?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Two green tea types, leaf grade and sanding, were extracted at different time intervals: 20, 40, and 120 min at a constant temperature of 50°C. The extracts were analyzed by GC/MS technique. The major compounds identified were myristic acid, palmitic acid, stearic acid, oleic acid, 1H-purine-2,6-dione, caffeine, linoleic acid, diethyl ester, and 1H-purine-6-amine. Stearic acid, palmitic acid, linoleic acid, and myristic acid were more abundantly present in the leaf-grade variety than sanding. However, some levels of acetic acid, cyclobutanol, hexadecanoic acid, octadecanoic acid, 9-octadecenoic acid, and caffeine were also found in both the tea types. Most of the volatile compounds were detected between 20–40-min time of extraction. The 40-min time of extraction also showed the maximum content of polyphenols and antioxidants in both the tea types. Thus, 40 min was suggested as the most suitable time for maximum extraction of bioactive volatiles, antioxidants, and polyphenols from green tea.
Public Interest Statement
The article reports about the extraction time required to release maximum bioactive components from green tea. This could be helpful for industrialists to optimize the process for extracting tea components. In the present era, the general public is more prone to various diseases like cancer, diabetes, cardiovascular diseases and others. Green tea is reported for its health-promoting activities such as anti-proliferative, anti-hypertensive, anti-atherosclerotic, hypo-cholesterolemic, and hypo-lipidemic activities, which are mostly attributed to the flavonoids, antioxidants, and total phenols present in it. The article reports that processing time effects the type and amount of compounds released from green tea. The consumers can process green tea at the domestic level, at suggested time for extraction, and can avail maximum health benefits from it.
Competing interests
The authors declare no competing interest.
1. Introduction
Green tea (Camellia sinensis) is native to the southern regions of China and some parts of India, Laos, Thailand, Vietnam, and Myanmar (Balentine, Harbowy, & Graham, Citation1998). Tea was first known to be discovered as a medicinal drink in China around 2737 BC. It was then introduced to Japan during the thirteenth century and to Europe in the sixteenth century, then to America, Africa, and other regions of the world (Chan, Fong, Cheung, Huang, & Chen, Citation1992; Graham, Citation1992). Tea is presently cultivated in over 30 countries around the world and the tea beverage is second only to water in terms of worldwide consumption (Chan et al., Citation1992). It grows best in tropical and subtropical areas with adequate rainfall, good drainage, and slightly acidic soil. The worldwide popularity of tea has increased not only because of its characteristic aroma and flavor, but also due to its health-promoting activities such as anti-proliferative, anti-hypertensive, anti-atherosclerotic, hypo-cholesterolemic, and hypo-lipidemic activities, which are mostly attributed to the flavonoids, antioxidants, and total phenols present in it (Ahmad et al., Citation2014, Citation2015; Chen, Zhu, Tsang, & Huang, Citation2001; Cheng, Citation2006; Kim, Goodner, Park, Choi, & Talcott, Citation2011). Various studies about the tea volatiles have already been reported (Amanda, Teobaldo, Ivan, & Edward, Citation1996; Bilia, Flamini, Taglioli, Morelli, & Vincieri, Citation2002; Kumar, Satyanarayan, Gopikrishna, & Solomon, Citation2012; Shah et al., Citation2015),
It is essential to determine the volatile as well as non-volatile components present in green tea for a better understanding of the physiological, pharmacological, and flavor-imparting properties of tea. To the best of the knowledge of the authors, the effect of extraction time on the volatile constituents of green tea has not been reported yet. In general, the analysis of volatile components is usually conducted using gas chromatography and mass spectrometry (GC–MS). The present study analyzes the effect of extraction time on the bioactive volatile components, antioxidants, and polyphenolic contents of green tea.
2. Materials and methods
2.1. Sample collection and preparation of the extract
Two green tea types, viz. leaf grade and Sanding, were purchased from the local market and stored at room temperature until use. To extract the sample, 2 g of each green tea variety were extracted in 50-ml distilled water at a constant temperature of 50°C, for different times 20, 40, and 120 min. The extracts obtained were concentrated under reduced pressure in a rotary evaporator (Rotary Equitron).
2.2. Instrument and chromatographic conditions
The concentrated pure extracts were dried in a vacuum oven at 60°C to get a powdered residue. The residue was dissolved in 20-ml methanol and collected in corked glass test tubes. The extracts were analyzed on a GC–MS system Shimadzu QP2010 Plus with 2010 GC. An Omega SPTm column 0.25 mm ID, film thickness of 0.25 μm was used with nitrogen as the carrier gas. The injector temperature was 270°C with split ratio of 10:0. The GC oven temperature was programmed to hold at 100°C for 2 min and then increased to 200°C at 15°C/min and held for 2 min, and finally increased to 240°C at 20°C/min and held for 18 min. Ion source temperature was 230°C and the interface temperature was set at 280°C. Mass spectra were collected over the range of m/z 40–650. Each compound was identified using WILEY library 8 L.
2.3. Free radical scavenging activity
The antioxidant activity of the tea extract was determined according to the method of Gani et al. (Citation2014) with some modifications. Eighty microliters of the sample were mixed with 200 μL of 0.05% DPPH in a total volume of 4-ml methanol and allowed to react in the dark for 30 min. The results were expressed as percent inhibition using the relation.
2.4. Determination of total phenol content
Total phenol content of the extract was determined according to the method of Jan et al. (Citation2015) with modifications. One hundred and fifty microliters of the extract, 2,400 μL of nanopure water, and 150 μL of 0.25 N folin ciocalteu’s reagent were combined and then mixed well by shaking. The mixture was allowed to react for 3 min then 300 μL of 1 N Na2CO3 solution was added and mixed well again by shaking. The solution was incubated at room temperature in the dark for 2 h. The absorbance was measured at 725 nm using a spectrophotometer and the results were expressed as milligram of gallic acid equivalents (GAE) per gram of the extract.
2.5. Stastical analysis
Analysis was carried out in triplicates and results were represented as means ± standard deviation. The data were assessed by analysis of variance (ANOVA) and significant differences were considered at p ≤ 0.05 using Duncan’s Multiple Range Test in SPSS software (version 16.0.).
3. Results and discussion
3.1. Identification and analysis of various components
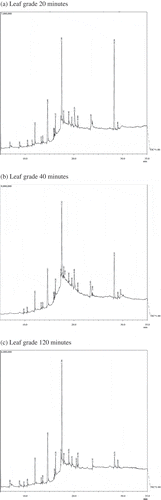
GC–MS chromatograms of aqueous extract of the different types of green tea viz. leaf-grade and Sanding samples for different time periods of extraction are given in Figures and . The number of volatile constituents as depicted by the peaks varied to some extent according to the type of tea sample, possibly due to varied environmental, genetic, and processing conditions and the time of extraction. On comparison of the mass spectra of the constituents with the library WILEY8.LIB, different compounds were characterized and identified (Tables and ). The relative percentages of some major compounds which are present, in almost, in both the samples are presented in Table . They were identified as myristic acid, palmitic acid, stearic acid, oleic acid, 1H-purine-2, 6-dione, caffeine, linoleic acid, diethyl ester, and 1H-purine-6-amine. However, compounds such as stearic acid, palmitic acid, linoleic acid, and myristic acid were more abundantly present in the leaf grade than sanding. Varying levels of acetic acid, cyclobutanol, hexadecanoic acid, octadecanoic acid, 9-octadecenoic acid, and caffeine were also found in both the tea types. The amount of compounds released in the extract was observed to be influenced by the type of tea and time of extraction employed: for example, 9-octadecenoic acid, oleic acid, hexadecanoic acid, and palmitic acid showed the maximum peak area at 20 min in leaf-grade tea, while in sanding, the maximum peak area was seen at 120 min of extraction. Similarly, 1H-purine-2, 6-dione and caffeine showed the maximum peak area at 40 min of extraction in leaf-grade tea, whereas sanding showed the maximum peak area at 20 min of extraction. Further increase in extraction time leads to a decrease in the relative percentage of most of the physiologically important volatile compounds. Most of the compounds detected were health benefiting and physiologically important as mentioned in Table .
Figure 2. (a)–(c) Showing GC–MS chromatograph of sanding-grade tea type at different extraction times
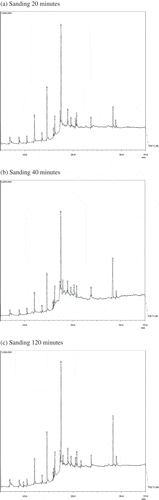
Table 1. (a)–(c) Showing the identification and relative percentage of volatile compounds in leaf-grade type of green tea at 50°C in 20-, 40-, and 120-min time of extraction
Table 2. (a)–(c) Showing the identification and relative percentage of volatile compounds in sanding type of green tea at 40°C in 20-, 40-, and 120-min time of extraction
Table 3. Relative percentages of some major compounds present in both types of samples
Table 4. Biological activity of some phytocomponents identified in the green extracts
3.2. Total phenolic content
The effect of extraction time on the polyphenolic content of green tea extracts is depicted in Table . The results revealed that with increase in time of extraction from 20 to 40 min in both types of green tea, the content of polyphenols in the extract increased significantly from 120.79 to 137.59 GAE/g and 116.59 to 131.37 GAE/g, respectively, but further increase in time of extraction up to 120 min decreased the polyphenols to 118.76 and 127.45 GAE/g, respectively. Hence, the maximum amount of total phenols was observed at 40 min of extraction and thus suggesting it as the most efficient extraction time for maximum release of phenolic components in both the types of green tea. The decrease in total polyphenols at prolonged time of extraction may be due to the thermal degradation of the polyphenols. Thermal degradation of heat-susceptible phenolic compounds leads to the decrease in the total phenolic content (Randhir, Kwon, & Shetty, Citation2008). The decrease in total phenolic content could be due to the alteration in molecular structure of phenolic compounds, which may lead to the decrease in extractability due to the degree of polymerization (Altan, McCarthy, & Maskan, Citation2009). Perva-Uzunalić et al. (Citation2006) also showed the tendency of catchins to degrade during prolonged extraction in some green tea types (Fanning Belas and China) and also found highest extraction efficiency at 80°C for 20–30 min of extraction.
Table 5. Effect of extraction time on total phenolic content (TPC) and antioxidant activity of two green tea types
3.3. DPPH free radical scavenging activity
It is well known that the antioxidant activity of plant extracts containing polyphenolic components is due to their capacity to be donors of hydrogen atoms or electrons and to capture the free radicals (Shon, Kim, & Sung, Citation2003). Free radicals are known to be a major factor in biological damages. In brief, the reduction capacity of DPPH was determined by the decrease in its absorbance at 517 nm, which is reduced by the antioxidant (Duh, Citation1998). The extent of the reaction depends on the hydrogen-donating ability of the antioxidant (Bondet, Brand-Williams, & Berset, Citation1997).
The scavenging ability of both extracts of green tea at 20 and 120 min did not vary significantly (p ≤ 0.05), whereas it was significantly (p ≤ 0.05) higher at 40-min time of extraction (Table ). The prolonged extraction time decreases the antioxidant capacity of green tea. This may be due to the thermal degradation of the antioxidant components at higher extraction time. The decrease in antioxidant activity at higher extraction time may also be due to the decrease in polyphenols because polyphenols exhibit antioxidant properties (Baba et al., Citation2014). The leaf-grade variety showed comparatively better antioxidant activity than sanding.
4. Conclusion
The present study confirmed the presence of various health-benefiting compounds using GC–MS. Leaf grade was found to be richer in stearic acid, palmitic acid, linoleic acid, and myristic acid than sanding. The antioxidant and phenolic content of both tea types were found to be higher at 40-min time of extraction, whereas prolonged extraction time of 120 min decreased the polyphenols and antioxidants from both types of green tea. It was concluded that 20–40 min extraction time is the most suitable for maximum retention of bioactive compounds in green tea.
Acknowledgment
The authors are highly thankful to the Department of Biotechnology, Government of India.
Additional information
Funding
Notes on contributors
Adil Gani
Adil Gani is an assistant professor in the Department of Food Science and Technology, University of Kashmir, Srinagar. He is currently working on different projects viz. Nutraceutical potential of β- glucan, its utilization for making functional foods, and as an encapsulating material for target delivery of probiotics. Wheat flour modification by bacterial, enzymatic, and chemical interventions to combat coeliac disorders. Extraction of resistant starch from rice and horse chestnut and its utilization as an encapsulating agent for targeted delivery into the colon. Safety, Quality, and Nutraceutical Status of Kradi—A traditional Dairy-based fermented food of Himalayan Regions of J&K. Apart from this, he is also associated in teaching, training, and other related activities of the University/Department.
References
- Ahmad, M. , Baba, W. N. , Shah, U. , Gani, A. , Gani, A. , Riyaz, A. , & Masoodi, F. A. (2014). Nutraceutical properties of the green tea polyphenols. Journal of Food Processing and Technology . doi:10.4172/2157-7110.1000390
- Ahmad, M. , Baba, W. N. , Wani, T. A. , Gani, A. , Gani, A. , Shah, U. , … Masoodi, F. A. (2015). Effect of green tea powder on thermal, rheological & functional properties of wheat flour and physical, nutraceutical & sensory analysis of cookies. Journal of Food Science and Technology , 52 , 5799–5807.10.1007/s13197-014-1701-3
- Altan, A. , McCarthy, K. L. , & Maskan, M. (2009). Effect of extrusion process on antioxidant activity, total phenolics and β-glucan content of extrudates developed from barley-fruit and vegetable by-products. International Journal of Food Science Technology , 44 , 1263–1271.10.1111/ifs.2009.44.issue-6
- Amanda, J. J. , Teobaldo, L. , Ivan, M. , & Edward, J. C. (1996). Identification and quantitation of alkaloids in coca tea. Forensic Science International , 77 , 179–189.
- Baba, N. W. , Rashid, I. , Shah, A. , Ahmad, M. , Gani, A. , & Masoodi, F. A. (2014). Effect of microwave roasting on antioxidant and anti-cancerous activities of barley flour. Journal of the Saudi Society of Agricultural Sciences , 27 , 143–154.
- Balentine, D. A. , Harbowy, M. E. , & Graham, H. N. (1998). Caffeine (pp. 35–68). Boca Raton, FL: CRC Press.
- Bilia, A. R. , Flamini, G. , Taglioli, V. , Morelli, I. , & Vincieri, F. F. (2002). GC–MS analysis of essential oil of some commercial Fennel teas. Food Chemistry , 76 , 307–310.10.1016/S0308-8146(01)00277-1
- Bondet, V. , Brand-Williams, W. , & Berset, C. (1997). Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. LWT - Food Science and Technology , 30 , 609–615.10.1006/fstl.1997.0240
- Chan, P. T. , Fong, W. P. , Cheung, Y. L. , Huang, Y. , & Chen, Z. Y. (1992). Jasmine green tea epicatechins are hypolipidemic in hamsters fed a high-fat diet. Journal of Nutrition , 129 , 1094–1101.
- Chen, Z. Y. , Zhu, Q. Y. , Tsang, D. , & Huang, Y. (2001). Degradation of green tea catechins in tea drinks. Journal of Agricultural and Food Chemistry , 49 , 477–482.10.1021/jf000877h
- Cheng, T. O. (2006). All teas are not created equal. International Journal of Cardiology , 108 , 301–308.10.1016/j.ijcard.2005.05.038
- Duh, P. D. (1998). Antioxidant activity of burdock (Arctium lappa Linné): Its scavenging effect on free-radical and active oxygen. Journal of the American Oil Chemists’ Society , 75 , 455–461.10.1007/s11746-998-0248-8
- Gani, A. , Rasool, N. , Shah, A. , Ahmad, M. , Gani, A. , & Masoodia, F. A. (2014). DNA scission inhibition, antioxidant, and antiproliferative activities of water chestnut (Trapa natans) extracted in different solvents. Cyta-Journal of Food . doi:10.1080/19476337.2014.992967
- Gopalakrishnan, S. , & Vadivel, E. (2011). GC-MS analysis of some bioactive constituents of Mussaenda frondosa Linn. International Journal of Pharma and Biosciences , 2 , 313–320.
- Graham, H. N. (1992). Green tea composition, consumption, and polyphenol chemistry. Preventive Medicine , 21 , 334–350.10.1016/0091-7435(92)90041-F
- Jan, U. , Gani, A. , Ahmad, M. , Shah, U. , Baba, W. N. , Wani, I. A. , … Gani, A. (2015). Characterization of cookies made from wheat flour blended with buckwheat flour and effect on antioxidant properties. Journal of Food Science and Technology . doi:10.1007/s13197-015-1773-8
- Jananie, R. K. , Priya, V. , & Vijayalakshm, K. (2001). Determination of bioactive components of cynodondactylon by GC-MS analysis. New York Science Journal , 4 , 16–20.
- Kim, Y. , Goodner, K. L. , Park, J. , Choi, J. , & Talcott, S. T. (2011). Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chemistry , 129 , 1331–1342.10.1016/j.foodchem.2011.05.012
- Kugelman, A. , & Durand, M. (2011). A comprehensive approach to the prevention of bronchopulmonary dysplasia. Pediatric Pulmonology , 46 , 1153–1165.10.1002/ppul.v46.12
- Kumar, N. , Satyanarayan, R. J. , Gopikrishna, G. , & Solomon, A. K. (2012). GC-MS determination of bioactive constituents of cycasbeddomei cones. International Journal of Pharma and Bio Sciences , 3 , 344–350.
- Mathew, O. P. (2011). Apnea of prematurity: Pathogenesis and management strategies. Journal of Perinatology , 31 , 302–310.
- Muriel, P. , & Arauz, J. (2010). Coffee and liver diseases. Fitoterapia , 81 , 297–305.10.1016/j.fitote.2009.10.003
- Nehlig, A. J. , Daval, J-L. , & Debry, G. (1992). Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Research Reviews , 17 , 139–170.10.1016/0165-0173(92)90012-B
- Perva-Uzunalić, A. , Škerget, M. , Knez, Ž. , Weinreich, B. , Otto, F. , & Grüner, S. (2006). Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chemistry , 96 , 597–605.10.1016/j.foodchem.2005.03.015
- Randhir, R. , Kwon, Y. I. , & Shetty, K. (2008). Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innovative Food Science & Emerging Technologies , 9 , 355–364.10.1016/j.ifset.2007.10.004
- Shah, S. , Gani, A. , Ahmad, M. , Shah, A. , Gani, A. , & Masoodi, F. A. (2015). In-vitro antioxidant and antiproliferative activity of microwave extracted green tea and black tea (Camellia. sinensis): A comparative study. NutraFoods . doi:10.1007/s13749-015-0036-7
- Shon, M. Y. , Kim, T. H. , & Sung, N. J. (2003). Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chemistry , 82 , 593–597.10.1016/S0308-8146(03)00015-3
- Van, R. M. D. (2008). Coffee consumption and risk of type 2 diabetes cardiovascular diseases and cancer. Applied Physiology Nutrition and Metabolism , 33 , 1269–1283.