?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A central composite rotatable design was applied to analyse the effects of independent variables [soaking time (ST), germination time (Gt) and temperature (GT)] on responses [antioxidant activity (AoxA), total phenolic contents (TPC) and flavonoid contents (TFC)]. The results indicated that with increase in ST, Gt and GT, AoxA, TPC (free/bound) and TFC (free/bound) of foxtail millet increased significantly. The best combination of germination bioprocess variables for producing optimized germinated foxtail millet flour with the highest AoxA (90.5%), TPC (45.67 mg gallic acid equivalent (GAE)/100 g sample) and TFC (30.52–43.96 mg RU/g sample) were found with soaking time of 15.84 min having germination temperature of 25°C. The optimized germinated foxtail millet flour was nutritionally rich as it produced higher protein (14.32 g/100 g), dietary fibre (27.42 g/100 g), calcium (25.62 mg/kg), iron (54.23 mg/kg), magnesium (107.16 mg/kg) and sodium (69.45 mg/kg) per kg as compared to un-germinated foxtail millet flour.
Public Interest Statement
The foxtail millet is a minor millet. It has many nutritional and medicinal properties and is recommended in ayurvedic and unani products. The present study was carried out to optimize the germinations conditions of millet seed in order to enhance its nutritional status. Also, analysing the effect of germination on seed composition, bioactive compounds and other nutrients bioavailability. The study shows that the germination enhanced and modified foxtail millet composition, availability of higher amount of bioactive compounds such as total phenolics, antioxidants, total flavonoid, dietary fibre, protein, minerals and lowered anti-nutritional factors. This results in offering the inherent health benefitting substances especially phytochemicals to the consumers from millets. Furthermore, germinated foxtail millet grains can be used in food formulations and product development especially functional/health foods. Also the flour of the germinated seeds can be used added in composite flours, in the preparation of various bakery and home-made products.
Competing interests
The authors declare no competing interest.
1. Introduction
According to world agriculture production report, millets are one of the most important drought-resistant crops and depict 6th rank among cereal crops (Devi, Vijayabharathi, Sathyabama, Malleshi, & Priyadarisini, Citation2011). Foxtail millet (Setaria italica) also called as Kangni, tenai, kakun and navane, is generally grown as a rain-fed crop in India, besides China and Bangladesh. It has been reported that foxtail millet has many nutritional and medicinal properties and is recommended in ayurvedic and unani products by the practitioners (Adekunle, Citation2012). Foxtail millet is non-glutinous, like buckwheat and quinoa, and is a non-acid-generating food, hence considered as easily digestible food (Prashant, Namakkal, & Chandra, Citation2005), also possesses the higher amount of proteins and minerals (Pawar & Pawar, Citation1997), act as a potential functional food ingredient and a supplementary protein source to most cereals, due to its high lysine content (Mohamed, Zhu, Issoufou, Fatmata, & Zhou, Citation2009).
The availability of minerals increased during germination is due to the catabolism of anti-nutrients like polyphenol and saponins which hinder the bioavailability of minerals (Grewal & Jood, Citation2006). Coulibaly and Chen (Citation2011) described that germination of foxtail millet for 3 days resulted in production of high concentration of minerals. The consumption of sprouts as functional food can be considered very important in reducing human diseases related with oxidative stress due to the presence of antioxidants (Silva, Pereira, & Azevedo, Citation2013). A negligible amount of research on its antioxidant property has been carried out by researchers (Asharani, Jayadeep, & Malleshi, Citation2010). Until now no research has been done on foxtail millet commercially available, which is utilized in various forms for human consumption.
For optimizing complex processes, response surface methodology (RSM) is an efficient statistical technique. The main advantage of RSM is to reduce the number of experimental trials needed to evaluate multiple parameters and their interactions with less laborious and time-consuming (Irakoze, Haihua, Qin, & Huiming, Citation2010). The extraction process variables, such as anthocyanins, phenolic compounds, polysaccharides, vitamin E and protein from varied materials can be optimizing by using RSM (Chandrika & Fereidoon, Citation2005). Mora-Escobedo, Paredes-Lopez, and Dominguez (Citation1991) also carried out the optimization of germination bioprocess of amaranth. Yet no research has been done based pn optimization of germination bioprocess to increase the bioactive compounds of foxtail millet grain. The aim of the present research was to reveal the appreciable bioactive, phytochemical and antioxidant potency of the less explored commercially available foxtail millet and optimization of the germination parameters to get the promising results
2. Materials and methods
2.1. Procurement of raw material
Foxtail millet grains were purchased from authorized seed centre located in Punjab (India). Seed were 3 months old and were of appropriate viability when tested during an initial check. The seeds were stored at 4°C for further analysis. The AR grade chemicals (Folin–Ciocalteu reagent, gallic acid (GA), sodium carbonate, ethanol, ethyl acetate, HCL, ammonium thiocyanate, Iron(III)chloride, tannic acid, hexane, NaOH and aluminium chloride purchased from Loba Chemie Pvt. Ltd, Mumbai; 2,2-diphenyl picryl hydrazyl (DPPH) purchased from Fluka Goldie, Mumbai; acetone and methanol (HPLC grade) purchased from Ranbaxy, New Delhi) were used for study.
2.2. Preparation of germinated foxtail millet flours
Foxtail millets were washed and soaked in tap water at room temperature at variable time intervals (8–16 h). Soaked seeds were germinated in a pilot-scale seed germinator (Seed Germinator Macro Scientific Works Pvt. Ltd, India) at different temperatures (19.55–46.55°C) (Kamkar, Koocheki, Nassiri Mahallati, & Rezvani Moghaddam, Citation2006) and time periods (13.18–46.82 h). A relative humidity of 80–90% within the chamber was maintained by using trays with water. The resulting bioprocessed foxtail millet seeds were dried (45°C/8 h) to final moisture content of 7–8%, cooled to 25°C and ground in a lab grinder to obtain germinated foxtail millet flours. The un-germinated foxtail millet flour was used as control. The resulting flours were packed and kept at 4°C in air tight containers for further analysis.
2.3. RSM experimental design and optimization
RSM is a commonly employed tool in analysing experimental data resulting in the optimization of processes or products. It is useful for the simultaneous optimization of the process variables (independent variables) to obtain desired response variables (dependent variables). RSM was used to derive the optimum levels of the independent variables using a three-factor five-level central composite rotatable design which dictated 20 experimental combinations including six replicates at the centre point (Table ). The independent variables chosen were ST, GT and Gt, which varied from 8 to 18.72 h, 25 to 46.54°C and 20 to 46.81 h, respectively. The three responses selected were total phenolic contents (TPC), total flavonoid contents (TFC) and antioxidant activity (AoxA) of germinated foxtail millet flour. The data was analysed by multiple regressions using the least-squares method. A second-order polynomial equation was used to express the responses as a function of the independent variables which is given below:
Table 1. Experimental designTable Footnote a used to obtain different combinations of soaking time, germination temperature/germination time for producing germinated foxtail flours, and experimental results for response variables
where Y k is the response variable; Y 1 is the TPC (mg GAE/100 g); Y 2 is the AoxA (%); and Y 3 is the TFC (RU/g); x i represented the coded independent variables (x 1 is the soaking time, x 2 is the germination temperature, x 3 is the germination time,); where β k0 was the value of the fitted response at the centre point of the design, i.e. point (0, 0, 0). β ki , β kii and β kij were the linear, quadratic and cross-product regression coefficients, respectively. The test of statistical significance was performed on the total error criteria, with a 95% confidence level. For each response, the significant terms in the model were determined by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test comparison among means with 5% of significance level. The statistical software Design Expert version 7.0.0 (Stat-Ease, Minneapolis, MN, USA) was used for the RSM analyses.
This study was conducted to find the best soaking and germination conditions of foxtail millet grain that would maximize the TPC, AoxA and TFC, and minimize the antinutritional factors. The RSM model could have developed the equations that could predict the quality of the germinated foxtail millet flour. This work highlighted the different biochemical modifications that occurred in foxtail millet grain during soaking and germination which resulted in improvement of the nutraceutical properties of foxtail millet flour acceptable to brewers and infant foods.
2.4. Chemical composition
The chemical composition like fat and protein content of the foxtail millet flours (raw and optimized germinated samples) were determined by the method of AOAC (Citation1995). Minerals (calcium, magnesium, iron and sodium) were analysed by atomic absorption spectrophotometry (AAS4141, India) after digestion with concentrated nitric acid (AOAC 985.35, Citation2012). Total dietary fibre of samples was determined by using method determined by IS-11062 (Citation1984).
2.5. Extraction of free and bound phenolic compounds
Free phenolic compounds were extracted by the method of Dewanto, Wu, and Liu (Citation2002). One gram of ground sample was blended with 10 mL of 80% chilled ethanol for 10 min and then centrifuged at (2,500 g for 10 min). The supernatant was concentrated under vacuum at 45°C and stored at −20°C until its evaluation. Bound phenolic compounds were extracted from the residue according to method of Adom and Liu (Citation2002). The residue of the flour samples obtained after extraction of soluble phenolics was hydrolyzed at room temperature with 2M NaOH with continuous stirring for 4 h. The resulting mixture was set to pH 2 by adding 6 M HCl and extracted five times with hexane to remove fatty acids released upon alkali digestion. The bound phenolic compounds were extracted five times with 10 mL of ethyl acetate and dried under vacuum at 35°C. The bound phenolic compounds were reconstituted in 2 mL of methanol–water (50:50 v/v). The extract was frozen and stored at −20°C until further evaluation.
2.6. Estimation of TPC and TFC
The TPC and TFC of free and bound extracts from ground samples were determined spectrophotometrically using the methods of Prior, Wu, and Schaich (Citation2005) and Miliauskas, Venskutonis, and van Beek (Citation2004), respectively. The absorbance was measured using a spectrophotometer (HACH DR 6000, Germany). Total phenolics were expressed as milligrams of gallic acid equivalents (mg GAE)/g sample (db), while total flavonoids were expressed as milligrams rutin equivalents (mg Ru)/g sample (db). All measurements were made in triplicate.
2.7. Antioxidant activity
The AoxA of native and processed foxtail millet extracts were measured by the DPPH radical scavenging method (Blois, Citation1958). An aliquot of the sample extract was added to 1 M methanolic solution of DPPH. The mixture was kept for 30 min for incubation. After measuring the absorbance at 517 nm, activity was expressed as percentage DPPH scavenging relative to control using the following equation:
2.8. Assay of antinutritional factors
Antinutritional factors such as phytic acid and tannins were determined by following methods:
Phytates were quantitatively determined according to the method as described by Wheeler and Ferrel (Citation1971). A 4 g of the grounded sample was soaked in 100 mL of 2% HCl for 3 h and then filtered through Watt man No. 1 filter paper. Twenty-five millilitre of the filtrate was placed in a 100-mL conical flask and an indicator consisting of 5 mL of 0.3% ammonium thiocyanate solution was added. Distilled water (53.5 mL) was added to the mixture after setting it to proper acidity; this was titrated with a standards iron(III) chloride solution, having about 0.00195 g of iron per millilitre, till a brownish-yellow colour appeared, which remained for 5 min.
where T is the titre value; M is the Molar mass of phytate.
Tannins were determined according to the Vanillin–HCl method of Price and Butler (Citation1987) as modified by Chang, Collins, Bailey, and Coffey (Citation1994). Five gram of defatted foxtail millet was used for extraction of tannins by using acidic methanol. One mL of appropriately diluted extract was put in a test tube and 5 mL of freshly prepared Vanillin–HCl reagent was added to it slowly with mixing and colour developed was read at 500 nm. The standard curve of tannic acid was prepared according to AOAC (Citation2004) for measurement the concentration of tannin in the samples [plotting the concentration of tannin acid (mg) against the corresponding reading of spectrophotometer in absorbance]. The results were showed as mg/100 g dry weight basis with the help of following equation:
where C is the Concentration corresponding to the Optical density.
3. Results and discussion
3.1. Experimental design
Predictive regression equation models for AoxA, TPC and TFC were selected as given below. The AoxA, TPC and TFC of germinated foxtail millet flours varied from 62.45 to 90.54%, 23.03 to 45.67 mg GAE/100 g and 30.52 to 43.96 mg RU/g, respectively (Table ). Analysis of variance showed that AoxA, TPC and TFC were significantly dependent on ST, Gt and GT. Predictive models using un-coded variables for the response variable (AoxA, TPC and TFC) were:
ANOVA showed that the fitted models were suitable showing significant regression, low residual values, no lack of fit with satisfactory determination coefficients (R 2) of 0.94, 0.94 and 0.99 for the responses (TPC, AoxA and TFC), respectively, and the relative dispersion of the experimental points from the predictions of the models (CV) was found to be <10%. These results indicated the suitability of the model to be used in optimizing the conditions of germination for the foxtail millet.
3.2. Optimization
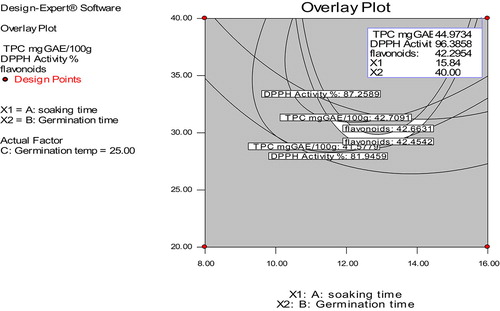
A numerical multi-response optimization technique of RSM was applied to determine the optimum combination of soaking time, germination time and germination temperature for the production of germinated foxtail millet flour with highest AoxA, TPC and TFC. The superimposition of contour plots was utilized to determine the best combination of process variables for production of germinated foxtail millet flour as shown in Figure . The central point of the optimization region in Figure corresponds to the optimum combination of process variables [Soaking time (ST) = 15.84 h, Germination temperature (GT) = 25°C and germination time (Gt) = 40 h]. The predicted values of TPC, AoxA and TFC were 44.97 mg GAE/100 sample, 96.38% and 42.28 mg RU/g, respectively, while experimental values obtained were 90.54%, 45.67 mg GAE, 43.96 RU mg/g, respectively. Least difference was observed between the experimental and predicted values indicating the suitability of the model to be used in optimizing the germination process for foxtail millet.
3.3. Effect of germination on the nutritional components
Germination affects protein and lipid contents of foxtail millet seeds (Table ). The protein content of optimized germinated foxtail millet after 46.5 h germination showed a significant (p < 0.05) increase as compared to the raw seeds. This increase in protein content may be attributed to biological synthesis of new amino acids during germination decrease in carbohydrates (Mbithi-Mwikya, Camp, Yiru, & Huyghebaert, Citation2000). Lestienne, Icard-Vernière, Mouquet, Picq, and Trèche (Citation2005) reported a slight but significant increase in protein content of finger millet during germination. Similar results were noticed by other researcher (Wanasundara, Wanasundara, & 7 Shahidi, Citation1999). Fat content was affected by germination period. Fat content for raw and optimized flour was 4.4 and 3.6%, respectively (Table ), which was significantly (p < 0.05) lower than un-germinated sample. The fat content decreased after germination because of its use as energy source during germination process. (Kanensi, Ochola, Gikonyo, & Makokha, Citation2011; Mostafa & Rahma, Citation1987; Mubarak, Citation2005) reported a significant decrease in fat content during germination in soybean, mung and amaranth seeds, respectively.
Table 2. Chemical composition, phenolic content, flavonoid content, antinutritional factors and mineral contents of foxtail millet
3.4. Effect of germination on the dietary fibres
After germination bioprocess, the total dietary fibres in foxtail seeds increased from 22.20 to 27.42% (Table ). The fibre content increased significantly (p < 0.05) with increasing germination time due to modification of structure of cell wall polysaccharides of the seeds, possibly by affecting the intactness of tissue histology and disrupting the protein carbohydrate interaction. This involved extensive cell wall biosynthesis and therefore the production of new dietary fibre (Martin-Cabrejas, Ariza, & Esteban, Citation2003). A similar result of effect of germination on the dietary fibre in foxtail millet was reported by Duodu (Citation2014).
3.5. Effect of germination on phenolics and flavonoids
Comparing the non-germinated and germinated foxtail millet, free, bound and the total phenolics content increased significantly (p < 0.05) from 9.79 to 21.75 mg (GAE)/100 g, 23.38 to 35.42 mg (GAE)/100 g and 33.17 to 57.17 mg (GAE)/100 g, respectively (Table ). The concentration of phenolic compounds in foxtail millet seeds increased due to cell wall-degrading enzymes, which became active during germination and modified the cell wall structure of the grain. The significance of this lies in the fact that phenolic compounds such as hydroxycinnamates (e.g. ferulic and p-coumaric acids) remain bound to non-starch polysaccharides in grain cell walls through associations such as ester and ether bonds. The bound phenolic compounds get liberated by the action of cell wall-degrading enzymes (mainly esterases) on these bonds (Duodu, Citation2014). Free, bound and total flavonoids of foxtail millet increased significantly (p < 0.05) after germination from 12.43 to 25.74 mg RU/g, 15.67 to 31.98 mg RU/g and 27.10 to 57.72 mg RU/g, respectively. Similar findings were obtained with amaranth for free, bound and total phenolic and free, bound and TFC during germination (Perales-Sánchez et al., Citation2014).
3.6. Effect of germination on mineral contents
Germination increased iron, magnesium, calcium and sodium contents of foxtail millet flour significantly (p < 0.05) (Table ). Phytic acid as powerful chelating agent reduces the bioavailability of mineral content by the formation of insoluble complexes (Lestienne et al., Citation2005; Wheeler & Ferrel, Citation1971). During the germination phytase gets activated which (myo-inositol hexakinase-phosphate phosphor-hydrolases) is a phytate-specific phosphatases that hydrolyzes phytate to inositol and free orthophosphate and releases minerals. Hübner, O’Neil, Cashman, and Arendt (Citation2010) also observed an increase in mineral content in oat and barley during germination.
3.7. Effect of germination on antinutritional factors
After germination bioprocess, the tannin and phytate contents in foxtail seeds decreased from 2.803 to 0.983 mg/100 g and 0.341 to 0.102 mol/kg, respectively (Table ). The antinutritional factors decreased significantly (p < 0.05) with increase in germination time due to hydrolytic activity of the enzyme phytase that increases during germination (Sinha & Kawatra, Citation2003). Throughout the germination, phytate phosphorus hydrolysis into inositol monophosphate by the phytase activity to contributes to the decrease in phytic acid. During the periods of germination procedure, a significant decrease in tannin content could be indicated due to the leaching of soluble tannin compounds during soaking which further gets reduced during subsequent germination (Hussain, Uddin, & Aziz, Citation2011).
4. Conclusions
TPC, AoxA and TFC of optimized germinated foxtail millet flour were affected by the soaking time, germination time and germination temperature. The present study showed that optimized germinated foxtail millet flour contained higher TPC, AoxA, TFC, dietary fibre, protein, magnesium, calcium, iron, sodium and lowered antinutritional factors. Germinated foxtail millet may offer the inherent health benefits especially phytochemicals to the consumer. Furthermore, the results of this study explore the possible potential utilization of germinated millet grains in food formulations and product development especially functional/health foods in developing countries.
Additional information
Funding
Notes on contributors
Seema Sharma
Seema Sharma is a doctoral fellow at Sant Longowal Institute, Sangrur, Punjab. Her research is on nutritional aspects of minor millets and their utilization in product development.
Dharmesh C. Saxena
Dharmesh C. Saxena is professor at Sant Longowal Institute, Sangrur, Punjab with specialization in Cereal Technology. He has published a number of research papers in journals of repute, author and editor of books.
Charanjit Singh Riar
Charanjit Singh Riar is professor at Sant Longowal Institute, Sangrur, Punjab with specialization in Cereal Processing & Technology. He has published a number of research papers in journals of repute, books and book chapters. The group of authors is working on germination and its impact on nutritional status of varied food grains including cereals, pulses and legumes, in vitro digestibility studies and protein profile. The work is in progress to study the impact of germination on extraction of bioactive compounds from minor millets and product developments.
References
- Adekunle, A. A. (2012). Agricultural innovation in sub-Saharan Africa: Experiences from multiple stakeholder approaches . Forum for Agricultural Research in Africa, Ghana. 9988-8373-2-4.
- Adom, K. K. , & Liu, R. H. (2002). Antioxidant Activity of Grains. Journal of Agricultural and Food Chemistry , 50 , 6182–6187.10.1021/jf0205099
- AOAC . (1995). Official methods of analysis (15th ed.). Washington, DC: Author.
- AOAC . (2004). Official methods of analysis (13th ed.). Washington, DC: Author.
- AOAC . (2012). Official methods of analysis (19th ed.). Washington, DC: Author.
- Asharani, V. T. , Jayadeep, A. , & Malleshi, N. G. (2010). Natural antioxidants in edible flours of selected small millets. International Journal of Food Properties , 13 , 41–50.10.1080/10942910802163105
- Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature , 181 , 1199–1200.10.1038/1811199a0
- Chandrika, L. P. , & Fereidoon, S. (2005). Optimization of extraction of phenolic compounds from wheat by using response surface methodology. Journal of Food Chemistry , 93 , 7–56.
- Chang, M. J. , Collins, J. L. , Bailey, J. W. , & Coffey, D. L. (1994). Cowpeas tannins related to cultivar, maturity, dehulling and heating. Journal of Food Science , 59 , 1034–1036.10.1111/jfds.1994.59.issue-5
- Coulibaly, A. , & Chen, J. (2011). Evaluation of energetic compounds, antioxidant capacity, some vitamins and minerals, phytase and amylase activity during germination of foxtail millet. American Journal of Food Technology , 6 , 40–51.
- Devi, P. B. , Vijayabharathi, R. , Sathyabama, S. , Malleshi, N. G. , & Priyadarisini, V. B . (2011). Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: A review. Journal of Food Science & Technology . doi:10.1007/s13197-011-0584-9
- Dewanto, V. , Wu, X. , & Liu, R. H. (2002). Processed sweet corn has higher antioxidant activity. Journal of Agricultural and Food Chemistry , 50 , 4959–4964.10.1021/jf0255937
- Duodu, K. G. (2014). Effects of processing on phenolic phytochemicals in cereals and legumes. Cereal Foods World , 59 , 64–70.10.1094/CFW-59-2-0064
- Grewal, A. , & Jood, S. (2006). Effect of processing treatments on nutritional and antinutritional contents of green gram. Journal of Food Biochemistry , 30 , 535–546.10.1111/jfbc.2006.30.issue-5
- Hübner, F. , O’Neil, T. , Cashman, K. D. , & Arendt, E. K. (2010). The influence of germination conditions on beta-glucan, dietary fibre and phytate during the germination of oats and barley. European Food Research Technology , 231 , 27–35.
- Hussain, I. , Uddin, M. B. , & Aziz, M. G. (2011). Optimization of antinutritional factors from germinated wheat and mungbean by response surface methodology. International Food Research Journal , 18 , 957–963.
- Irakoze, P. C. , Haihua, L. , Qin, K. Zhou , & Huiming, Z. (2010). Optimization of ultrasonic extraction of polysaccharides from Chinese malted sorghum using response surface methodology. Pakistan Journal of Nutrition , 4 , 336–342.
- IS-11062 . (1984). Indian standard method for estimation of total dietary fibre in foodstuff .
- Kamkar, B. , Koocheki, A. , Nassiri Mahallati, M. , & Rezvani Moghaddam, P. (2006). Cardinal temperatures for germination in three millet species (Panicum miliaceum, Pennisetum glaucum and Setaria italica). Asian Journal of Plant Sciences , 5 , 316–319.
- Kanensi, O. J. , Ochola, S. , Gikonyo, N. K. , & Makokha, A. (2011). Optimization of the period of steeping and germination for amaranth grain. Journal of Agricultural and Food Technology , 1 , 101–105.
- Lestienne, I. , Icard-Vernière, C. , Mouquet, C. , Picq, C. , & Trèche, S. (2005). Effects of soaking whole cereal and legume seeds on iron, zinc, and phytate contents. Food Chemistry , 89 , 421–425.10.1016/j.foodchem.2004.03.040
- Martin-Cabrejas, M. A. , Ariza, N. , & Esteban, R. (2003). Effect of germination on the carbohydrate composition of the dietary fibre of peas (Pisum sativum L.). Journal of Agriculture & Food Chemistry , 51 , 1254–1259.
- Mbithi-Mwikya, S. , Camp, J. V. , Yiru, Y. , & Huyghebaert, A. (2000). Nutrient and antinutrient changes in finger millet (Eleusine coracan) during sprouting. Lebensm LWT-Food Science & Technology , 33 , 9–14.
- Miliauskas, G. , Venskutonis, P. R. , & van Beek, T. A. (2004). Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry , 85 , 231–237.10.1016/j.foodchem.2003.05.007
- Mohamed, T. K. , Zhu, K. , Issoufou, A. , Fatmata, T. , & Zhou, H. (2009). Functionality, in vitro digestibility and physicochemical properties of two varieties of defatted foxtail millet protein concentrates. International Journal of Molecular Science , 10 , 5224–5238.
- Mora-Escobedo, R. , Paredes-Lopez, O. , & Dominguez, J. (1991). Optimization of a germination procedure by response surface methodology. LWT-Food Science & Technology , 24 , 518–522.
- Mostafa, M. M. , & Rahma, E. H. (1987). Chemical and nutritional changes in soybean during germination. Food Chemistry , 23 , 257–275.10.1016/0308-8146(87)90113-0
- Mubarak, A. E. (2005). Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chemistry , 89 , 489–495.10.1016/j.foodchem.2004.01.007
- Pawar, V. S. , & Pawar, V. D . (1997). Malting characteristics and biochemical changes of foxtail millet. Journal of Food Science & Technology , 34, 416–418.
- Perales-Sánchez, J. X. K. , Reyes-Moreno, C. , Gómez-Favela, M. A. , Milán-Carrillo, J. , Cuevas-Rodríguez, E. O. , Valdez-Ortiz, A. , & Gutiérrez-Dorado, R. (2014). Increasing the antioxidant activity, total phenolic and flavonoid contents by optimizing the germination conditions of amaranth seeds. Plant Foods Human Nutrition , 69 , 196–202.
- Prashant, S. H. , Namakkal, S. R. , & Chandra, T. S. (2005). Effect of the antioxidant properties of millet species on oxidative stress and glycemic status in alloxan-induced rats. Nutrition Research , 25 , 1109–1120.
- Price, M. L. , & Butler, L. C . (1987). Tannin and nutrition ( Station Bulletin No. 272). West Lafayette, IN: Purdue University, Agriculture Experiment Station.
- Prior, R. L. , Wu, X. , & Schaich, K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agriculture & Food Chemistry , 53 , 4290–4302.
- Silva, L. R. , Pereira, M. J. , & Azevedo, J. (2013). Glycine max (L.) Merr., Vigna radiata L. and Medicago sativa L. sprouts: A natural source of bioactive compounds. Food Research International , 50 , 167–175.10.1016/j.foodres.2012.10.025
- Sinha, R. , & Kawatra, A. (2003). Effect of processing on phytic acid and polyphenol contents of cowpeas [Vigna unguiculata (L) Walp]. Plant Foods for Human Nutrition , 58 , 1–8.10.1023/B:QUAL.0000040322.01063.d4
- Wanasundara, P. K. J. P. D. , Wanasundara, U. N. , & 7 Shahidi, F. (1999). Changes in flax (Linumus itatissimum L.) seed lipids during germination. Journal of Oil & Chemical Society , 76 , 41–48.
- Wheeler, E. L. , & Ferrel, R. E. (1971). A method for phytic acid determination in wheat fractions. Cereal Chemistry , 48 , 312–316.