Abstract
Natural antioxidants recently have gained popularity since synthetic ones have toxic and carcinogenic effects. In the present study, effect of temperature (120, 150 and 180°C) and cinnamaldehyde on oxidative stability of several oils (olive, hazelnut and palm oils) and fats (milkfat and butter) was examined. In order to compare the results with the synthetic antioxidant, butylated hydroxy toluene (BHT) was used at a concentration of 200 ppm. This level is the legal maximum limit allowed. Experiments were conducted by using a PetroOxy device, a rapid small scale oxidation stability test. According to induction time values obtained by PetroOxy device, the stability of oils drastically decreased with increasing temperature. Cinnamaldehyde had no significant effect (p > 0.05) on all fat and oil samples compared to control (no antioxidant added) and BHT added samples. BHT significantly increased induction times of all fat and oil samples at lower temperatures. However, it was not effective at 180°C (p < 0.05). It can be concluded that cinnamaldehyde could not be considered as a good alternative to BHT for preservation of fats and oils at high temperatures.
Public Interest Statement
The lipid oxidation generated during frying causes formation of rancid odours and flavours in the food product, contributing to decreases in nutritional quality and safety caused by the formation of potentially toxic secondary compounds. In addition, lipid oxidation reactions decrease the shelf life of oil. Therefore, it is very important to prevent/delay lipid oxidation from both economic and human health point of views. Addition of natural antioxidants and precursors present in the plant kingdom to oils is the best way of enhancing oxidative and flavour stability. Cinnamaldehyde is one of the active components available in cinnamon. This research provides information on the antioxidant activity and stability of cinnamaldehyde at frying temperatures in various fats and oils.
Competing interests
The authors declare no competing interests.
1. Introduction
Fats and oils have an important role in human life for healthy diet. Edible oils are known as good solvent/carrier for vitamins A, D, E and K. Moreover, they are good energy sources for the body. They are indispensable components of our diet due to their content of essential fatty acids. Fats and oils are not stable products; therefore, they deteriorate through a variety of chemical reactions in a short time, especially at high temperatures. The most important of these reactions is lipid oxidation reaction.
Through lipid oxidation the chemical structure of oil molecules is altered and the oil may eventually reach a level where it is no longer used for getting high quality products and it must be discarded (Kathirvel & Rupasinghe, Citation2011). The lipid oxidation also causes rancid odours and flavours of the food products. Besides the oil shelf life, it results in decreases in nutritional quality and safety caused by the formation of potentially toxic secondary compounds.
The most preferable way to inhibit the lipid oxidation mechanism is the use of antioxidants. Because of perceived harmful effects of synthetic antioxidants, investigations have focused on the using of natural antioxidants to stabilise the oils. Many studies have proven the effectiveness of natural antioxidants in protection of the oils from lipid oxidation (Al-Bandak & Oreopoulou, Citation2011; Chen et al., Citation2014; Inanc & Maskan, Citation2012; Rodrigues et al., Citation2012).
Plants are well known for their antioxidant properties because of their contents of mainly phenolic compounds. These compounds can be used to delay the oil deterioration. They can also protect the human body from many chronic diseases (Yong, Citation2007). Extracts, essential oils of plants and their active components are commonly used as natural antioxidants for stabilising the oils. Many studies with these plant-derived compounds as natural antioxidants in food products led the researchers to conclude that some plant compounds could be considered as proper alternatives to synthetic antioxidants. However, the essential oils have been observed to have an effect on the oxidative stability of edible oils, especially during storage (Al-Jaber, Awaad, & Moses, Citation2011; Chen et al., Citation2014; Maestri, Nepote, Lamarque, & Zygadio, Citation2006; Tomaino et al., Citation2005). In our previous study, various essential oils showed poor antioxidant activity during the accelerated oxidation test of corn oil, presumably due to low concentration of active components and interference of extraneous pro-oxidative materials in the essential oils (İnanç & Maskan, Citation2013).
In the current study, it was, thus, aimed to test the antioxidant effect of cinnamaldehyde, which is an active component of cinnamon, on fats and oils at frying temperatures. The effects of temperature (120, 150, 180°C) and cinnamaldehyde on the oxidative stability of several oils (olive, hazelnut and palm oils) and fats (milkfat and butter) were examined. The results were evaluated by means of the induction period of each sample measured by PetroOxy device. The results were compared with butylated hydroxy toluene (BHT) to understand whether the stability of fats/oils could be provided by using cinnamaldeyde as an antioxidant instead of synthetic ones.
2. Materials and methods
2.1. Materials
In this study, the materials used were different oils (refined olive, hazelnut and palm oils) and fats (milkfat and butter), an active component of cinnamon; cinnamaldehyde (≥95%) as natural antioxidants and a synthetic antioxidant, BHT. Oils and fresh butter were purchased from a local market in Gaziantep-Turkey. The fats and oils used did not contain any antioxidants originally. The selected fats and oils had different degrees of saturation/unsaturation. Milkfat is different from butter. It was obtained by melting butter and then separating the water phase from the medium. Oil phase was used in the study named as milkfat. Cinnamaldehyde and BHT were obtained from the Sigma Aldrich Co. (St. Louis, MO).
2.2. Sample preparation
Fats (milkfat and butter) were melted and 200 ppm antioxidant (cinnamaldehyde or BHT) was added slowly under stirring in order to distribute antioxidants homogeneously. In the case of oils, antioxidants added and stirred only.
2.3. Determination of ınduction period by accelerated oxidation method
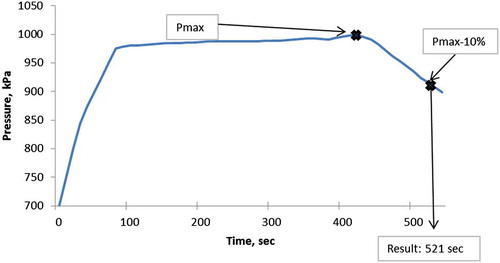
Induction period which is an indicator of oxidative stability of oil samples was measured as a response. Induction period is defined as the time prior to acceleration of lipid oxidation. From this definition, it can be easily understood that the substances having higher induction period have better oxidative stability. In this study, Petrotest PetroOxy device (Petrotest Instruments GmbH & Co. KG, Dahlewitz, Germany) which is basically similar to the Rancimat method was employed to measure the induction times of the samples. This device is new in the food science and technology researches. Therefore, it has not been widely used in oxidative stability test of foods up to now. The PetroOXY has a small hermetically sealed test chamber. Within the test chamber a small amount of sample, about 5 ml, is exposed to pure oxygen (99.9997% purity) at a pressure of 700 kPa and heated to the desired temperature. These test conditions provide with a very fast artificial ageing process. As the oil sample oxidises, it consumes the oxygen in the sealed test chamber resulting in a pressure drop. The induction period is determined as time elapsed between starting a test and the breaking point (Figure ). It is defined as a pressure drop of 10% below the maximum pressure detected in the test chamber as it warms up to test temperature as described by Neumann, Jebens, and Wierzbicki (Citation2008). Fat and oil samples were prepared as cinamaldehyde-added or BHT-added at a concentration of 200 ppm. The induction periods of samples with or without antioxidant were measured using the PetroOxy under 700 kPa of oxygen and the temperatures of 120, 150 and 180°C.
The data were analysed for significant differences by one-way analysis of variance and compared by Duncan’s multiple range test at the 5% significance level using SPSS 16.0 software.
3. Results and discussions
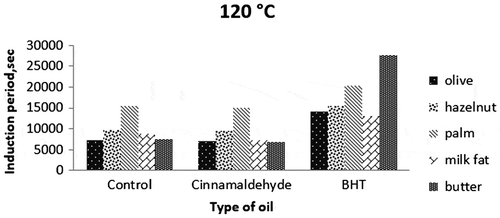
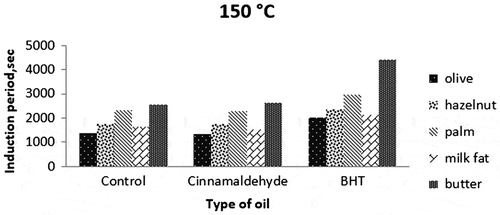
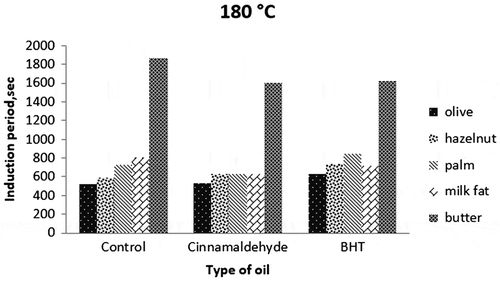
The induction periods of all samples were determined from PetroOxy in the presence of cinnamaldehyde, an active component used as a natural antioxidant and BHT as a synthetic antioxidant (200 mg/kg oil) at elevated temperatures (120, 150 and 180°C).
Previous studies have shown that cinnamaldehyde can act as an antioxidant at moderate temperatures (Singh, Maurya, deLampasona, & Catalan, Citation2007). Although it is known that cinnamon has a considerable antioxidant activity with numerous studies, these studies do not contain the activity of cinnamon at high temperatures (Brewer, Citation2011; Özcan & Arslan, Citation2011; Yanishlieva, Marinova, & Pokorný, Citation2006). In the present study, cinnamaldehyde only increased the induction period of hazelnut oil by 6% at 180°C (Table ). However, it generally did not affect significantly (p > 0.05) induction periods of all fat/oil samples, probably due to its volatility and low resistance to high temperatures. This result is correlated with one study, in which cinnamon oil was found not to exhibit antioxidant activity at high temperature despite its good activity at storage temperature (Tomaino et al., Citation2005). Therefore, it is probable that cinnamaldehyde could not increase the induction period of the corn oil due to its low resistance to heat. In addition to this, lack of synergy of pure cinnamaldehyde with other active components in cinnamon might be other reason for low activity of it in the samples. Because, the antioxidative effect depends on the environmental conditions (temperature, humidity, etc.), the degree of unsaturation of the oil and the content and chemical structure of other oil minor compounds present exert a synergistic effect. Marmesat, Morales, Velasco, and Dobarganes (Citation2010) also reported that some antioxidants rapidly degrade and cannot act as antioxidants at high temperatures in some oils. Also, the volatility of cinnamaldehyde may be another reason that it couldn’t preserve the fats and oils against oxidation in this study.
Table 1. Induction periods (IP) of fat/oil samples with or without antioxidants at different temperatures
BHT is known as a strong synthetic antioxidant commercially used in vegetable oils for extending the storage life. However, many studies showed that it is also effective in retarding the oxidative deterioration of oils at high temperatures (Chen et al., Citation2014; Shahidi, Citation2005). In the present study, the synthetic antioxidant BHT was found to be effective in the induction periods of all fat/oil samples at all temperatures (Figures ). According to Figures () and statistical analysis, antioxidative effect of BHT on all samples was clearly realised to be significantly positive (p < 0.05). The highest induction period (2,7582 s) was from butter sample with BHT at 120°C. It is followed by BHT-added palm oil at the same temperature (20,226 s). The effect of BHT was very low on the samples at 180°C because of dominant negative effect of high temperature on destabilisation of the antioxidants.
Variations between olive and hazelnut oils could partly be explained by differences in the degree of saturation and level of vitamin E, with high oleic content hazelnut oil being the most stable oil at all temperatures. The higher induction periods for palm oil compared to hazelnut and olive oils may be due to high saturated (stable) palm and stearic acid contents (about 50%). Palm oil was also found to be more stable than milk fat rich in monounsaturated fatty acids (80%). The stability of palm oil to oxidation is due to its palmitic acid content, a saturated fatty acid. An interesting result was obtained with butter (an oil–water emulsion). Its induction periods were higher than those of the other samples studied almost at all the temperatures. It can be explained by the protective role of water that was described by Dana, Blumenthal, and Saguy (Citation2003). They suggested that water bubbles injected into heated oil may provide protection via a distillation effect that drives out volatile oxidised substances and free radicals generated during the deep-frying process. The water in butter vaporises immediately at frying temperatures (120–180°C). It is hypothesised that volatile pro-oxidants are removed with the water, inducing a delay in deterioration. The results indicate a role for water in preventing lipid oxidation and decomposition in butter at these temperatures, suggesting that butter may be a preferable alternative for frying compared to other oils (Kirkhus et al., Citation2015).
4. Conclusion
The results of accelerated oxidation experiments revealed that temperature had a very significant negative effect on the oxidative stability of all fats and oils. It was also concluded that cinnamaldehyde did not have sufficient antioxidant activity to inhibit or retard the lipid oxidation in all samples. Thus, cinnamaldehyde cannot be considered as an alternative to BHT.
Additional information
Funding
Notes on contributors
Tuğba İnanç Horuz
Tuğba İnanç Horuz is a PhD student at University of Gaziantep, Faculty of Engineering, Food Engineering Department. She is working as a research assistant in the same faculty.
Medeni Maskan
Medeni Maskan is a professor at University of Gaziantep, Engineering Faculty, Food Engineering Department currently. His research programme focuses on Fats and Oils, Dehydration of Food Materials and Extrusion Technology. In particular, he is interested in essential oils and extracts with antioxidant potential, including the evaluation of the effects of frying process on the antioxidant property of the essential oils and extracts. He has done numerous studies on natural antioxidants and their uses in vegetable oils.
References
- Al-Bandak, G. , & Oreopoulou, V. (2011). Inhibition of lipid oxidation in fried chips and cookies by Majorana syriaca . International Journal of Food Science and Technology , 46 , 290–296.10.1111/ifs.2011.46.issue-2
- Al-Jaber, N. A. , Awaad, A. S. , & Moses, J. E. (2011). Review on some antioxidant plants growing in Arab world. Journal of Saudi Chemical Society , 15 , 293–307.10.1016/j.jscs.2011.07.004
- Brewer, M. S. (2011). Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Comprehensive Reviews in Food Science and Food Safety , 10 , 221–247.10.1111/crf3.2011.10.issue-4
- Chen, X. Q. , Zhang, Y. , Zu, Y. G. , Yang, L. , Lu, Q. , & Wang, W. (2014). Antioxidant effects of rosemary extracts on sunflower oil compared with synthetic antioxidants. International Journal of Food Science and Technology , 49 , 385–391.10.1111/ijfs.12311
- Dana, D. , Blumenthal, M. M. , & Saguy, I. S. (2003). The protective role of water injection on oil quality in deep fat frying conditions. European Food Research Technology , 217 , 104–109.10.1007/s00217-003-0744-x
- Inanc, T. , & Maskan, M. (2012). The potential application of plant essential oils/extracts as natural preservatives in oils during processing: A review. Journal of Food Science and Engineering , 2 , 1–9.
- İnanç, T. , & Maskan, M. (2013). Testing the antioxidant effect of essential oils and BHT on corn oil at frying temperatures: A response surface methodology. Journal of the American Oil Chemists’ Society , 90 , 1845–1850.10.1007/s11746-013-2351-8
- Kathirvel, P. , & Rupasinghe, H. P. V. (2011). Fish oil: Production, consumption and health benefits: Plant-derived antioxidants as potential omega-3 PUFA stabilizers . Hauppauge, NY: Nova Science Publishers.
- Kirkhus, B. , Lamglait, A. , Storrø, I. , Vogt, G. , Olsen, E. , Lundby, F. , & Standal, H. (2015). The role of water in protection against thermal deterioration of liquid margarine. Journal of the American Oil Chemists’ Society , 92 , 215–223.10.1007/s11746-014-2589-9
- Maestri, D. M. , Nepote, V. , Lamarque, A. L. , & Zygadio, J. A. (2006). Natural products as antioxidants. Research Signposts , 1 , 105–135.
- Marmesat, S. , Morales, A. , Velasco, J. , & Dobarganes, M. C. (2010). Action and fate of natural and synthetic antioxidants during frying. Grasas y Aceites , 61 , 333–340.
- Neumann, A. , Jebens, T. , & Wierzbicki, V. (2008). A method for determining oxidation stability of petrodiesel, biodiesel, and blended fuels. American Laboratory , 40 , 22–26.
- Özcan, M. M. , & Arslan, D. (2011). Antioxidant effect of essential oils of rosemary, clove and cinnamon on hazelnut and poppy oils. Food Chemistry , 129 , 171–174.10.1016/j.foodchem.2011.01.055
- Rodrigues, N. , Malheiro, R. , Casal, S. , Asensio-S.-Manzanera, M. C. , Bento, A. ,& Pereira, J. A. (2012). Influence of spike lavender (Lavandula latifolia Med.) essential oil in the quality, stability and composition of soybean oil during microwave heating. Food and Chemical Toxicology , 50 , 2894–2901.10.1016/j.fct.2012.05.020
- Shahidi, F. (2005). Bailey’s ındustrial oil and fat products . New York, NY: Wiley.10.1002/047167849X
- Singh, G. , Maurya, S. , deLampasona, M. P. , & Catalan, C. A. N. (2007). A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food and Chemical Toxicology , 45 , 1650–1661.10.1016/j.fct.2007.02.031
- Tomaino, A. , Cimino, F. , Zimbalatti, V. , Venuti, V. , Sulfaro, V. , De Pasquale, A. , & Saija, A. (2005). Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chemistry , 89 , 549–554.10.1016/j.foodchem.2004.03.011
- Yanishlieva, N. V. , Marinova, E. , & Pokorný, J. (2006). Natural antioxidants from herbs and spices. European Journal of Lipid Science and Technology , 108 , 776–793.10.1002/(ISSN)1438-9312
- Yong, Y. S. (2007). Determination of synthetic phenolic antioxidants in food items using HPLC and total antioxidants using FIA approaches (Master thesis). Malaysia: Universiti Sains Malaysia.