Abstract
Potato is an ideal candidate for the delivery of functional ingredients due to its high worldwide consumption. The metabolites in cooked tubers of eight diploid potato genotypes from Colombia were explored. Potato tubers were harvested, cooked,lyophilized, and then stored at −80°C. Metabolites were extracted from flesh samples and analyzed using liquid chromatography and high-resolution mass spectrometry. A total of 294 metabolites were putatively identified, of which 87 metabolites were associated with health-benefiting roles for humans, such as anticancer and anti-inflammatory properties. Two metabolites, chlorogenic acid and N-Feruloyltyramine were detected in high abundance and were mapped on to the potato metabolic pathways to predict the related biosynthetic enzymes: hydroxycinnamoyl-CoA quinate transferase (HQT) and tyramine hydroxycinnamoyl transferase (THT), respectively. The coding genes of these enzymes identified nonsynonymous single-nucleotide polymorphisms (nsSNPs) in AC09, AC64, and Russet Burbank, with the highest enzyme stability found in AC09. This is consistent with the highest presence of hydroxycinnamic acids in the AC09 genotype. The metabolites detected at high fold change, their functional ingredient properties, and their enhancement through breeding to improve health of the indigenous communities’ of Colombia are discussed.
Public Interest Statement
Potato plays a significant role in global food security as one of the most consumed non-grain crops worldwide. It thus has great potential as an excellent source of functional foods. Based on a comprehensive metabolomics approach we have identified a few functional-foods-related metabolites in cooked potato genotypes, and indicated their potential functional role on human nutrition and health. The polymorphism observed in the genes that biosynthesize these metabolites can be used to develop cisgenetic cultivars based on genome editing approach.
Competing interests
The authors declare no competing interest.
1. Introduction
Potato (Solanum tuberosum L.) is an important tuber food crop for human consumption around the world. It originated in South America over 10,000 years ago (CDC, Citation1999a), but did not spread to Europe until the late 1500s (CDC, Citation1999b). Now, there are more than 4,000 edible varieties of potato which play an important role in world food security (CIP, Citation2014). Potato is the fourth most important staple crop for humans after maize, rice, and wheat (FAOSTAT, Citation2014). More than one billion people consume potato in the world and global production has exceeded 300 million metric tons (CIP, Citation2014). Most of the potato grown around the world is tetraploid, whereas diploid potato (S. tuberosum Group Phureja) is grown mainly in the Andean regions of Colombia, where it is mainly consumed as soup by the indigenous communities.
Potato has the potential to contribute to world food security by providing food to the poor and by providing food with nutritional value to developed countries. In comparison with other food crops, potato has higher yield per unit growing area. In North America, potato has a yield of 40.6 mt ha−1 on average, which is higher than anywhere else (Camire, Kubow, & Donnelly, Citation2009). Thus, potato has a great effect on nutrition and health around the world. Potato is an important source of carbohydrates, protein, dietary fiber, minerals, and vitamins, and has been shown to contribute to the prevention of nutrient deficiencies. Interestingly, potato also contains a large number of functional ingredients which can contribute to human health (Ezekiel, Singh, Sharma, & Kaur, Citation2013). A functional ingredient is defined as belonging to a diverse group of compounds that can have beneficial effects on human health (Kruger & Mann, Citation2003). For example, potato is a good source of polyphenols, and contains 530–1,770 μg g−1 phenolics (Al-Saikhan, Howard, & Miller, Citation1995). Phenolics have become interesting functional ingredients mainly because of their antioxidants capacities. Condensed tannins have been shown to have anticarcinogenic, cardiovascular, and cholesterol-lowering properties (Dykes & Rooney, Citation2006; Gondim Junior, Barros, Silva, & Vasconcelos, Citation2005). Avenanthramides are shown to be anti-inflammatory and antioxidant (Bratt et al., Citation2003). Lignans are believed to reduce the risk of breast and prostate cancer (Cotterchio et al., Citation2006; Hooper & Cassidy, Citation2006). Alkylresorcinols are believed to have antibacterial and antifungal properties (Ross, Kamal-Eldin, & Åman, Citation2004). Potato is also a good source of flavonoids. Among potato varieties flavonoid concentrations varies between 200 and 300 μg g−1 FW (Lewis, Walker, Lancaster, & Sutton, Citation1998). Quercetin has been shown to play a role in protecting against cardiovascular disease (Finotti & Di Majo, Citation2003), diabetes, and the human immunodeficiency virus (HIV) (Li et al., Citation2000). Baicalin was recently shown to be anti-inflammatory and anti-HIV-1 (Li et al., Citation2000). Potato is a good source of carotenoids, and the main carotenoids present are lutein, zeaxanthin, violaxanthin, and neoxanthin. Carotenoids can play an important role in human health. β-Carotene is valued for its capacity to reduce the risk of lung cancer (Ziegler, Mayne, & Swanson, Citation1996). Lycopene has been reported to reduce the risk of breast (Zhang et al., Citation1997) and prostate cancer (Giovannucci et al., Citation1995; Mills, Beeson, Phillips, & Fraser, Citation1989). Lutein and zeaxanthin potentially protect against eye diseases (Snodderly, Citation1995; Yeum, Taylor, Tang, & Russell, Citation1995). The anthocyanin fraction of potato cultivar CO112F2–2 showed a potent ability to induce apoptosis in prostate cancer cells (Reddivari, Vanamala, Chintharlapalli, Safe, & Miller, Citation2007). Anthocyanins from purple potato showed the ability to suppress the growth of stomach cancer cells. Besides, some compounds in potato have been shown to play a role in preventing breast cancer (Kallio et al., Citation2008), colon and liver cancer (Lee et al., Citation2004), lung cancer (Shih, Chen, Wu, Jeng, & Wang, Citation2007), and cardiovascular disease by lowering cholesterol levels (Camire et al., Citation2009).
Metabolomics has become an excellent tool to detect a large number of biochemical compounds. Metabolomics is the comprehensive and quantitative analysis of all small molecules in a biological system (Fiehn, Citation2001). Metabolomics has recently been applied to different disciplines enabling comprehensive large-scale analyses of various biochemical compounds. Nuclear magnetic resonance (NMR) and mass spectrometry (MS) are currently the two main techniques used in metabolomics studies. NMR spectroscopy is used to profile low molecular metabolites. Mass spectroscopy has been widely used to analyze large population of metabolites. Gas chromatography and mass spectrometry (GC–MS) has been used to profile volatile metabolites (Pasikanti, Ho, & Chan, Citation2008), whereas liquid chromatography and high-resolution mass spectrometry (LC-HRMS) enabled identification of both volatile and nonvolatile metabolites (Allwood & Goodacre, Citation2010). Thousands of metabolites can be detected and quantified based on LC-HRMS (De Vos et al., Citation2007; Tolstikov, Lommen, Nakanishi, Tanaka, & Fiehn, Citation2003). Metabolites identified this way belonged to several chemical groups such as alkaloids, phenolics, flavonoids, polyamines, and fatty acids (Moco et al., Citation2006; Rischer et al., Citation2006). Recently, several metabolites against biotic stress have been identified based on a non-targeted metabolomics approach using LC-HRMS (Pushpa, Yogendra, Gunnaiah, Kushalappa, & Murphy, Citation2014; Yogendra et al., Citation2014; Yogendra, Kushalappa, Sarmiento, Rodriguez, & Mosquera, Citation2015b). However, studies including the large-scale discovery of functional ingredient-related metabolites in potato tubers, especially of the diploid potato, are lacking. Therefore, the objective of this study was to identify the functional ingredient-related metabolites in cooked diploid potato cultivars based on metabolic profiling. Eight potatoes advanced breeding genotypes were produced under field conditions and tubers were harvested at maturity. Metabolites were extracted from cooked tubers and identified using LC-HRMS. Metabolites with significantly higher abundance and major health benefits were selected as candidates. These metabolites were mapped in metabolic pathways to predict their biosynthetic genes. Due to sample availability, candidate genes were sequenced in genotypes AC09, AC64, and Russet Burbank; nonsynonymous single-nucleotide polymorphisms (nsSNPs) causing amino acid differences were used to explain the variations in the abundance of the metabolites. The potential application of these genes in developing potato cultivars with increased levels of FI through cisgenics is discussed.
2. Materials and methods
2.1. Potato genotypes
The diploid potato genotypes (Solanum tuberosum L. Group Phureja) used in this study were hybrids produced through crosses among four Criolla potato cultivars: Criolla Colombia, Criolla Guaneña, Criolla Galeras, and Criolla Latina by the Universidad Nacional de Colombia (Developed by Dr. Luis-Ernesto Rodriguez). The hybrids were selected based on agronomic characteristics, tuber yield potential, and resistance to Phytophthora infestans, Ralstonia solanacearum, Spongospora subterranea, Rhizoctonia solani, and potato yellow vein virus (PYVV). These hybrid genotypes are of medium height when erect with a vegetative period between 110 and 150 days, tubers are spherical with yellow skin and flesh, as well as shallow-depth eyes. From this collection eight genotypes were selected for this study.
2.2. Potato production and sample collection
Eight potato genotypes with good agronomic qualities were field grown in Nariño, Colombia. Each genotype was grown in three replicates. Recommended fertilization practices were followed. Potato tubers were harvested and stored at 4°C until use. The tubers were boiled for 30 min, skin peeled, ground in liquid nitrogen, lyophilized, and shipped with dry ice to McGill University for further analysis (Peña et al., Citation2015).
2.3. Metabolite extraction and analysis
Metabolites were extracted from the lyophilized potato flesh samples using 60% aqueous methanol with 0.1% formic acid (De Vos et al., Citation2007). Metabolites were analyzed using the liquid chromatography and high-resolution mass spectrometry (LC-HRMS) system (LC-ESL-LTQ Orbitrap, Thermo Fisher, Waltham, MA, USA) in negative ionization mode, fitted with a relatively polar reverse phase Kinetex column XB-C18 (5 cm × 2.1 mm) (Phenomenex, AC, USA) (Bollina et al., Citation2010). Mass resolution was set at 60,000 at 400 m/z. First, all samples were run to obtain the MS1 files and then the samples of AC50 and AC51 were re-run to get the MS/MS fragmentations using a normalized collision-induced dissociation energy of 35 eV. All the data obtained were recorded in centroid mode (Yogendra et al., Citation2015a).
2.4. LC-HRMS output data processing
The data files were converted into mzXML and analyzed using MZmine-2 software for mass detection, chromatogram deconvolution, identification of peaks, and retention time alignment across the samples (Pluskal, Castillo, Villar-Briones, & Orešič, Citation2010). For peak identification, wavelets were used with a signal-to-noise ratio (S/N) threshold of 5, wavelet scales of 0.2–5 min, and peak duration of 0.0–5.0. RANSAC alignment was used for retention time alignment with an m/z tolerance from 0.001 to 5.0 ppm, a retention time tolerance of 0.5 min, iterations of 10,000, and a threshold value of 0.5 s. The accurate mass, retention time, and their relative intensity were exported to MS Excel; peaks that were not consistent across replications and those annotated as isotopes and adducts were eliminated from further analyses.
2.5. Identification of metabolites
Putative identification of each metabolite was made based on: (i) the monoisotopic mass (MS-1) match with metabolites reported in different databases with an accurate mass error of (AME < 5 ppm): plant metabolic network (PMN, Citation2013), LIPIDMAPS, and KEGG (Kushalappa & Gunnaiah, Citation2013); (ii) MS/MS fragmentation pattern of metabolites in house spiked MS/MS library MASSBANK, METLIN, and MS2T; (iii) in silico fragmentation as described previously (Gunnaiah, Kushalappa, Duggavathi, Fox, & Somers, Citation2012). The metabolites with putative identification were classified according to chemical groups based on referenced databases such as PubChem and PMN (Kushalappa & Gunnaiah, Citation2013). The metabolites identified in cooked potatoes were further confirmed using an in-house uncooked tuber metabolite database based on the Shepody cultivar.
2.6. Functional ingredients-related properties of metabolites
Functional ingredient properties of the identified metabolites were searched in databases and the relevant literature. Anticancer related metabolites were confirmed through the NPACT database (Mangal, Sagar, Singh, Raghava, & Agarwal, Citation2013).
2.7. Statistical analysis
Treatments were replicated three times and assigned to a randomized complete block design (RCBD). The abundances of metabolites were normalized relative to abundance in the genotype AC04 to derive relative fold change for a given metabolite. All the data were subjected to analysis of variance to compare eight different genotypes and to indicate significance based on one-way ANOVA using MS Excel. Tukey’s test was applied to reveal the statistical significance of differences between genotype pairs.
2.8. Cloning and sequencing of functional ingredient biosynthetic candidate genes from the phenylpropanoid pathway
PCR amplification was done in genotypes AC09, AC64, and Russet Burbank, for which the uncooked samples were available, for the coding region of HQT and THT candidate genes using potato genomic DNA (200 ng μl−1) as a template and Taq Polymerase (Takara, CA, USA). The PCR cycling protocol was as follows: 95°C for 3 min–one cycle; 95°C for 30 s, 56–57°C for 30 s, and 72°C for 90 s–40 cycles; final extension at 72°C for 8 min. The amplified PCR products were cloned into the pGEM-T Easy Vector (Promega, UK). The ligated products were transformed into competent cells of E.coli. After getting positive colony PCR results, the plasmids were isolated using the Zyppy™ Plasmid Miniprep Kit (Zymo Research, CA, USA) and sent for DNA sequencing, which was done at Genome Québec in Montreal, Canada, using the ABI automated DNA sequencer. DNA sequences were aligned using BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html) and then were converted to amino acid sequences using the ExPASy translate tool (http://web.expasy.org/translate/). Multiple sequence alignments were conducted for the amino acid sequences of AC09, AC64, and Russet Burbank genotypes; and GenBank sequences for HQT and THT candidate genes using the ClustalX tool, with default parameters. Amino acid substitutions were identified between AC09 and AC64 genotypes, and their effects on protein stability was predicted by estimating the relative stability changes (ΔΔG value) upon protein mutation using I-Mutant2.0 (Capriotti, Fariselli, & Casadio, Citation2005), where ΔΔG < 0 indicates the reduced stability of proteins.
3. Results
3.1. Metabolic profiles of cooked potatoes
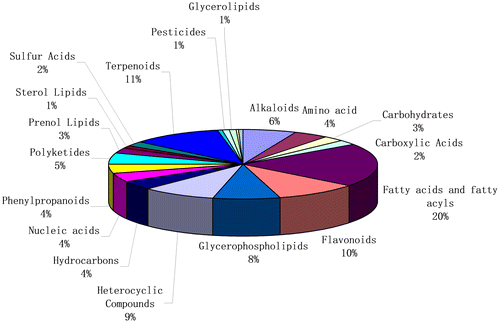
A total of 294 metabolites were detected in the cooked potatoes from 8 genotypes. The metabolites belonged to different chemical groups, including 20% fatty acids and acyls, 10% terpenoids, 10% flavoniods, 6% alkaloids, 4% amino acids, and 4% phenylpropanoids (Figure ). The chemical groups of heterocyclic compounds, glycerophospholipids, polyketides, nucleic acids, hydrocarbons, prenol lipids, and carbohydrates comprised 9, 8, 5, 4, 4, 3, and 3%, respectively. Other metabolites belonging to chemical groups including sulfur compounds, carboxylic acids, sterol lipids, and amides were also detected.
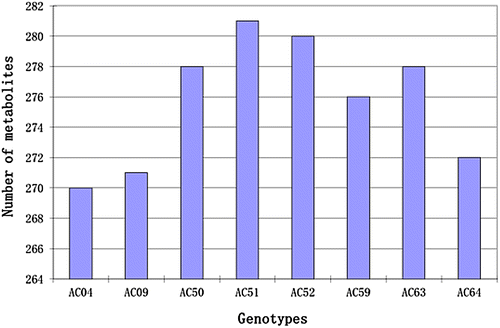
Among the eight genotypes, the highest number of metabolites was identified in genotype AC51 and AC52, with 281 and 280 metabolites, respectively. A total of 278 metabolites were identified in both AC50 and AC63. AC59 and AC64 had 276 and 272 metabolites, respectively. The lowest numbers of metabolites were identified in AC04 and AC09 with 270 and 271 metabolites, respectively (Figure ).
3.2. Functional ingredients related metabolites in cooked diploid potatoes
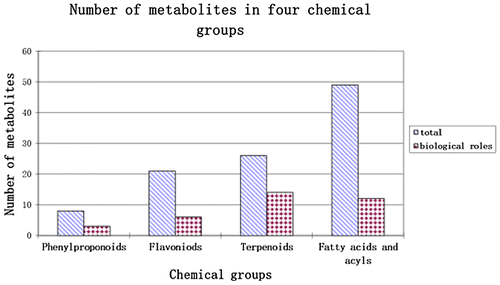
Out of 294 metabolites identified in cooked Colombian diploid potatoes from 8 genotypes, 87 were reported to exhibit important human health benefits, including anticancer, anti-inflammatory, antimicrobial, antioxidant, and anti-HIV properties (Cavin, Hostettmann, Dyatmyko, & Potterat, Citation1998; Chen et al., Citation2004; Zhu et al., Citation2012). Three out of eight phenylpropanoids identified have shown health benefits like antidiabetic, antioxidant, and anticancer effects. Out of 21 metabolites that were classified as flavonoids, six have anticancer effects, and two have anti-inflammatory and antimicrobial properties. Out of 26 terpenoid metabolites, 14 have human health benefits including anticancer, antioxidant, and antiapoptotic effects. The 49 fatty acids and acyls have exhibited diverse health-related benefits such as anti-HIV-1, antimicrobial, antiviral, and anti-inflammatory properties (Figure ).
3.3. Relative abundances of functional ingredients metabolites among genotypes
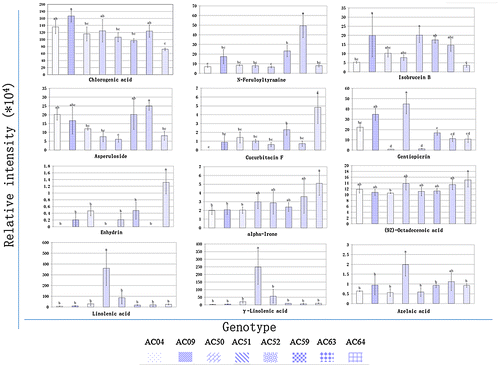
Out of 87 metabolites with reported human health benefits, only 46 have been previously reported in plants. Both the intensities and fold change of metabolites significantly varied among the eight genotypes. The fold change was calculated relative to the abundance in genotype AC04. The genotype AC51 had the highest fold changes of 46 and 80, for the metabolites linolenic acid and γ-Linolenic acid, respectively. The genotype AC63 had the highest fold change of 7 for the metabolite N-Feruloyltyramine, followed by AC 59 (FC = 3.3) and AC09 (FC = 2.5). A high fold change of 5 was observed in AC64 for the metabolite Cucurbitacin F (Table , Figure ).
Table 1. Fold change in abundances of functional ingredients metabolites in diploid cooked potato (Solanum tuberosum Group Phureja)
3.4. Impact of cooking on metabolite structure
The occurrence of compounds detected in cooked potato was confirmed based on an in-house metabolite library of compounds detected in uncooked potato, from the cultivar Shepody (Solanum tuberosum L.). Several metabolites such as chlorogenate, N-Feruloyltyramine, linoleic acid, and γ-Linolenic acid were detected in both cooked and uncooked potato (Table , Figure ).
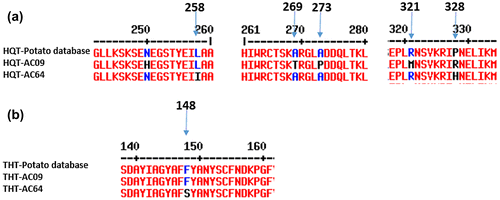
3.5. Difference in coding sequences of FI biosynthetic genes between AC09 and AC64 genotypes
The DNA of two FI metabolite biosynthetic genes, HQT and THT, was sequenced in AC09 and AC64, for which uncooked samples were available. These sequences were compared to potato database sequences, and multiple sequence alignment of amino acids was done to identify polymorphisms. The amino acids from the AC09 genotype varied compared to AC64 at six positions for HQT, and at only one position for THT (Table , Figure ). The relative free energy ΔΔG > 0 indicated that AC09 has a greater enzyme stability than AC64for all the amino acid substitutions, in both HQT and THT (Table ). The amino acid substitutions at positions 250, 258, 269, 273, 321, and 328 for HQT, in AC64 relative to AC09, yielded negative ΔΔG values of −2.39, −0.35, −1.77, −1.55, −0.73, and −2.17, respectively; meaning lower enzyme stability values in AC64 as compared to AC09. Likewise, at positions 148, it yielded ΔΔG values of −2.16 in AC64 for THT. The decrease (<1.0) in protein stability leads to a reduced expression of HQT and THT genes in AC64.
Table 2. Amino acid sequences of FI metabolites biosynthetic genes from AC09 and AC64 genotypes and potato database sequences
4. Discussion
Potato has the potential to contribute to global food security by providing food to the poor and also by providing food with increased nutritional value to developed countries. Indigenous communities in the Nariño area of Colombia are impoverished and their food security is precarious. The people there consume potatoes mainly as cooked potato soup rather than French fries or potato chips. In this study, we have identified several metabolites with important health benefits, and their role in improving diet and health is discussed.
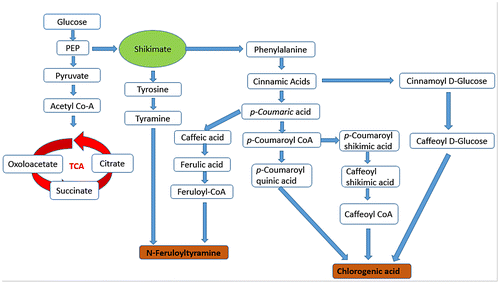
4.1. Chlorogenic acid
This hydroxycinnamic acid was detected in all eight genotypes, and the abundance of this compound significantly varied among all genotypes. AC09 had the highest fold change of 1.2, while AC64 had the lowest fold change of 0.5. The concentration of chlorogenate was previously reported to range from 9.65 ± 0.49 to 18.7 ± 12.10 mg 100 g−1 of fresh weight among potato cultivars (Dao & Friedman, Citation1992). In boiled potatoes, the highest amount was 31.9 ± 1.0 mg 100 g−1 of freeze-dried weight (Dao & Friedman, Citation1992). Chlorogenic acid can also be found in bamboo, Phyllostachys edulis (Kweon, Hwang, & Sung, Citation2001), and in many other plants such as apple (mean values 30–60 mg kg−1), berry fruits, and grapes (Clifford, Citation1999). High amounts of phenolic compounds have been detected in peach (Cheng & Crisosto, Citation1995), prunes (184 mg 100 g−1) (Stacewicz-Sapuntzakis, Bowen, Hussain, Damayanti-Wood, & Farnsworth, Citation2001), and green coffee bean extracts (Khalesi et al., Citation2014). Chlorogenate has been reported to show antioxidant properties, such as the protection of blood granulocytes from oxidative stress (Bouayed, Rammal, Dicko, Younos, & Soulimani, Citation2007; Marinova, Toneva, & Yanishlieva, Citation2009), and it exhibits scavenging abilities of organic free radicals and peroxy radicals (Kono et al., Citation1997). It can also increase the resistance of LDL (low-density lipoprotein) and exhibit antilipidemic and antidiabetic effects (Shafi & Tabassum, Citation2013). Furthermore, it shows anticarcinogenic effects, such as the inhibition of chemical-induced carcinogenesis in rats and hamsters (Rakshit et al., Citation2010), antiproliferative effects on HepG2 cells (Zhang, Guo, Shangguan, Zheng, & Wang, Citation2012), and apoptosis induction of Bcr-Abl+ chronic myeloid leukemia (CML) cell lines (Rakshit et al., Citation2010).
4.2. N-Feruloyltyramine
This hydroxycinnamic acid amide was detected in all eight genotypes and its abundance was the highest in AC63 (FC = 7.1), followed by AC59 (FC = 3.3), AC09 (FC = 2.5) and AC64 (FC = 1.2). In addition, N-Feruloyltyramine in potato imparts resistance to late blight disease (Yogendra et al., Citation2014, Citation2015a). This compound was previously reported in Tinospora crispa Miers as exhibiting antioxidant and radical scavenging properties (Cavin et al., Citation1998). It has also been reported to show strong anti-inflammatory effects mediated by the inhibition of arachidonate 5-lipoxygenase (Al-Taweel et al., Citation2012). It also exhibits strong anti-nitric oxide (Anti-NO) activity (Yokozawa, Wang, Chen, & Hattori, Citation2000). It also has anti-obesity properties because of its inhibition of pancreatic lipase (Ahn et al., Citation2013).
4.3. Effect of amino acid substitutions on FI metabolite biosynthetic gene expression and metabolite abundance
The biosynthetic genes HQT and THT, that biosynthesized chlorogenic acid and N-Feruloyltyramine metabolites, were analyzed in AC09 and AC64 genotypes; as fresh uncooked samples were available only for these two genotypes. The coding regions of HQT and THT were sequenced to identify polymorphisms. High fold change metabolites in AC09 genotype were considered to be due to variation in biosynthetic gene sequence between AC09 and AC64 genotypes, which resulted in changes in the amino acids they code for, consequently affecting gene expression and enzyme stability (Capriotti et al., Citation2005). The amino acid substitutions of H250N, L258I, T269A, P273A, M321R, and R328H in HQT and F148S in THT in AC64 genotype, which were different from that of high abundance genotypes tested here, were used to assess enzyme stability (Table , letters indicate amino acids). Further studies are needed that explore other genotypes in order to find one with very high amounts of these metabolites. We have identified only the genes directly involved in the biosynthesis of these metabolites. However, these genes are generally regulated by several other genes, such as transcription factors and phytohormones, and these also should be explored for their potential application in breeding.
4.4. Genome editing to improve functional ingredient metabolites
The functional genes we have identified here, especially concerning chlorogenic acid, and the associated regulatory genes to be identified in future can be used to replace the nonfunctional genes in commercial potato cultivars in order to enhance FI metabolites. Since potato is difficult to breed, owing to sexual incompatibility, the latest genome editing technologies can be used to replace nonfunctional genes with the functional genes that we reported here (Shan, Wang, Li, & Gao, Citation2014). Transgenic approaches have been used to increase the carotenoids in potato, by overexpressing three genes CrtB, CrtI, and CrtY from bacteria, which resulted in a 20-fold increase in carotenoids and a 3,600-fold of β-carotene (Diretto et al., Citation2007). The overexpression of a bacterial phytoene synthase increased the carotenoids content from 5.6 to 35 μg g−1 DW(Ducreux et al., Citation2005). However, the public is against transgenics, and they prefer cisgenic (gene transfer from sexually compatible plants) (Telem et al., Citation2013). Cisgenic approaches have been used to enhance the antioxidant capacity of apples (Espley et al., Citation2007) and to improve the apple scab resistance (Vanblaere et al., Citation2011) as well as the late blight resistance of potato (Park et al., Citation2005).
5. Conclusion
In this study, we have reported several functional ingredient metabolites and ranked them based on the abundance relative to one genotype, AC04. The metabolites were selected based on their major health benefits such as anticancer, anti-inflammatory, antimicrobial, antioxidant, and anti-HIV properties. The results showed that different genotypes can be explored for important metabolites which showed health benefiting properties. Two metabolites, chlorogenic acid and N-Feruloyltyramine, were further explored for variations in gene expression, allelic variation in gene sequence, and to select candidate genes. Following validation, the FI metabolites or the genes involved in the biosynthesis of most significant FI metabolites can be used as potential biomarkers in breeding to improve the amounts of these metabolites. Specific genotypes with high abundance of functional ingredients reported here can be used in the Colombian breeding programs to increase a specific metabolite. Alternatively, similar studies can be conducted on other potato cultivars to explore high abundance of a given metabolite, and in turn this cultivar can be used in breeding programs to enhance the compound based on cisgenics (Jacobsen, Citation2013).
Kushalappa-Sup-Tables.docx
Download MS Word (18.3 KB)Acknowledgements
Liyao Ji, conducted all the analysis and wrote the manuscript; Kalenahalli N. Yogendra, assisted in metabolic profiling, gene sequencing and edited the manuscript; Kareem A. Mosa, assisted in metabolic profiling; Ajjamada C. Kushalappa, obtained funding, coordinated the work, and edited manuscript; Clara Piñeros-Niño, produced potato and collected samples; Teresa Mosquera and Carlos Narvaez, coordinated the experiments in Colombia.
Additional information
Funding
Notes on contributors
Ajjamada C. Kushalappa
Our group is working on improving food security, by providing food with increased nutritional value and also biotic stress resistance, of developing countries, especially the indigenous communities in the Nariño region of Colombia. We use systems biology approach to discover genes with desired traits, and replace them in commercial cultivars based on genome editing. A special focus is accorded to biochemical characterization and innovative functional food ingredients’ formulation.
References
- Ahn, J. H., Kim, E. S., Lee, C., Kim, S., Cho, S. H., Hwang, B. Y., & Lee, M. K. (2013). Chemical constituents from Nelumbo nucifera leaves and their anti-obesity effects. Bioorganic and Medicinal Chemistry Letters, 23, 3604–3608.10.1016/j.bmcl.2013.04.013
- Allwood, J. W., & Goodacre, R. (2010). An introduction to liquid chromatography–mass spectrometry instrumentation applied in plant metabolomic analyses. Phytochemical Analysis, 21, 33–47.10.1002/(ISSN)1099-1565
- Al-Saikhan, M., Howard, L., & Miller, J. (1995). Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum, L.). Journal of Food Science, 60, 341–343.10.1111/jfds.1995.60.issue-2
- Al-Taweel, A. M., Perveen, S., El-Shafae, A. M., Fawzy, G. A., Malik, A., Afza, N., & Iqbal, L. (2012). Bioactive phenolic amides from Celtis africana. Molecules, 17, 2675–2682.10.3390/molecules17032675
- Bollina, V., Kumaraswamy, G. K., Kushalappa, A. C., Choo, T. M., Dion, Y., Rioux, S., … Hamzehzarghani, H. (2010). Mass spectrometry-based metabolomics application to identify quantitative resistance-related metabolites in barley against Fusarium head blight. Molecular plant pathology, 11, 769–782.
- Bouayed, J., Rammal, H., Dicko, A., Younos, C., & Soulimani, R. (2007). Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. Journal of the Neurological Sciences, 262, 77–84.10.1016/j.jns.2007.06.028
- Bratt, K., Sunnerheim, K., Bryngelsson, S., Fagerlund, A., Engman, L., Andersson, R. E., & Dimberg, L. H. (2003). Avenanthramides in oats (Avena sativa L.) and structure−antioxidant activity relationships. Journal of Agricultural and Food Chemistry, 51, 594–600.10.1021/jf020544f
- Camire, M. E., Kubow, S., & Donnelly, D. J. (2009). Potatoes and human health. Critical Reviews in Food Science and Nutrition, 49, 823–840.10.1080/10408390903041996
- Capriotti, E., Fariselli, P., & Casadio, R. (2005). I-Mutant2.0: Predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Research, 33, W306–W310.10.1093/nar/gki375
- Cavin, A., Hostettmann, K., Dyatmyko, W., & Potterat, O. (1998). Antioxidant and lipophilic constituents of Tinospora crispa. Planta Medica, 64, 393–396.10.1055/s-2006-957466
- CDC. (1999a). The potato, then and now. Early beginnings. Canada’s Digital Collections archived by Library and Archives Canada. Retrieved from http://epe.lacbac.gc.ca/100/205/301/ic/cdc/potato/history/beginnings.asp
- CDC. (1999b). The potato, then and now. Potato migration to Europe. Canada’s Digital Collections archived by Library and Archives Canada. Retrieved from http://epe.lacbac.gc.ca/100/205/301/ic/cdc/potato/history/migration.asp
- Chen, C.-Y., Milbury, P. E., Kwak, H.-K., Collins, F. W., Samuel, P., & Blumberg, J. B. (2004). Avenanthramides and phenolic acids from oats are bioavailable and act synergistically with vitamin C to enhance hamster and human LDL resistance to oxidation. The Journal of nutrition, 134, 1459–1466.
- Cheng, G. W., & Crisosto, C. H. (1995). Browning potential, phenolic composition, and polyphenoloxidase activity of buffer extracts of peach and nectarine skin tissue. Journal of the American Society for Horticultural Science, 120, 835–838.
- CIP. (2014). International year of the potato, potato world. Retrieved from http://cipotato.org/
- Clifford, M. N. (1999). Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. Journal of the Science of Food and Agriculture, 79, 362–372. doi:10.1002/(SICI)1097-0010(19990301)79:3<362:AID-JSFA256>3.0.CO;2-D
- Cotterchio, M., Boucher, B. A., Manno, M., Gallinger, S., Okey, A., & Harper, P. (2006). Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. The Journal of nutrition, 136, 3046–3053.
- Dao, L., & Friedman, M. (1992). Chlorogenic acid content of fresh and processed potatoes determined by ultraviolet spectrophotometry. Journal of Agricultural and Food Chemistry, 40, 2152–2156. doi:10.1021/jf00023a022
- De Vos, R. C., Moco, S., Lommen, A., Keurentjes, J. J., Bino, R. J., & Hall, R. D. (2007). Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nature Protocols, 2, 778–791.10.1038/nprot.2007.95
- Diretto, G., Al-Babili, S., Tavazza, R., Papacchioli, V., Beyer, P., & Giuliano, G. (2007). Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. Plos One, 2, e350. doi:10.1371/journal.pone.0000350
- Ducreux, L. J. M., Morris, W. L., Hedley, P. E., Shepherd, T., Davies, H. V., Millam, S., & Taylor, M. A. (2005). Metabolic engineering of high carotenoid potato tubers containing enhanced levels of β-carotene and lutein. Journal of Experimental Botany, 56, 81–89. doi:10.1093/jxb/eri016
- Dykes, L., & Rooney, L. W. (2006). Sorghum and millet phenols and antioxidants. Journal of Cereal Science, 44, 236–251.10.1016/j.jcs.2006.06.007
- Espley, R. V., Hellens, R. P., Putterill, J., Stevenson, D. E., Kutty-Amma, S., & Allan, A. C. (2007). Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. The Plant Journal, 49, 414–427. doi:10.1111/j.1365-313X.2006.02964.x
- Ezekiel, R., Singh, N., Sharma, S., & Kaur, A. (2013). Beneficial phytochemicals in potato—A review. Food Research International, 50, 487–496.10.1016/j.foodres.2011.04.025
- FAOSTAT. (2014). Food and agriculture organization of the united nations statistics division. Retrieved from http://faostat.fao.org/site/339/default.aspx
- Fiehn, O. (2001). Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comparative and Functional Genomics, 2, 155–168.10.1002/cfg.82
- Finotti, E., & Di Majo, D. (2003). Influence of solvents on the antioxidant property of flavonoids. Nahrung/Food, 47, 186–187.10.1002/food.200390043
- Giovannucci, E., Ascherio, A., Rimm, E. B., Stampfer, M. J., Colditz, G. A., & Willett, W. C. (1995). Intake of carotenoids and retino in relation to risk of prostate cancer. JNCI Journal of the National Cancer Institute, 87, 1767–1776.10.1093/jnci/87.23.1767
- Gondim Junior, M. G. C., Barros, R., Silva, F. R. d., & Vasconcelos, G. J. N. d. (2005). Occurence and biological aspects of the clitoria tree psyllid in Brazil. Scientia Agricola, 62, 281–285.
- Gunnaiah, R., Kushalappa, A. C., Duggavathi, R., Fox, S., & Somers, D. J. (2012). Integrated metabolo-proteomic approach to decipher the mechanisms by which wheat QTL (Fhb1) contributes to resistance against Fusarium graminearum. Plos One, 7, e40695.10.1371/journal.pone.0040695
- Hooper, L., & Cassidy, A. (2006). A review of the health care potential of bioactive compounds. Journal of the Science of Food and Agriculture, 86, 1805–1813.10.1002/(ISSN)1097-0010
- Jacobsen, E. (2013). Cisgenesis: A modern way of domesticating traits of the breeders’ gene pool. CAB Reviews, 8, 56. doi:10.1079/PAVSNNR20138056
- Kallio, P., Kolehmainen, M., Laaksonen, D. E., Pulkkinen, L., Atalay, M., Mykkänen, H., & Niskanen, L. (2008). Inflammation markers are modulated by responses to diets differing in postprandial insulin responses in individuals with the metabolic syndrome. The American journal of clinical nutrition, 87, 1497–1503.
- Khalesi, S., Sun, J., Buys, N., Jamshidi, A., Nikbakht-Nasrabadi, E., & Khosravi-Boroujeni, H. (2014). Green tea catechins and blood pressure: a systematic review and meta-analysis of randomised controlled trials. European Journal of Nutrition, 53, 1299–1311. doi:10.1007/s00394-014-0720-1
- Kono, Y., Kobayashi, K., Tagawa, S., Adachi, K., Ueda, A., Sawa, Y., & Shibata, H. (1997). Antioxidant activity of polyphenolics in diets. Biochimica et Biophysica Acta (BBA) - General Subjects, 1335, 335–342. 10.1016/S0304-4165(96)00151-1
- Kruger, C. L., & Mann, S. W. (2003). Safety evaluation of functional ingredients. Food and Chemical Toxicology, 41, 793–805.10.1016/S0278-6915(03)00018-8
- Kushalappa, A. C., & Gunnaiah, R. (2013). Metabolo-proteomics to discover plant biotic stress resistance genes. Trends in Plant Science, 18, 522–531.10.1016/j.tplants.2013.05.002
- Kweon, M.-H., Hwang, H.-J., & Sung, H.-C. (2001). Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis). Journal of Agricultural and Food Chemistry, 49, 4646–4655. doi:10.1021/jf010514x
- Lee, K.-R., Kozukue, N., Han, J.-S., Park, J.-H., Chang, E.-Y., Baek, E.-J., … Friedman, M. (2004). Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. Journal of Agricultural and Food Chemistry, 52, 2832–2839.10.1021/jf030526d
- Lewis, C. E., Walker, J. R., Lancaster, J. E., & Sutton, K. H. (1998). Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanum tuberosum L. Journal of the Science of Food and Agriculture, 77, 45–57.10.1002/(ISSN)1097-0010
- Li, B. Q., Fu, T., Dongyan, Y., Mikovits, J. A., Ruscetti, F. W., & Wang, J. M. (2000). Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochemical and Biophysical Research Communications, 276, 534–538.10.1006/bbrc.2000.3485
- Mangal, M., Sagar, P., Singh, H., Raghava, G. P., & Agarwal, S. M. (2013). NPACT: Naturally occurring plant-based anti-cancer compound-activity-target database. Nucleic Acids Research, 41, D1124–D1129.10.1093/nar/gks1047
- Marinova, E. M., Toneva, A., & Yanishlieva, N. (2009). Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chemistry, 114, 1498–1502.10.1016/j.foodchem.2008.11.045
- Mills, P. K., Beeson, W. L., Phillips, R. L., & Fraser, G. E. (1989). Cohort study of diet, lifestyle, and prostate cancer in adventist men. Cancer, 64, 598–604.10.1002/(ISSN)1097-0142
- Moco, S., Bino, R. J., Vorst, O., Verhoeven, H. A., de Groot, J., van Beek, T. A., … De Vos, C. R. (2006). A liquid chromatography-mass spectrometry-based metabolome database for tomato. Plant Physiology, 141, 1205–1218.10.1104/pp.106.078428
- Park, T. H., Gros, J., Sikkema, A., Vleeshouwers, V. G., Muskens, M., Allefs, S., … van der Vossen, E. A. (2005). The late blight resistance locus Rpi-blb3 from Solanum bulbocastanum belongs to a major late blight R gene cluster on chromosome 4 of potato. Molecular Plant-Microbe Interactions, 18, 722–729.10.1094/MPMI-18-0722
- Pasikanti, K. K., Ho, P., & Chan, E. (2008). Gas chromatography/mass spectrometry in metabolic profiling of biological fluids. Journal of Chromatography B, 871, 202–211.10.1016/j.jchromb.2008.04.033
- Peña, C., Restrepo-Sánchez, L. P., Kushalappa, A., Rodríguez-Molano, L. E., Mosquera, T., & Narváez-Cuenca, C. E. (2015). Nutritional contents of advanced breeding clones of Solanum tuberosum group Phureja. LWT - Food Science and Technology, 62, 76–82. doi:10.1016/j.lwt.2015.01.038
- Pluskal, T., Castillo, S., Villar-Briones, A., & Orešič, M. (2010). MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics, 11, 395.10.1186/1471-2105-11-395
- PMN. (2013). PMN. Retrieved from www.plantcyc.org
- Pushpa, D., Yogendra, K. N., Gunnaiah, R., Kushalappa, A. C., & Murphy, A. (2014). Identification of late blight resistance-related metabolites and genes in potato through nontargeted metabolomics. Plant Molecular Biology Reporter, 32, 584–595.10.1007/s11105-013-0665-1
- Rakshit, S., Mandal, L., Pal, B. C., Bagchi, J., Biswas, N., Chaudhuri, J., & Bandyopadhyay, S. (2010). Involvement of ROS in chlorogenic acid-induced apoptosis of Bcr-Abl+ CML cells. Biochemical Pharmacology, 80, 1662–1675.10.1016/j.bcp.2010.08.013
- Reddivari, L., Vanamala, J., Chintharlapalli, S., Safe, S. H., & Miller, J. C. (2007). Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways. Carcinogenesis, 28, 2227–2235.10.1093/carcin/bgm117
- Rischer, H., Oresic, M., Seppanen-Laakso, T., Katajamaa, M., Lammertyn, F., Ardiles-Diaz, W., & Van Montagu, M. C. E. (2006). Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proceedings of the National Academy of Sciences, 103, 5614–5619.10.1073/pnas.0601027103
- Ross, A. B., Kamal-Eldin, A., & Åman, P. (2004). Dietary alkylresorcinols: Absorption, bioactivities, and possible use as biomarkers of whole-grain wheat-and rye-rich foods. Nutrition Reviews, 62, 81–95.10.1111/nure.2004.62.issue-3
- Shafi, S., & Tabassum, N. (2013). Antihyperglycemic and lipid lowering activities of ethanolic extract of eriobotrya japonica seeds in alloxan induced diabetic rats. European Scientific Journal, 9(21). doi:10.1079/PAVSNNR20138056
- Shan, Q., Wang, Y., Li, J., & Gao, C. (2014). Genome editing in rice and wheat using the CRISPR/Cas system. Nature Protocols, 9, 2395–2410.10.1038/nprot.2014.157
- Shih, Y.-W., Chen, P.-S., Wu, C.-H., Jeng, Y.-F., & Wang, C.-J. (2007). α-Chaconine-reduced metastasis involves a PI3K/Akt signaling pathway with downregulation of NF-κB in human lung adenocarcinoma A549 cells. Journal of Agricultural and Food Chemistry, 55, 11035–11043.10.1021/jf072423r
- Snodderly, D. M. (1995). Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. The American journal of clinical nutrition, 62, 1448S–1461S.
- Stacewicz-Sapuntzakis, M., Bowen, P. E., Hussain, E. A., Damayanti-Wood, B. I., & Farnsworth, N. R. (2001). Chemical composition and potential health effects of prunes: A functional food? Critical Reviews in Food Science and Nutrition, 41, 251–286. doi:10.1080/20014091091814
- Telem, R. S., Wani, S., Singh, N. B., Nandini, R., Sadhukhan, R., Bhattacharya, S., & Mandal, N. (2013). Cisgenics—A sustainable approach for crop improvement. Current Genomics, 14, 468–476.10.2174/13892029113146660013
- Tolstikov, V. V., Lommen, A., Nakanishi, K., Tanaka, N., & Fiehn, O. (2003). Monolithic silica-based capillary reversed-phase liquid chromatography/electrospray mass spectrometry for plant metabolomics. Analytical Chemistry, 75, 6737–6740.10.1021/ac034716z
- Vanblaere, T., Szankowski, I., Schaart, J., Schouten, H., Flachowsky, H., Broggini, G. A. L., & Gessler, C. (2011). The development of a cisgenic apple plant. Journal of Biotechnology, 154, 304–311.10.1016/j.jbiotec.2011.05.013
- Yeum, K.-J., Taylor, A., Tang, G., & Russell, R. M. (1995). Measurement of carotenoids, retinoids, and tocopherols in human lenses. Investigative Ophthalmology & Visual Science, 36, 2756–2761.
- Yogendra, K., Pushpa, D., Mosa, K., Kushalappa, A., Murphy, A., & Mosquera, T. (2014). Quantitative resistance in potato leaves to late blight associated with induced hydroxycinnamic acid amides. Functional & integrative genomics, 14, 285–298. doi:10.1007/s10142-013-0358-8
- Yogendra, K. N., Kumar, A., Sarkar, K., Li, Y., Pushpa, D., Mosa, K. A., Duggavathi, R., & Kushalappa, A. C. (2015a). Transcription factor StWRKY1 regulates phenylpropanoid metabolites conferring late blight resistance in potato. Journal of Experimental Botany, 66, 7377–7389. doi:10.1093/jxb/erv434
- Yogendra, K. N., Kushalappa, A. C., Sarmiento, F., Rodriguez, E., & Mosquera, T. (2015b). Metabolomics deciphers quantitative resistance mechanisms in diploid potato clones against late blight. Functional Plant Biology, 42, 284–298.
- Yokozawa, T., Wang, T. S., Chen, C. P., & Hattori, M. (2000). Inhibition of nitric oxide release by an aqueous extract of Tinospora tuberculata. Phytotherapy Research, 14, 51–53.10.1002/(ISSN)1099-1573
- Zhang, S., Tang, G., Russell, R. M., Mayzel, K. A., Stampfer, M. J., Willett, W. C., & Hunter, D. J. (1997). Measurement of retinoids and carotenoids in breast adipose tissue and a comparison of concentrations in breast cancer cases and control subjects. The American journal of clinical nutrition, 66, 626–632.
- Zhang, Q. F., Guo, Y. X., Shangguan, X., Zheng, G., & Wang, W. J. (2012). Antioxidant and anti-proliferative activity of Rhizoma Smilacis Chinae extracts and main constituents. Food Chemistry, 133, 140–145.10.1016/j.foodchem.2012.01.008
- Zhu, W., Pang, M., Dong, L., Huang, X., Wang, S., & Zhou, L. (2012). Anti-inflammatory and immunomodulatory effects of iridoid glycosides from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on uric acid nephropathy rats. Life Sciences, 91, 369–376.10.1016/j.lfs.2012.08.013
- Ziegler, R. G., Mayne, S. T., & Swanson, C. A. (1996). Nutrition and lung cancer. Cancer Causes & Control, 7, 157–177.