?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Moisture sorption isotherms of rice-based instant soup mix at temperature range 15–45°C and relative humidity from 0.11 to 0.86 were determined using the standard gravimetric static method. The experimental sorption curves were fitted by five equations: Chung-Pfost, GAB, Henderson, Kuhn, and Oswin. The sorption isotherms of soup mix decreased with increasing temperature, exhibited type II behavior according to BET classification. The GAB, Henderson, Kuhn, and Oswin models were found to be the most suitable for describing the sorption curves. The isosteric heat of sorption of water was determined from the equilibrium data at different temperatures. It decreased as moisture content increased and was found to be a polynomial function of moisture content. The study has provided information and data useful in large-scale commercial production of soup and have great importance to combat the problem of protein-energy malnutrition in underdeveloped and developing countries.
Public Interest Statement
Rice-based instant soup mix belongs to a class of food powders that is hygroscopic by nature. Moisture sorption isotherms have an important role to play in the quantitative approach to the prediction of the shelf life of dried foods due to their sensitivity to moisture changes. Equations for fitting water sorption isotherms in foods are important in aspects of food preservation by dehydration, packaging, and storage life of a food product. Moisture sorption isotherms of rice-based instant soup mix obtained in the temperature range of 15–45°C were sigmoid type II, common to most foods. Compatibilities of food items can be explored, which are to be packaged together, can be determined with good reliability.
Competing interests
The authors declare no competing interest.
1. Introduction
Soup mix is a product of complex heterogeneous food category. Instant soup plays an important role in the nutrition of people as they satisfy the present and future consumer requirements (Singh & Prasad, Citation2014b), also mainly depends upon its ingredients as an important criterion that contributes to the final formulation of the product. It is often served as the starter, first course or entree before the main meal as it stimulates appetite and provides quick nourishment, which is mainly responsible for the improvement of appetite and gastrointestinal responses (Cecil, Francis, & Read, Citation1999).
The commercial production of soups depends on characteristic behavior before and after preparation. An easy and convenient way of making a soup is to use a soup base in the form of granule and powder material (Prasad & Singh, Citation2014b). The quality of most dried foods depends to a great extent upon their physical, chemical, and microbiological stability. All dehydrated products absorb moisture at moderate and high water activities that leads to flavor and quality deterioration.
Moisture sorption isotherm food product describes the relationship between equilibrium moisture content and relative humidity at constant temperature (Jamali et al., Citation2006). It gives an insight into the moisture binding characteristics, sorption mechanisms, and interaction of food biomaterial with water. The moisture sorption properties of foods have been shown to be influenced by food composition, processing treatment, temperature, and relative humidity (Iglesias & Chirife, Citation1976a). In addition, it is also extremely important for predicting storage life and appropriate packaging material (Gal, Citation1987). There are four common practical application of isotherms related to food processing: drying (in order to save energy and establish the optimal processing conditions), mixing (to determine the water activity of the mixture from the isotherms of each component), packaging (to determine the permeability of the packing), and storage (to lengthen the shelf life of foods) (Labuza, Citation1984).
Static gravimetric method was preferred to determine the sorption isotherms of food materials by many authors (Aguerre, Suarez, & Viollaz, Citation1983; Bianco, Pollio, Resnik, Boente, & Larumbe, Citation1997; Gencturk, Bakshi, Hong, & Labuza, Citation1986; Iguaz & Vírseda, Citation2007; Reddy & Chakraverty, Citation2004). In static gravimetric methods, saturated salt solutions were used, wherein a sample of known mass is stored in an enclosure, such as a desiccator, and is allowed to reach equilibrium with the surrounding atmosphere of known relative humidity (RH) maintained by the saturated salt solutions. The sample is weighed at regular intervals until a constant weight is reached, which is assumed to be the equilibrium point.
The moisture sorption isotherms of food and agricultural materials have described through EMC/ERH equations by many researchers (van den Berg & Bruin, Citation1981). Many empirical and semi-empirical equations describing the sorption characteristics of foods have been suggested in the literature for the dependence between the equilibrium moisture content (EMC) and the RH of the air (ASAE, Citation1995). Literature reported through numerous studies evidence the importance of the characterization of EMC for predicting the stability and shelf life of various products, i.e. corn starch (Peng, Chen, Wu, & Jiang, Citation2007), bitter orange leaves (Mohamed, Kouhila, Jamali, Lahsasni, & Mahrouz, Citation2004), green tea in powder and granules (Arslan & Togrul, Citation2005), millets (Raji & Ojediran, Citation2011), and onion powder (Debnath, Hemavathy, & Bhat, Citation2002). The ability to predict the moisture content during storage under a variety of conditions is very important for reducing the cost and the cycle time of product development.
Water availability in the reactions of food degradation depends both on water content and on the properties of the diffusion surface. The water sorption isotherms can be used to calculate the net heat of sorption which provides an indication of the binding energy of water molecules and has a bearing on the energy balance of drying operations (Iglesias & Chirife, Citation1982). Sorption isotherms at different temperatures enable an evaluation of the isosteric heat of sorption, which determines the interaction between an adsorbent and adsorbate. The isosteric heat of sorption is of great importance for designing equipment for dehydration processes and the qualitative understanding of the state of water on the food surface (Mulet, Garcı́a-Pascual, Sanjuán, & Garcı́a-Reverter, Citation2002; Tolaba, Peltzer, Enriquez, & Lucı́a Pollio, Citation2004), because the isotherms can be predicted at other temperatures by means of the integrated Clausius–Clapeyron equation (Rizvi, Citation1986).
There is no reported research on the moisture sorption characteristics of rice-based instant soup mix even though it is commonly commercialized and consumed around the world in infusions. Therefore, the present work was studied for the moisture sorption behavior of rice-based instant soup mix at different temperatures further experimental data will be analyzed by using five sorption isotherm equations to find the most appropriate model to describe the sorption data and to estimate the net isosteric heat of sorption.
2. Materials and methods
2.1. Sample preparation
The optimized level of soup ingredients (rice flour, 5.537 g; Moringa leaf powder, 0.890 g; blended oil, 0.526 g) obtained response surface methodology (RSM) (Prasad & Singh, Citation2014b) was used to prepare the dry soup mix for sorption studies. Rice flour, Moringa leaf powder, and oil blend were used as process-independent variables for optimization of ingredients level. The development of rice-based instant soup mix was based on the previous results obtained in previous studies (Prasad & Singh, Citation2014a; Singh & Prasad, Citation2013a, Citation2013b, Citation2013c, Citation2014a; Singh, Yadav, & Prasad, Citation2014). The initial moisture content of soup mix sample was 8.95 ± 0.55% determined by measuring loss in mass in a hot air oven at 105°C (Association of Official Analytical Chemists, Citation2002).
2.2. Chemicals
Lithium chloride, potassium acetate, magnesium chloride, potassium carbonate, calcium nitrate, sodium nitrite, sodium chloride, and potassium chromate were obtained from Himedia Laboratories Limited, Mumbai and Sisco Research Laboratories Pvt. Ltd., Mumbai.
2.3. Experimental procedure
The static gravimetric method for sorption study was used, which involves the use of saturated salt solutions to maintain a fixed equilibrium relative humidity (Spiess & Wolf, Citation1987). The mass transfer between the product and the ambient atmosphere was assured by natural diffusion of the water vapor and the water activity of the product equals the relative humidity of the atmosphere at equilibrium conditions. To achieve different relative humidity environments, aqueous solutions of lithium chloride (LiCl), potassium acetate (KC2H3O2), magnesium chloride (MgCl2), potassium carbonate (K2CO3), calcium nitrate (Ca(NO3)2), sodium nitrite (NaNO2), sodium chloride (NaCl), and potassium chromate (K2CrO4) to have RH of 11.1, 22.9, 32.9, 43.9, 51.8, 64.8, 75.5, and 86.5, respectively, were used (Bizot, Riou, & Multon, Citation1987; Greenspan, Citation1977; Rizvi, Citation1986; Young, Citation1967). A small quantity of thymol was added to the containers that had saturated salts with water activities higher than 0.6, in order to prevent microbial growth. In order to prevent fungal growth at relative humidity over 51.8%, sodium azide was mixed into solutions. The sorption apparatus was placed in constant temperature cabinets maintained at the temperatures of 15, 25, 35, and 45°C and allowed to equilibrate at respective temperatures for 3 days before the samples were placed in them.
Weighed quantity (5.00 ± 0.02 g) of the sample was spread as a single layer and placed in the respective sorption containers for each temperature. The gain or loss in weight of the samples in each desiccator was monitored at regular interval. The total time required for removal, weighing, and replacing the samples in the desiccators was approximately 30 s. The EMC was acknowledged when three consecutive weight measurements showed. The experiment was carried out in triplicate for each sample and the average values of EMC were measured.
2.4. Mathematical modeling
Numerous models have been cited in the literature for the sorption isotherms (ASAE, Citation1995; Basunia & Abe, Citation2001; Lahsasni, Kouhila, Mahrouz, & Fliyou, Citation2003; Mohamed et al., Citation2004; van den Berg & Bruin, Citation1981). In this study, the suitability of five model equations (Table ) to describe moisture sorption isotherms of soup mix was assessed. The experimental data were fitted to the models using a nonlinear regression program (Statistica for Windows, Citation1995) to find the best equation for the soup mix sorption isotherms.
Table 1. Sorption isotherm models used
2.5. Isosteric heat of sorption
Isosteric heat of sorption indicates the binding energy of sorbed water by solid particles. The net isosteric heat of sorption provides an estimation of heat required to be removed in excess of latent heat of vaporization of pure water for extracting sorbed water at a particular moisture content. Since, sorption data are obtained at different temperatures, it is possible to evaluate the net heat of sorption Q
st
(kJ mol−1) at different moisture contents using the best-fitted isotherm model (Aviara & Ajibola, Citation2002). Estimation of isosteric heat of sorption from isotherms at two or more temperatures allows prediction of isotherms at any temperature. The heat of sorption can be explained by the Clausius–Clapeyron equation (Iglesias & Chirife, Citation1976b; Okos, Narsimhan, Ingh, & Weitmauuer, Citation1992) as follows:(1)
(1)
Integrating Equation (1), assuming that the net isosteric heat of sorption (Q
st) is temperature independent, gives the following:(2)
(2)
where, Q st is the net isosteric heat (kJ mol−1) of sorption and R is the universal gas constant (8.314 J mol−1 K−1).
2.6. Statistical analysis
The quality of fitting of experimental data to the selected mathematical models was tested with different criteria, namely coefficient of determination (R
2), the mean relative percent error (% E), and root mean square error (RMSE). The coefficient of correlation R
2 was one of the primary criteria for selecting the best equation to define the sorption curves. In addition to R
2, the various statistical parameters such as % E, RMSE and χ
2 were used to determine the quality of the fit (Chen & Morey, Citation1989). The mean relative percentage deviation modulus (% E) is widely adopted throughout the literature, with a modulus value below 10% indicative of a good fit for practical purposes (Lomauro, Bakshi, & Labuza, Citation1985). The measures of errors using % E, RMSE, and χ
2 are defined as:(3)
(3)
(4)
(4)
(5)
(5)
3. Results and discussion
3.1. Moisture sorption profiles
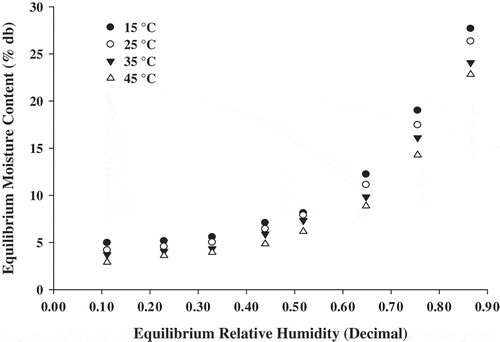
The mean experimental values of equilibrium moisture content based on triplicate measurements for respective water activities at 15, 25, 35, and 45°C for sorption is presented in Figure . Rice-based instant soup mix was reached at equilibrium moisture content in 10 days for sorption. The resulting sigmoid shaped curves reflecting a type II isotherm (Brunauer, Emmett, & Teller, Citation1938), which is typical to the most of the foods. This type of sorption isotherm can be divided into three regions as reported by van den Berg and Bruin (Citation1981). In which, first region conforming to ERH ≤ 20% relates to adsorption of monomolecular film of water, the second region indicates adsorption of additional layers over this monolayer at ERH of 20–70%, and the third region for ERH > 70% correspond to condensation of water in pores of the product followed by dissolution of soluble materials (Kumar, Jha, Jain, Sahu, & Arora, Citation2012). In the second and third regions of the sorption profile, water molecules penetrate newly created pores of the already-swollen structure and are mechanically entrapped in the void spaces. Consequently, water uptake particularly at a higher equilibrium relative humidity would be markedly influenced by the stability of the microporous structure (Kumar et al., Citation2012). This trend may be due to reduction in the total number of active sites for water binding as a result of physical or chemical changes in the product induced by temperature (Mazza & LeMaguer, Citation1980).
The isotherms demonstrate an increase in equilibrium moisture content with increasing equilibrium relative humidity. The Figure also shows that the equilibrium moisture content decreased with increasing temperature at constant equilibrium humidity; thus, indicating that soup mix became less hygroscopic. The steep acceleration of sorption of the sample at high water activities could be due to solubilization of sugars (Rao et al., Citation2006). The water binding capacity of soup mix at a higher temperature was low. This may be due to protein and starch content present in soup mix. Proteins and carbohydrates are known to have more water binding capacity at low temperatures than at high temperatures (Kumar et al., Citation2012; Rao et al., Citation2006). The Figure represents that at all water activities, the equilibrium moisture content was lower at higher temperature.
More specifically, there is a significant influence of temperature on the equilibrium moisture content of rice-based instant soup mix in the range of 15–45°C. The higher excitation state of water molecules at a higher temperature thus decreasing the attractive forces between them may explain this result (Arslan & Togrul, Citation2005; Bahloul, Boudhrioua, & Kechaou, Citation2008; Basunia & Abe, Citation2001; Mohamed et al., Citation2004; Nourhène, Neila, Mohammed, & Nabil, Citation2008). The results of this study help in determination of package systems that maximize product protection. Further, the compatibilities of food items, which are to be packaged together, can be determined with good reliability as major outcome of this research work.
3.2. Mathematical modeling
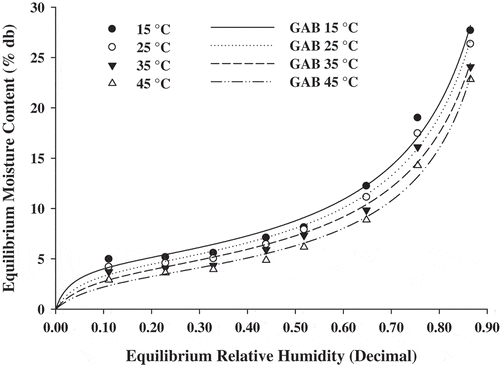
All isotherm models were fitted in the forms of EMC = f(ERH,T) and ERH = f(EMC,T), as minimizing the error in the prediction of EMC or ERH generates different constants in the fitted equation. The experimental results obtained in terms of model and statistical parameters of different sorption models for rice-based instant soup mix are represented in Table . According to correlation coefficient (R 2) and other statistical parameters, the Chung-Pfost, GAB, Henderson, Kuhn, and Oswin models could be considered the best to describe experimental data. It can be seen in Table that the highest value of R 2 (0.992) was obtained for the GAB model followed by the 0.991 for Kuhn model. The lowest R 2 was obtained for the Chung-Pfost equation, thus this model was less effective in fitting the data. The GAB model appears to be one of the most suitable for describing the relationship between water content, equilibrium relative humidity, and temperature throughout the intervals considered.
Table 2. Statistical parameters for sorption models
Figure shows the comparison between experimental and calculated (GAB model) data of sorption isotherms of soup mix obtained at 15, 25, 35, and 45°C. The data resemble the characteristic sigmoid shape of the type II pattern isotherm according to Brunauer’s classification (Brunauer et al., Citation1938) which is frequently found for biological and food materials (Bandyopadhyay, Das, & Sharma, Citation1987; Caurie, Citation1970; Chetana, Srinivasa, & Reddy, Citation2005; Ghodake, Goswami, & Chakraverty, Citation2007; Patil & Singh, Citation1998).
3.3. Isosteric heat of sorption
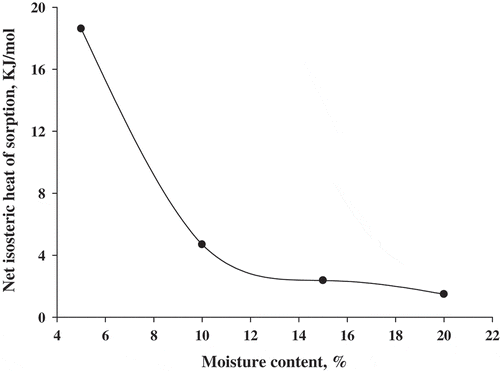
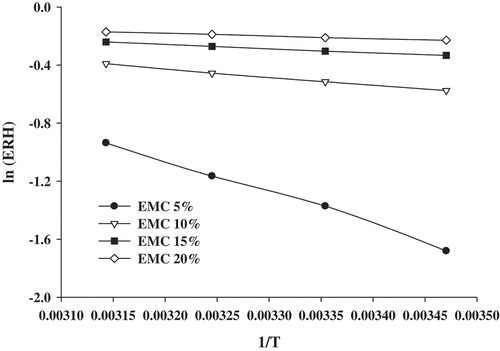
The net isosteric heat of sorption and desorption determined by applying Equation (2) to the sorption EMC data as obtained from GAB model have been presented as a function of moisture in Figure . Heat of sorption: the values of Q st were calculated from the slope of the plot between the values of ln(e.r.h) and 1/T at constant moisture content as shown in Figures and . The representation of the net isosteric heats of sorption for different moisture contents is shown in Figure .
Figure 3. ln (e.r.h) vs. 1/T graphs for calculating the heat of sorption of rice-based instant soup mix
It can be observed that the net isosteric heat decreases when the moisture content increases for sorption. These trends are similar in both food and pharmaceutical products (Acosta, Nuevas, Rodriguez, Pardillo, & Ramos, Citation2000; Ertekin & Sultanoğlu, Citation2001; Hossain, Bala, Hossain, & Mondol, Citation2001; Tsami, Citation1991). The same behavior was observed for various materials, as presented by Kaya and Kahyaoglu (Citation2007) for safflower petals and tarragon, Yanniotis and Zarmboutis (Citation1996) for pistachio nuts, McLaughlin and Magee (Citation1988) for potatoes, and Palou, López-Malo, and Argaiz (Citation1997) for cookies and corn snacks.
4. Conclusion
The influence of temperature on the sorption behavior for rice-based instant soup mix was considered significant at temperature ranges from 15 to 45°C. The equilibrium moisture content increased with an increase in the equilibrium relative humidity, at a constant temperature and decreased with an increase in the temperature, for constant values of equilibrium relative humidity. It can be concluded from this study that rice-based instant soup mix belongs to a class of food powders that is hygroscopic by nature. The GAB model was found to be the best model to describe the experimental sorption data for rice-based instant soup mix. The isosteric heat of sorption curve showed a regular fall with increasing moisture content and approached the heat of vaporization of free water at higher moisture content. It may also be used to optimize the processing conditions and to evaluate the quality of ingredients used in the commercial production of soup mix with rice flour as a base material.
Acknowledgment
The first author acknowledges the receipt of fellowship from Sant Longowal Institute of Engineering and Technology (Deemed University), Longowal during his Doctoral studies.
Additional information
Funding
Notes on contributors
Yogender Singh
Yogender Singh is a postdoctoral fellow and conducted his doctoral research on studies on development of rice-based functional instant soup mix at Sant Longowal Institute of Engineering and Technology, Sangrur, Punjab, India.
Kamlesh Prasad
Kamlesh Prasad is a professor at Sant Longowal Institute of Engineering and Technology, Sangrur, Punjab, India with specialization in engineering properties of foods, fruits and vegetable processing, and non-destructive analysis of biomaterials.
References
- Acosta, J. , Nuevas, L. , Rodriguez, I. , Pardillo, E. , & Ramos, A. (2000). The moisture sorption isotherms of cefotaxime sodium salt. Drying Technology , 18 , 469–477.10.1080/07373930008917717
- Aguerre, R. J. , Suarez, C. , & Viollaz, P. E. (1983). Moisture desorption isotherms of rough rice. Journal of Food Technology , 18 , 345–351.
- Arslan, N. , & Togrul, H. (2005). The fitting of various models to water sorption isotherms of tea stored in a chamber under controlled temperature and humidity. Journal of Stored Products Research , 42 , 112–135.
- ASAE . (1995). Moisture relationship of plant-based agricultural products ( ASAE Standard D245.5). St. Joseph, MI: American Society of Agricultural and Biological Engineers.
- Association of Official Analytical Chemists . (2002). Official methods of analysis (17th ed.). Washington, DC: Author.
- Aviara, N. A. , & Ajibola, O. O. (2002). Thermodynamics of moisture sorption in melon seed and cassava. Journal of Food Engineering , 55 , 107–113.10.1016/S0260-8774(02)00023-7
- Bahloul, N. , Boudhrioua, N. , & Kechaou, N. (2008). Moisture desorption–adsorption isotherms and isosteric heats of sorption of Tunisian olive leaves (Olea europaea L.). Industrial Crops and Products , 28 , 162–176.10.1016/j.indcrop.2008.02.003
- Bandyopadhyay, P. , Das, H. , & Sharma, G. P. (1987). Moisture adsorption characteristics of casein, lactose, skim milk and chhana powder. Journal of Food Science & Technology , 24 , 6–11.
- Basunia, M. A. , & Abe, T. (2001). Moisture desorption isotherms of medium-grain rough rice. Journal of Stored Products Research , 37 , 205–219.10.1016/S0022-474X(00)00022-9
- Bianco, A. M. , Pollio, M. L. , Resnik, S. L. , Boente, G. , & Larumbe, A. (1997). Comparison of water sorption behaviour of three rice varieties under different temperatures. Journal of Food Engineering , 33 , 395–403.10.1016/S0260-8774(97)00029-0
- Bizot, H. , Riou, N. , & Multon, J. L. (1987). Guide pratique pour la de′termination des isothermes et de lactivite′ de leau. Sciences des Aliments: nume′ro hors se′rie .
- Brunauer, S. , Emmett, P. H. , & Teller, E. (1938). Adsorption of gases in multimolecular layers. Journal of the American Chemical Society , 60 , 309–319.10.1021/ja01269a023
- Caurie, M. (1970). A new model equation for predicting safe storage moisture level for optimum stability of dehydrated foods. Journal of Food Technology , 5 , 301–307.
- Cecil, J. E. , Francis, J. , & Read, N. W. (1999). Comparison of the effects of a high-fat and high-carbohydrate soup delivered orally and intragastrically on gastric emptying, appetite, and eating behaviour. Physiology and Behavior , 67 , 299–306.10.1016/S0031-9384(99)00069-4
- Chen, C. C. , & Morey, R. V. (1989). Comparison of four EMC/ERH equations. Transactions of the ASAE , 32 , 983–989.10.13031/2013.31103
- Chetana, R. , Srinivasa, P. C. , & Reddy, S. R. Y. (2005). Moisture sorption characteristics of milk burfi, an traditional Indian sweet, using sugar substitutes. European Food Research and Technology , 220 , 136–141.10.1007/s00217-004-1007-1
- Debnath, S. , Hemavathy, J. , & Bhat, K. K. (2002). Moisture sorption studies on onion powder. Food Chemistry , 78 , 479–482.10.1016/S0308-8146(02)00161-9
- Ertekin, F. K. , & Sultanoğlu, M. (2001). Moisture sorption isotherm characteristics of peppers. Journal of Food Engineering , 47 , 225–231.10.1016/S0260-8774(00)00120-5
- Gal, S . (1987). The need for and practical applications of sorption data. In R. Jowitt , F. Escher , B. Hallstrom , H. Mefert , W. Spiess , & G. Vos . (Eds.), Physical properties of foods (pp. 13–25). London: Elsevier Applied Science.
- Gencturk, M. B. , Bakshi, A. S. , Hong, Y. C. , & Labuza, T. P. (1986). Moisture transfer properties of wild rice. Journal of Food Process Engineering , 8 , 243–261.10.1111/jfpe.1986.8.issue-4
- Ghodake, H. M. , Goswami, T. K. , & Chakraverty, A. (2007). Moisture sorption isotherms, heat of sorption and vaporization of withered leaves, black and green tea. Journal of Food Engineering , 78 , 827–835.10.1016/j.jfoodeng.2005.11.023
- Greenspan, L. (1977). Humidity fixed points of binary saturated aqueous solutions. Journal of Research of the National Bureau of Standards Section A: Physics and Chemistry , 81A , 89–96.10.6028/jres.081A.011
- Hossain, M. D. , Bala, B. K. , Hossain, M. A. , & Mondol, M. R. A. (2001). Sorption isotherms and heat of sorption of pineapple. Journal of Food Engineering , 48 , 103–107.10.1016/S0260-8774(00)00132-1
- Iglesias, H. A. , & Chirife, J. (1976a). Prediction of the effect of temperature on water sorption isotherms of food material. International Journal of Food Science & Technology , 11 , 109–116.10.1111/ifs.1976.11.issue-2
- Iglesias, H. A. , & Chirife, J. (1976b). Isosteric heats of water vapour sorption on dehydrated foods. Part II: Hysteresis and heat of sorption, comparison with BET theory. Lebensmittel-Wissenschaft und Technologie , 9 , 123–127.
- Iglesias, H. A. , & Chirife, J. (1982). Handbook of food isotherms: Water sorption parameters for food and food components (pp. 170–175). New York, NY: Academic Press.
- Iguaz, A. , & Vírseda, P. (2007). Moisture desorption isotherms of rough rice at high temperatures. Journal of Food Engineering , 79 , 794–802.10.1016/j.jfoodeng.2006.03.002
- Jamali, A. , Kouhila, M. , Mohamed, L. A. , Jaouhari, J. T. , Idlimam, A. , & Abdenouri, N. (2006). Sorption isotherms of Chenopodium ambrosioides leaves at three temperatures. Journal of Food Engineering , 72 , 77–84.10.1016/j.jfoodeng.2004.11.021
- Kaya, S. , & Kahyaoglu, T. (2007). Moisture sorption and thermodynamic properties of safflower petals and tarragon. Journal of Food Engineering , 78 , 413–421.10.1016/j.jfoodeng.2005.10.009
- Kumar, A. , Jha, A. , Jain, P. , Sahu, J. K. , & Arora, S. (2012). Moisture sorption characteristics of lal peda at different storage temperatures. Food Research International , 49 , 373–378.10.1016/j.foodres.2012.07.050
- Labuza, T. P . (1984). Moisture sorption: Practical aspects of isotherm, measurement and use (p. 149). St. Paul, MN: American Association of Cereal Chemists.
- Lahsasni, S. , Kouhila, M. , Mahrouz, M. , & Fliyou, M. (2003). Moisture adsorption–desorption isotherms of prickly pear cladode (Opuntia ficus indica) at different temperatures. Energy Conversion and Management , 44 , 923–936.10.1016/S0196-8904(02)00094-8
- Lomauro, C. J. , Bakshi, A. S. , & Labuza, T. P. (1985). Evaluation of food moisture sorption isotherm equations. Part I. Fruit vegetable and meat products. LWT – Food Science and Technology , 18 , 111–117.
- Mazza, G. , & LeMaguer, M. (1980). Dehydration of onion. Some theoretical and practical considerations. Journal of Food Technology , 15 , 181–194.
- McLaughlin, C. P. , & Magee, T. R. A. (1988). Determination of sorption isotherm and the isosteric heats of sorption for potatoes. Journal of Food Engineering , 35 , 267–280.
- Mohamed, L. A. , Kouhila, M. , Jamali, A. , Lahsasni, S. , & Mahrouz, M. (2004). Moisture sorption isotherms and heat of sorption of bitter orange leaves (Citrus aurantium). Journal of Food Engineering , 67 , 491–498.
- Mulet, A. , Garcı́a-Pascual, P. , Sanjuán, N. , & Garcı́a-Reverter, J. (2002). Equilibrium isotherms and isosteric heats of morel (Morchella esculenta). Journal of Food Engineering , 53 , 75–81.10.1016/S0260-8774(01)00142-X
- Nourhène, B. , Neila, B. , Mohammed, K. , & Nabil, K. (2008). Sorptions isotherms and isosteric heats of sorption of olive leaves (Chemlali variety): Experimental and mathematical investigations. Food and Bioproducts Processing , 86 , 167–175.10.1016/j.fbp.2007.10.010
- Okos, M. R. , Narsimhan, G. , Ingh, R. K. , & Weitmauuer, A. C. (1992). Food dehydration. In D. R. Heldman & D. B. Lund (Eds.), Hand book of food engineering (pp. 339–382). New York, NY: Marcel Dekker.
- Palou, E. , López-Malo, A. , & Argaiz, A. (1997). Effect of temperature on the moisture sorption isotherms of some cookies and corn snacks. Journal of Food Engineering , 31 , 85–93.10.1016/S0260-8774(96)00019-2
- Patil, G. R. , & Singh, S. (1998). Water activity lowering behaviour of pumpkin syrup solids in the intermediate moisture range. Journal of Food Science and Technology , 35 , 236–241.
- Peng, G. , Chen, X. , Wu, W. , & Jiang, X. (2007). Modeling of water sorption isotherm for corn starch. Journal of Food Engineering , 80 , 562–567.10.1016/j.jfoodeng.2006.04.063
- Prasad, K. , & Singh, Y. (2014a, August 24–27). Dehydration and quality characterization of moringa oleifera leaves . In Proceedings of 19th International Drying Symposium (IDS 2014) . Lyon.
- Prasad, K. , & Singh, Y. (2014b). Rice based vegetable supplemented functional instant soup mix: Development and optimization (p. 84). Saarbrücken: Scholar’s Press.
- Raji, A. O. , & Ojediran, J. O. (2011). Moisture sorption isotherms of two varieties of millet. Food and Bioproducts Processing , 89 , 178–184.10.1016/j.fbp.2010.06.001
- Rao, K. J. , Dhas, P. H. , Emerald, F. M. E. , Ghosh, B. C. , Balasubramanyam, B. V. , & Kulkarni, S. (2006). Moisture sorption characteristics of channa podo at 5°C and 35°C. Journal of Food Engineering , 76 , 453–459.
- Reddy, B. S. , & Chakraverty, A. (2004). Equilibrium moisture characteristics of raw and parboiled paddy, brown rice, and bran. Drying Technology , 22 , 837–851.10.1081/DRT-120034266
- Rizvi, S. S. H. (1986). Thermodynamic properties of food in dehydration. In M. A. Rao , S. S. H. Rizvi , A. K. Datta , & J. Ahmed (Eds.), Engineering properties of foods (pp. 133–214). New York, NY: Marcel Dekker.
- Singh, Y. , & Prasad, K. (2013a). Physical characteristics of some of the paddy varieties as affected by shelling and milling operations. Oryza , 50 , 174–180.
- Singh, Y. , & Prasad, K . (2013b, December 6–7). Comparative behavior of chemical and pasting characteristics of basmati rice brokens and corn flour. In Proceedings of 6th International Conference on Fermented foods, health status and social well-being (pp. 98–99, Swedish South Asian Network on Fermented Foods). Anand: Anand Agricultural University.
- Singh, Y. , & Prasad, K. (2013c). Moringa oleifera leaf as functional food powder: Characterization and uses. International Journal of Agriculture and Food Science Technology , 4 , 317–324.
- Singh, Y. , & Prasad, K. (2014a). Multivariate classification of promising paddy cultivars on the basis of physical properties. Pertanika Journal of Tropical Agriculture Science , 37 , 327–342.
- Singh, Y. , & Prasad, K. (2014b). Soup: Integrated approach to nutrition, health benefits. Food & Beverage News , 6 , 20.
- Singh, Y. , Yadav, P. , & Prasad, K. (2014). Fatty acid profile optimization in edible oil blend through linear programming. Asian Journal of Chemistry , 26 , 1145–1150.
- Spiess, W. E. L. , & Wolf, W. (1987). Critical evaluation of methods to determine moisture sorption isotherms. In L. B. Rockland & L. R. Beuchat (Eds.), Water activity: Theory and applications to food (pp. 215–233). New York, NY: Marcel Dekker.
- Statistica for Windows . (1995). Computer program manual . Tulsa, OK: StatSoft.
- Tolaba, M. P. , Peltzer, M. , Enriquez, N. , & Lucı́a Pollio, M. (2004). Grain sorption equilibria of quinoa grains. Journal of Food Engineering , 61 , 365–371.10.1016/S0260-8774(03)00143-2
- Tsami, E. (1991). Net isosteric heat of sorption in dried fruits. Journal of Food Engineering , 14 , 327–335.10.1016/0260-8774(91)90022-K
- van den Berg, C. , & Bruin, S . (1981). Water activity and its estimation in food systems: theoretical aspects. In L. B. Rockland & G. F. Stewart (Eds.), Water Activity: Influences on Food Quality (pp. 1–61). New York, NY: Academic Press.10.1016/B978-0-12-591350-8.50007-3
- Yanniotis, S. , & Zarmboutis, I. (1996). Water sorption isotherms of pistachio nuts. LWT – Food Science and Technology , 29 , 372–375.10.1006/fstl.1996.0057
- Young, J. F. (1967). Humidity control in the laboratory using salt solutions, a review. Journal of Applied Chemistry , 17 , 241–245.