. One milliliter suspension of nematode from each sample was taken for counting the number of nematodes under stereomicroscope. An average of five counts was made to determine the density of nematodes in the suspension. The volume of water of nematode suspension was adjusted in order to reach 200 ± 5 nematodes. It was done by adding more water or decanting the excess amount, depending upon the situation. Two hundred freshly hatched J2 were inoculated per pot.
2.3. Preparation and sterilization of soil mixture
Sandy loam soil collected from the field of Botany department, AMU, Aligarh was passed through 10 mesh sieve. The soil and river sand were mixed in the ratio of 3:1 and 15-cm diameter clay pots were filled with 1 kg of soil per pot. Some water was poured in each pot just to wet the soil surface before transferring them to an autoclave for sterilization at 20 l.b.s. pressure for 20 min. Sterilized pots were allowed to cool down at room temperature before experimentation.
2.4. Organic manure
Twenty gram composted cow dung was integrated in the rhizosphere of lentil seedling by taking out soil layer shown in Tables and . Before using, the cow dung was allowed to decomposition in a cemented pit covered with polythene for a year with adequate amount of water added at intervals. Twenty gram composted cow dung contains about 60 mg N, 8.8 mg P, and 16.8 mg K.
2.5. Waste material
Eggshell and waste tea leaves were collected from AMU, canteen, and brought to laboratory. Eggshells were crushed to coarse powder and waste tea leaves were dried. Both the materials were amended with soil at the rate of 5-g per pot.
2.6. Bacterial inoculum
Charcoal soil based culture of Rhizobium sp. was obtained from Quarsi Farm, Aligarh. Hundred gram of Rhizobium sp. (lentil strain) culture was dissolved in 1,000-ml distilled water and 10 ml of the suspension containing 1 g inoculums were inoculated (Siddiqui et al., Citation2007).
2.7. Inoculation procedures
For each sample, soil around the roots was carefully removed suspension of nematode and/or bacteria, cow dung, waste material, inocula were poured around the roots uniformly and soil was replaced around the seedlings. For control, water was poured equally to inoculum suspension in the same way. Inoculations alone or in combination were done as shown in the Tables and .
2.8. Observations
Plants were uprooted 60 days after inoculation. Root systems were gently washed taking care to avoid losses and injury during the entire operation. For measuring length and weight, the plants were cut with sharp knife just above the base of root emergence zone. Lengths of plants were recorded in cm from the top of first leaves to longest root, respectively. The excess water was removed by putting two folds of blotting sheets for some time before weighing them separately. Plant fresh and dry weights were recorded in gm. For dry weight, shoots were kept in envelops for drying in oven running at 60°C for 2–3 days then weights were recorded.
Numbers of galls were counted under a stereomicroscope. Nematodes were extracted from 250 g subsamples from well mixed soil of each treatment by Cobb’s sieving and decanting technique followed by Baermann’s funnel (Southey, Citation1986). Nematodes suspensions were collected after 24 h and nematodes were counted in the counting dishes taking five replicates of 1 ml suspension from each sample. Means of five such counts were obtained and population of nematode per kg soil was calculated.
2.9. Statistical analysis
Data were analyzed statistically and Least Significant Difference (LSD) was calculated at P ≤ 0.05. DMRT (Duncan’s Multiple Range Test) was later employed to denote the significant difference between the treatments.
3. Results
Inoculation of Rhizobium (Rh), waste tea leaves (T), eggshell powder (E), and composted cow dung (CD) alone or in combination to plants without M. incognita caused significant increases in plant growth over control (Table ). Inoculation of Rh combined with CD, CD + T or E, CD + T + E gave the best increase in plant growth compared to control (Table ). Combination of Rh + CD was enough to increase plant growth (addition of T or E did not show any significant improvement). Plants that received E treatments produced lower plant length, weight, shoot dry weight, number of nodules, which were only higher than those of inoculated control. Plants treated with CD exhibited more or less similar improvement in shoot dry weight as recorded in T treated plants. Maximum improvement in shoot dry weight without nematode was observed when Rh was used with all the three waste material, but increase in shoot dry weight was similar to that caused by Rh with CD + E. Root nodulation was highly increased in plants inoculated with Rh. Inoculation of CD with Rh further increases root nodulation while T and E have almost non-significant effect on nodulation (Table ).
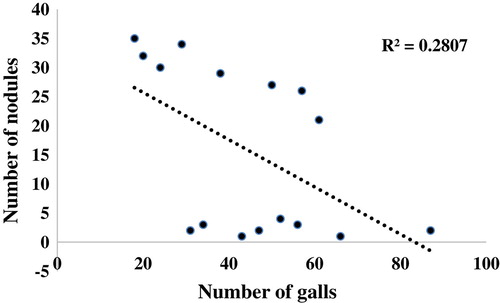
Inoculation of M. incognita caused significant reduction (halving weight) in plant growth compared to uninoculated plant. Inoculation of Rh, T, E, and CD alone or in combination caused significant increases in plant growth (Table ). Here again, Rh + CD + E (with or without T) gave the best increase in plant growth. The combined application of all treatments drastically decreased the nematode-related parameters such as nematode population, number of galls per root system and surprisingly increased plant length, weight, number of nodules per root system, and shoot dry weight (Table ). The different treatments alone or in combination significantly reduced galls and nematode multiplication. CD resulted in higher reduction in galls and nematode multiplication followed by E, then Rh, then T when used alone (Table ). However, use of Rh + CD + E (with or without T) caused a greater reduction in galls and nematode multiplication. Nodulation was very poor in absence of Rh, however, significant increment observed in the presence of Rh and in turn it further increases in presence CD, T, and or E. Highest root galling and nematode multiplication were observed in plants inoculated with M. incognita alone (Table ). Correlation analyses between number of galls and number of nodules per root system indicates (R 2 = 0.2807) illustrating that two variables are perfectly related in a linear sense (Figure ).
4. Discussion
The combined applications of organic fertilizers along with Rhizobium sp. gave surprising results by reducing nematode population which in turn increases the plant yield which is in the tune of Siddiqui et al. (Citation2007). Rhizobium sp. is reported to produce toxic metabolites inhibitory to many plants pathogens (Ehteshamul-Haque & Ghaffar, Citation1993) that may be one of the reasons for this effect. Rhizobium leguminosarum, for example, can cause mortality of RKN in vitro and can restrict and cease M. incognita reproduction (Ashoub & Amara, Citation2010). Rhizobium japonicum secretes rhizobitoxine which is inhibitory to pathogens (Chakraborty & Purkayastha, Citation1984). Increased level of phytoalexin was observed in seeds bacterized with R. leguminosarum which had adverse effect on plant pathogens (Chakraborty & Chakraborty, Citation1989). Reduced nematode multiplication in the presence of Rhizobium sp. can be due to the inhibitory effect of root nodule bacterium on nematodes (Siddiqui et al., Citation2007) or the systematic resistance induced by Rhizobium against RKN (Siddiqui & Shaukat, Citation2002, Citation2004). We have also observed nematode parasitism which has a negative effect on nodulation, as previously observed by Siddiqui and Husain (Citation1992). Reduction in number of nodules by nematode parasitism might be due to formation of Meloidogyne galls thus occupying space in roots (Barker & Hussey, Citation1976).
In the recent past, researchers have paid attention towards rhizobia due to its ability to act as bioagent against many phytopathogens (Siddiqui & Zehra, Citation2012). In the present study, it was found that Rhizobium sp. reduced root-knot population, enhanced plant yield with increased number of nodules per plant. The combined use of organic fertilizers with Rhizobium sp. resulted in higher nodulation which probably had greater adverse effect on nematode multiplication thereby a better plant growth. These findings are in agreement with Ashoub and Amara (Citation2010) and Siddiqui, Zareen, Shaukat, and Zaki (Citation2001). Reduction in root-knot galling and nematode population may be due to induction of resistance (Siddiqui & Shaukat, Citation2002, Citation2004).
Amendments with cow dung and Rhizobium sp. reduced the population of density of M. incognita. Higher plant yield was recorded in terms of plant length and shoot dry weight. Higher yield was noted may be due to improvement in the soil ecosystem as well as some toxic metabolites released by cow dung upon decomposition (Oka, Citation2010; Riegel & Noe, Citation2000). Organic manure has been reported to be rich in several compounds especially nitrogen and phenolics (Agyarko, Kwakye, Bonsu, Osei, & Fripong, Citation2006; Hassan, Chindo, Marley, & Alegbejo, Citation2010; Renco & Kovácik, Citation2012). Nitrogen after conversion into ammonia (Thoden, Korthals, & Termorshuizen, Citation2011) has been reported to kill several plant parasitic nematodes (Lazarovits, Tenuta, & Conn, Citation2001). Different workers using animal manures or organic residues as soil amendments registered a satisfactory results on the yield, in a variety of crops and also reduction in the population of plant parasitic nematodes (Chaudhary & Kaul, Citation2013; Iqbal et al., Citation2012; Orisajo, Afolami, Fademi, & Atungwu, Citation2008; Pakeerathan, Mikunthan, & Tharahani, Citation2009). All the treated plants showed significant and satisfactory results when compared to untreated control. Our findings are similar with earlier reports (Amulu & Adekunle, Citation2015; Nagaraju, Karemegam, & Kadalmani, Citation2010).
Amendments of waste leaves added organic matter which may provide better soil structure and suitable medium for plant growth. This may result in increased plant growth. Moreover, tea leaves had nicotine and may have adverse effect on nematode reproduction. Amendments of waste tea along with Rhizobium sp. showed significant increase in terms of plant length and weight (fresh and dry). However, this effect was lesser than the cow dung. Hassan, Rahman, Amin, Hoque, and Islam (Citation2001) recorded similar results and found that even low concentration of the organic matter like chicken litter, tea waste along with some other manure significantly checked the root-knot activity. Nchore, Waceke, and Kariuki (Citation2012) observed that tea residue may be used to control root-knot nematode infesting black night shade.
Egg shell is made up of chitin and application of chitin for the management of RKN may result in the buildup antagonistic fungi which may adversely affect nematode reproduction. Number of root galls and nematode population was drastically decreased which resulted better plant growth. Natasasmita (Citation1997) reported that application of shrimp shell powder drastically decreased the damaging potential of root-knot nematode. Natural chitinous materials have nematicidal activity and are potential to kill the nematodes (Natasasmita, Citation1997; Suganda, Citation1999). In brief, amendments of chitinous materials into the soil may increase the soil population of micro-organism which in turn parasitizes the plant parasitic nematodes.
Application of organic manure with egg shell and waste tea leaves plus Rhizobium sp. along with cow dung was better option for plant growth because of positive interaction. The positive interaction may result in the buildup antagonistic fungal population, greater nodulation, better soil structure, supply of nutrient, and suitable medium for plant to grow which provided integrated management of M. incognita beside better plant growth.
Poornima and Vadivelu (Citation1993) reported that oil cakes of neem, castor, and mahua boosted the growth of plant and reduced the population of M. incognita and R. reniformis on brinjal. Kumar and Vadivalu (Citation1993) reported that mustard oil cake at 5–10 g/kg wet compost was most effective in increasing the yield of mushroom and buttons and controlling the Aphelenchoides composticola. Patel and Patel (Citation1992) reported that poultry manure at 0.72 g/pot with 800 g soil increased plant height, fresh shoot, and root weights by 157, 500, and 390%, respectively, with 77% reduction in R. reniformis population. Crow, Guertal, and Rodriguez-Kabana (Citation1996) observed that response of M. arenaria and M. incognita to green manures and supplemental urea in glasshouse culture. When urea was applied at 200, 300, and 400 mg N/kg soil, rapeseed green manure was more effective than velvet bean manure at reducing galling of squash root caused by M. arenaria. Decreased viability of M. incognita eggs was observed from treatments that received rates > 200 mg N/kg soil with higher percentage of N from urea. Riegel, Fernandez, and Noe (Citation1996) observed effects of chicken litter on M. incognita in cotton and reported that at 92 and 184 days after planting nematode, population densities decreased linearly with increase in rates of litter. McSorley and Gallaher (Citation1997) observed that effects of yard waste compost treatment of Mesocriconema spp., M. incognita, and Pratylenchus spp. were in consistent whereas significant effects of compost on population densities of Paratrichodorus minor were found on four of six sampling occasion.
Application of Rhizobium with organic manures maximizes nematode control, increases plant growth parameters, and minimizes phytotoxicity or environmental hazards.
5. Conclusion
Our results clearly suggested that combined application of Rhizobium sp. with organic manures (especially cow dung and egg shell powder) drastically decreased the soil population of nematode as well as root galling of M. incognita. This combination may be sound alternatives for the management of M. incognita infesting lentil, especially in India. This IPM strategy may be suggested to the farmers/growers or related persons who are having great enthusiasm to establish a lentil production business. However, more attempts in this direction are needed to confirm actual rates and timings of amendments under field condition.
Cover image
Source: Authors.
Acknowledgement
Aligarh Muslim University is gratefully acknowledged for providing all necessary facilities during the course of the work.
Additional information
Funding
Notes on contributors
Rizwan Ali Ansari
The Aligarh Muslim University, Aligarh has been recognized for its outstanding contributions in the several aspects related to Plant Pathology and Nematology. Corresponding author, Rizwan Ali Ansari has been engaged with the development and formulation of different modules by exploiting the biological organisms and organic matters against the various economically important diseases infesting several agricultural crops. He has recently received a prestigious award by the Nematological Society of India (NSI) for his outstanding contribution in organic farming. In addition, thrust area of Ansari and his groups to promote organic farming across the world by utilizing the organic matters, mycorrhizal fungi, PGPR, biofertilizers, and some other beneficial organisms for sustainable management of plant parasitic nematodes through biological means as well as enrich the soil with nutrients necessary for plant growth and development.
References
- Abubakar, U. , Adamu, T. , & Manga, S. B. (2004). Control of Meloidogyne incognita (kofoid and white) chitwood (root-knot nematode) of Lycopersicon esculentus (tomato) using cowdung and urine. African Journal of Biotechnology , 3 , 379–381.10.5897/AJB
- Abubakar, U. , & Majeed, Q. (2000). Use of animal manure for the control of root-knot nematodes of cowpea. Journal of Agriculture and Environment , 1 , 29–33.
- Agyarko, K. , Kwakye, P. K. , Bonsu, M. , Osei, B. A. , & Fripong, K. A. (2006). Effects of organic soil amendments on root-knot nematodes, soil nutrients and growth of carrot. Journal of Agronomy , 5 , 641–646.
- Ali, S. S. (1997). Status of nematode problems and research in India. In S. B. Sharma (Ed.), Diagnosis of key nematode pests of chickpea and pigeon pea in their management. Proceedings of Regional Training Course (pp. 74–82). Patancheru: ICRISAT.
- Amulu, L. U. , & Adekunle, O. K. (2015). Comparative effects of poultry manure, cow dung, and carbofuran on yield of Meloidogyne incognita-infested okra. Journal of Agricultural Sciences and Technology , 17 , 495–504.
- Ashoub, A. H. , & Amara, M. T. (2010). Biocontrol activity of some bacterial genera against root-knot nematode, Meloidogyne incognita . Journal of American Science , 6 , 321–328.
- Barker, K. R. , & Hussey, R. S. (1976). Histopathology of nodular tissues of legumes infected with certain nematodes. Phytopathology , 66 , 851–855.10.1094/Phyto-66-851
- Callaham, D. , Deltredici, P. , & Torrey, J. G. (1978). Isolation and cultivation in vitro of the actinomycete causing root nodulation in comptonia. Science , 199 , 899–902. doi:10.1126/science.199.4331.899
- Chakraborty, U. , & Chakraborty, B. N. (1989). Interaction of Rhizobium leguminosarum and Fusarium solani f.sp. pisi in pea affecting disease development and phytoalexin production. Canadian Journal of Botany , 67 , 1698–1701.10.1139/b89-214
- Chakraborty, U. , & Purkayastha, R. P. (1984). Role of rhizobitoxine in protecting soybean roots from Macrophomina phaseolina infection. Canadian Journal of Microbiology , 30 , 285–289.10.1139/m84-043
- Chaudhary, K. K. , & Kaul, R. K. (2013). Efficacy of Pasteuria penetrans and various oil seed cakes in management of Meloidogyne incognita in chilli pepper (Capsicum annum L.). Journal of Agricultural Science and Technology , 15 , 617–626.
- Chen, J. , Abasi, G. S. , & Zuckerman, B. M. (1999). Suppression of Meloidogyne hapla and its damage to lettuce grown in a mineral soil amended with chitin and biocontrol organisms. Supplement to the Journal of Nematology , 31 , 719–725.
- Crow, W. T. , Guertal, E. A. , & Rodriguez-Kabana, R. (1996). Response of Meloidogyne arenaria and M. incognita to green manures and supplemental urea in glasshouse culture. Supplement to Journal of Nematology , 28 , 648–654.
- del Amor, F. M. , Serrano-Martínez, A. , Fortea, M. I. , Legua, P. , & Núñez-Delicado, E. (2008). The effect of plant-associative bacteria (Azospirillum and Pantoea) on the fruit quality of sweet pepper under limited nitrogen supply. Scientia Horticulturae , 117 , 191–196.10.1016/j.scienta.2008.04.006
- Ehteshamul-Haque, S. , & Ghaffar, A. (1993). Use of rhizobia in the control of root rot diseases of sunflower, okra, soybean and mungbean. Journal of Phytopathology , 138 , 157–163.10.1111/j.1439-0434.1993.tb01372.x
- FAO . (2013). Production of lentils by countries . UN Food & Agriculture Organization, Statistics Division. Retrieved March 24, 2015, from http://en.wikipedia.org/w/index.php?title=Lentil&oldid=655712616
- Hassan, M. A. , Chindo, P. S. , Marley, P. S. , & Alegbejo, M. D. (2010). Management of root knot nematodes (Meloidogyne spp.) on tomato (Lycopersicon lycopersicum) using organic wastes in Zaria, Nigeria. Plant Protection Sciences , 46 , 34–38.
- Hassan, S. M. E. , Rahman, M. S. , Amin, M. R. , Hoque, A. T. M. R. , & Islam, S. M. S. (2001). Effect of some organic substances on the root-knot disease of brinjal. Journal of Biological Sciences , 8 , 791–792.
- Hayat, R. , Ali, S. , Amara, U. , Khalid, R. , & Ahmed, I. (2010). Soil beneficial bacteria and their role in plant growth promotion: A review. Annals of Microbiology , 60 , 579–598. doi:10.1007/s13213-010-0117-1
- Huang, J. S. (1987). Interaction of nematode with rhizobia. In J. A. Veech & D. W. Dickson (Eds.), Vistas on nematology (pp. 301–306). Hyattsville, MD: Society of Nematologist.
- Hussaini, S. S. , & Seshadri, A. R. (1975). Interrelationship between Meloidogyne incognita and Rhizobium sp. on mung (Phaseolus aureus). Indian Journal of Nematology , 5 , 189–199.
- Hussey, R. S. , & Barker, K. R. (1976). Influence of nematode and light sources on growth and nodulation of soybean. Journal of Nematology , 8 , 48–52.
- Iqbal, M. A. , Khalid, M. , Shahzad, S. M. , Ahmad, M. , Soleman, N. , & Akhtar, N. (2012). Integrated use of rhizobium leguminosarum, plant growth promoting rhizobacteria and enriched compost for improving growth, nodulation and yield of lentil (Lens culinaris Medik.). Chilean Journal of Agricultural Research , 72 , 104–110.10.4067/S0718-58392012000100017
- Jones, J. T. , Haegeman, A. , Danchin, E. G. J. , Gaur, H. S. , Helder, J. , Jones, M. G. K. , Kikuchi, T. , … Perry, R. N. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology , 14 , 946–961. doi:10.1111/mpp.12057
- Jordan, D. C. (1984). Family III. Rhizobiaceae Conn 1938, 321 AI. In N. R. Krieg & J. G. Holt (Eds.), Bergey’s manual systematic bacteriology (Vol. 1, pp. 234–254). Baltimore, MD: Williams & Wilkins.
- Kloepper, J. W. , Lifshitz, R. , & Zablotowicz, R. M. (1989). Free-living bacterial inocula for enhancing crop productivity. Trends in Biotechnology , 7 , 39–44.10.1016/0167-7799(89)90057-7
- Kumar, S. , & Vadivalu, S. (1993). Inter-relationship between Meloidogyne incognita and rhizobium sp. black gram, vigna mungo (L) Hopper. Indian Journal of Nematology , 23 , 75–81.
- Lazarovits, G. , Tenuta, M. , & Conn, K. L. (2001). Organic amendments as a disease control strategy for soilborne diseases of high-value agricultural crops. Australasian Plant Pathology , 30 , 111–117.10.1071/AP01009
- Lechevalier, M. P. , & Lechevalier, H. A. (1989). Genus Frankia Brunchorst 1886, 174AL . In S. T. Williams , M. E. Sharpe , & J. G. Holt (Eds.), Bergey’s manual of systematic bacteriology (Vol. 4, pp. 2410–2417). Baltimore, MD: Williams & Wilkins.
- McSorley, R. , & Gallaher, R. N. (1996). Effect of yard-waste compost on nematode densities and maize yield. Supplement to the Journal of Nematology , 28 , 655–660.
- McSorley, R. , & Gallaher, R. N. (1997). Effect of compost and maize cultivars on plant parasitic nematodes. Supplement to the Journal of Nematology , 29 , 655–660.
- Muller, R. , & Gooch, P. S. (1982). Organic amendments in nematode control. An examination of the literature. Nematropica , 12 , 319–326.
- Nagaraju, M. , Karemegam, N. , & Kadalmani, B. (2010). Eco-friendly management of root-knot nematode Meloidogyne incognita using organic amendments on tomato. International Journal of Research in Pharmaceutical Sciences , 1 , 530–532.
- Natasasmita, S. (1997). Penggunaan tepung kulit udang dalam menekan serangan nematoda Meloidogyne spp. pada tanaman tomat (Lycopersicon esculentum Mill.). Journal of Agrikultura , 8 , 12–17.
- Nchore, S. B. , Waceke, J. W. , & Kariuki, G. M. (2012). Efficacy of selected agroindustrial wastes in managing root-knot nematodes on black nightshade in Kenya. ISRN Agronomy , 1–12, 364842. doi:10.5402/2012/364842
- Nutman, P. S. (1965). The relation between nodule bacteria and the legume host in the rhizosphere and in the rhizosphere and in the process of infection. In K. F. barker & W. C. Synder (Ed.), Ecology of soil-borne plant pathogens, prelude to biological control (pp. 231–247). Berkeley, CA: University of California Press.
- Oka, Y. (2010). Mechanisms of nematode suppression by organic soil amendments—a review. Applied Soil Ecology , 44 , 101–115.10.1016/j.apsoil.2009.11.003
- Orisajo, S. B. , Afolami, S. O. , Fademi, O. , & Atungwu, J. J. (2008). Effects of poultry litter and carbofuran soil amendments on Meloidogyne incognita attacks on cacao. Journal of Applied Biosciences , 7 , 214–221.
- Pakeerathan, K. , Mikunthan, G. , & Tharahani, N. (2009). Effects of different animal manures on Meloidogyne incognita (kofoid and white) on tomato. World Journal of Agriculture , 5 , 432–435.
- Patel, R. G. , & Patel, D. J. (1992). Comparative efficacy of some organic amendments and granular nematicides in the control of Rotylenchulus reniformis infecting pigeonpea. Indian Journal of Nematology , 22 , 14–18.
- Poornima, K. , & Vadivelu, S. (1993). Comparative efficacy of nematicides, oil cakes and plant extracts in the management of Meloidogyne incognita, Pratylenchus delattrei and Rotylenchulus reniformis on brinjal. Indian Journal of Nematology , 23 , 170–173.
- Renco, M. , & Kovácik, P. (2012). Response of plant-parasitic and free living soil nematodes to composted animal manure soil amendments. Journal of Nematology , 44 , 329–336.
- Riegel, C. , Fernandez, F. A. , & Noe, J. P. (1996). Meloidogyne incognita infested soil amended with chicken litter. Journal of Nematology , 28 , 369–378.
- Riegel, C. , & Noe, J. P. (2000). Chicken litter soil amendment effects on soilborne microbes and Meloidogyne incognita on cotton. Plant Disease , 84 , 1275–1281.10.1094/PDIS.2000.84.12.1275
- Siddiqui, I. A. , & Shaukat, S. S. (2002). Rhizobacteria-mediated induction of systemic resistance (ISR) in tomato against Meloidogyne javanica . Journal of Phytopathology , 150 , 469–473.10.1046/j.1439-0434.2002.00784.x
- Siddiqui, I. A. , & Shaukat, S. S. (2004). Systemic resistance in tomato induced by biocontrol bacteria against the root-knot nematode, Meloidogyne javanica is independent of salicylic acid production. Journal of Phytopathology , 152 , 48–54.10.1046/j.1439-0434.2003.00800.x
- Siddiqui, I. A. , Zareen, A. , Shauket, S. S. , & Zaki, M. J. (2001). Evaluation of rhizobia for control of Meloidogyne javanica in Vigna mungo . Pakistan Journal of Biological Sciences , 4 , 1124–1125.
- Siddiqui, Z. A. , Baghel, G. , & Akhtar, M. S. (2007). Biocontrol of Meloidogyne javanica by Rhizobium and plant growth-promoting rhizobacteria on lentil. World Journal of Microbiology and Biotechnology , 23 , 435–441. doi:10.1007/s11274-006-9244-z
- Siddiqui, Z. A. , & Husain, S. I. (1992). Interaction between Meloidogyne incognita race 3, Macrophomina phaseolina and Bradyrhizobium sp. in the root rot disease complex of chickpea, Cicer arietinum . Fundamental and Applied Nematology , 15 , 491–494.
- Siddiqui, Z. A. , & Mahmood, I. (1995). Role of plant symbionts in nematode management: A review. Bioresource Technology , 54 , 217–226.10.1016/0960-8524(95)00137-9
- Siddiqui, Z. A. , & Zehra, N. (2012). Biocontrol of wilt disease complex of pea using Pseudomonas fluorescens and Rhizobium sp. Archives of Phytopathology and Plant Protection , 45 , 2340–2346.10.1080/03235408.2012.727072
- Singh, S. , Hisamuddin , & Azam T. (2010). Interactive effects of Meloidogyne incognita and Macrophomina phaseolina on lentil. Indian Journal of Nematology , 40 , 31–34.
- Southey, J. F. (1986). Laboratory methods for work with plant and soil nematodes (Ministry of Agriculture, Fisheries and Food Reference Book 402, 202 pp.). London: HMSO.
- Stirling, G. R. (1991). Biological control of plant parasitic nematodes. Progress, problems and prospects . Wallingford: CAB International.
- Suganda, T. (1999). Natural chitinous amendment for controlling root-knot nematode (Meloidogyne spp.) of tomato. Journal Agrikultura , 10 , 17–19.
- Taha, A. H. Y. , & Raski, D. J. (1969). Inter relationship between root nodule bacteria, plant parasitic nematodes and their leguminous host. Journal of Nematology , 1 , 201–211.
- Thoden, T. C. , Korthals, G. W. , & Termorshuizen, A. J. (2011). Organic amendments and their influences on plant-parasitic and free-living nematodes: A promising method for nematode management. Nematology , 13 , 133–153.10.1163/138855410X541834
- Tiyagi, S. A. , Khan, A. V. , & Alam, M. M. (2001). Role of oil‐seed cakes for the management of plant‐parasitic nematodes and soil‐inhabiting fungi on lentil and mungben. Archives of Phytopathology and Plant Protection , 33 , 453–472.10.1080/03235400109383368
- Vance, C. P. , & Johnson, L. E. B. (1981). Nodulation: A plant disease perspective. Plant Disease , 65 , 118–128.10.1094/PD-65-118
- Wani, A. H. (2006). Management of root-knot nematode, Meloidogyne incognita, on okra and lentil by soil amendment with oil cakes and leaves of different plants. Nematologia Meditteranea , 34 , 83–87.
- Wani, A. H. , & Bhat, M. Y. (2011). Integrated management of root-knot nematode, Meloidogyne incognita infecting lentil using seed treatment and organic amendment. Plant Disease Research , 26 , 197. Retrieved from http://www.indianjournals.com/ijor.aspx?target=ijor:pdr&volume=26&issue=2&article=abs123
- Willer, H. , & Julia, L. (2014). The world of organic agriculture 2014: Summary. In H. Willer & L. Julia (Eds.), The world of organic agriculture. Statistics and emerging trends (pp. 25–32). Switzerland: International Federation of Organic Agriculture Movements (IFOAM).