Abstract
Lipase activity of commercially produced rice bran was inhibited with an aim of multiple product recovery. The initial values of free fatty acid were 8.72, 1.89, and 4.41% in bran from coarse, fine, and superfine cultivars which increased to 60.11, 51.70, and 75.33% level, respectively, after 40 days of ambient storage conditions. Storage temperature of −20°C and hydrochloric acid at 5% were found to be effective in controlling the lipase activity. Sodium hydroxide was less effective in controlling the lipase activity even at 10%. Hydrogen peroxide (30%) did not inhibit the activity but promoted oil degradation in rice bran. The initial peroxide value varied from 2.74 to 4.76 meq O2 per kg of oil which increased to 10.19–14.15 meq O2 per kg in untreated, while HCl-treated samples varied from 6.22 to 7.98 meq O2 per kg at 5% level after 40 days.
Public Interest Statement
Rice bran has highly nutritious oil and proteins. Rice bran shows high lipase activity after processing due to which rice bran oil is hydrolyzed into free fatty acids. Most of the industries inactivate lipase using high heat treatment mainly extrusion, but high temperature negatively affects rice bran protein isolation and its functional properties. So, chemical or low-temperature treatment was used to inhibit lipase activity which prevented hydrolysis of rice bran lipids and also enabled rice bran for better protein isolation.
Competing interests
The authors declare no competing interest.
1. Introduction
Rice bran is produced during the polishing of brown rice and constitutes 6–8% of the whole grain. It is rich in highly nutritional proteins, fats, dietary fibers, vitamins, and minerals such as iron, potassium, calcium, chlorine, magnesium, and manganese (Saunders, Citation1985). It contains about 14–16% protein, 12–23% fat, and 8–10% crude fiber (Malekian et al., Citation2000; Saunders, Citation1990).
Rice bran oil helps in reducing serum cholesterol level and prevents risk of heart diseases. Rice bran contains lipase enzyme that shows maximum activity at 35–40°C and pH 7.5–8.0 (Prabhakar & Venkatesh, Citation1986). Lipase and oil present in cells come in contact with each other due to rupturing of cells during rice milling. Lipase hydrolyzes triacylglycerols into free fatty acids (FFAs) that decrease the shelf life of rice bran and makes it unsuitable for human consumption (Barnes & Galliard, Citation1991).
Many treatments have been proposed to stabilize the rice bran by inactivating lipase (Saunders, Citation1990). Thermal treatments employed are dry heat, moist heat, microwave heat (Rhee & Yoon, Citation1984; Wu, Citation1990), extrusion cooking, and ohmic heating (Lakkakula, Lima, & Walker, Citation2004), whereas chemical method involves the use of hydrochloric acid (Prakash & Ramanatham, Citation1995), ethanol vapors, metal ions such as Fe3+, Ni2+ along with HCl (Munshi, Bhatia, Sekhon, & Sukhija, Citation1993), Li2+, and SeO2 (Gangadhara & Prakash, Citation2010). Thermal inactivation by extrusion of rice bran is commercially popular method as it produces pellets that facilitates oil recovery though solvent extraction. Extrusion treatment affects the solubility and functional properties of protein.
The present investigation was undertaken to inhibit the lipase activity in commercially produced rice bran from coarse, fine, and superfine cultivars with special consideration to multiple product recovery.
2. Materials and methods
2.1. Raw materials
On the basis of length to breath (L/B) ratio, rice varieties can be broadly classified into three categories, viz. superfine (L/B ratio > 3.0, long, and slender kernel, 4–5 times longer than its width), fine (L/B ratio 2.1–3.0, shorter, and wider kernel, 2–3 time longer than its width), and coarse (L/B ratio < 2.1, short, plump, and almost round kernel) (Cruz & Khush, Citation2000). Fine and superfine rice bran were procured from BN Exports Rice Mill, Amritsar, Punjab and coarse rice bran from KLA Rice Mill, Rudrapur, Uttarakhand, India. The rice bran was sieved (30 mesh, to remove husk and broken rice), packed in polyethylene bags and kept in deep freezer at −20°C.
2.2. Methods
2.2.1. Proximate composition
Proximate composition of rice was determined according to AACC (Citation1995) methods for moisture (44–15A), crude protein (46–12), ash (08–01), crude fat (30–2), fiber (32–05), and carbohydrates were calculated by difference method.
2.2.2. pH
The pH was determined using a pH Meter (SD Fine Chemicals Limited, Mumbai, India) on an aqueous suspension of rice bran (10gm/100 ml).
2.2.3. Oil extraction from rice bran
Rice bran (20 g) was taken in a 250 ml stoppered Erlenmeyer flask, 60 ml of hexane was added, and the mixture was kept in incubated shaker (Daihan Labtech Company Limited, Namyangju, South Korea) for 30 min at 130 rpm and 30°C, filtered, and vacuum evaporated (Buchi Labortechnik AG, Flawil, Switzerland) to get crude rice bran oil.
2.2.4. FFA estimation
FFAs were determined using standard method of AOAC (Citation1990). One gram of oil was dissolved in hot 10 ml of neutralized ethanol and titrated against standardized 0.1 N NaOH using phenolphthalein as an indicator. Results were expressed in term of percent oleic acid.
2.2.5. Peroxide value (AOAC, Citation1990)
Two grams of lipid extracted from rice bran was taken in a glass stoppered Erlenmeyer flask. About 30 ml of the acetic acid–chloroform (3:2) solution was added. The flask was swirled to dissolve the contents. One milliliter of saturated potassium iodide solution was added and again swirled for one minute. Thirty milliliters of distilled water was added immediately and shaked vigorously to liberate the iodine from the chloroform layer. Starch solution (1 ml) was added and titrated against 0.1 N sodium thiosulfate until the blue gray color disappeared. Results were expressed as milliequivalent of oxygen/kg of oil.
2.2.6. Statistical analysis
Analysis of variance (ANOVA) was carried out using Microsoft Excel Software. Fisher least significance difference (LSD) test was used to describe significant difference (p ≤ 0.05).
3. Results and discussion
3.1. Chemical composition
The proximate analysis of rice bran is presented in Table . The moisture content of rice bran varied from 9.6 to 14.7% (db). The moisture content of fine rice bran cultivar was significantly (p ≤ 0.05) lower (9.6%) as compared to other two cultivars (14.2–14.7%). The variation in moisture contents of the rice bran might be due to different processes followed by the rice millers and also environmental and genetic factors. The protein content of different cultivars of rice bran did not vary significantly (p ≥ 0.05) and was15.2–15.4% (db). The fat content varied from 21.5 to 28.5% (db). Fine rice bran cultivar showed significantly (p ≤ 0.05) the highest oil content followed by coarse and superfine bran. It was observed that fat and protein were the major components of rice bran. The fiber content of rice bran varied from 8.8 to 11.0% (db) with the highest content in fine rice bran. The ash content of coarse, fine, and superfine cultivars was 10.2, 9.3, and 9.6% db, respectively. Carbohydrate content was significantly (p ≤ 0.05) high in superfine rice cultivar (44.5%) followed by coarse (41.3%) and fine rice cultivar (35.9%). Statistical analysis showed that there was significant (p ≤ 0.05) difference in moisture, fat, fiber, and ash content of three cultivars, however, protein content showed non-significant (p ≥ 0.05) difference.
Table 1. Proximate composition of rice bran of coarse, fine, and superfine cultivars on dry basis (n = 3)
Saunders (Citation1990) and Juliano (Citation1985) reported that rice bran contained 8–12% moisture, 10–16% protein, 15–22% fat, 7–11.4% fiber, 6.6–9.9% ash, and 34.1–52.3% (db) carbohydrates. The moisture and oil content of commercial rice bran was 10.5–11.5% and 16.3–18.7%, respectively (Zullaikah, Lai, Vali, & Ju, Citation2005).Thus, the values obtained in the present study were close to the previously reported values.
3.2. Storage temperature
Temperature acts as an important factor to regulate enzyme activity. Rice bran lipase showed optimum activity at temperature 37°C. The rice bran was stored at −20 to 50°C to gauge the effect of temperature on lipase activity. Results showed a considerable effect of selected temperatures on the production of FFAs in rice bran (Figure ). FFAs content of rice bran did not vary significantly (p ≥ 0.05) at −20°C. FFAs in fine, superfine, and coarse cultivar were 1.8, 4.4, and 8.7% initially which increased to 3.6, 7.7, and 11.9%, respectively, after 40 days of storage. FFA level increased slowly during storage at 5°C and at the end of the storage it reached to 12.7, 32.9, and 29.3%, respectively, in fine, superfine, and coarse cultivars bran. In the rice bran of all cultivars, FFAs significantly (p ≤ 0.05) changed with temperature and storage time. Low-temperature (below 0°C) or refrigeration storage controlled the rice bran lipase activity (Amarasinghe, Kumarasiri, & Gangodavilage, Citation2009).
Figure 1. Effect of storage temperature on FFA % content of bran obtained from (a) course, (b) fine, and (c) superfine rice cultivars.
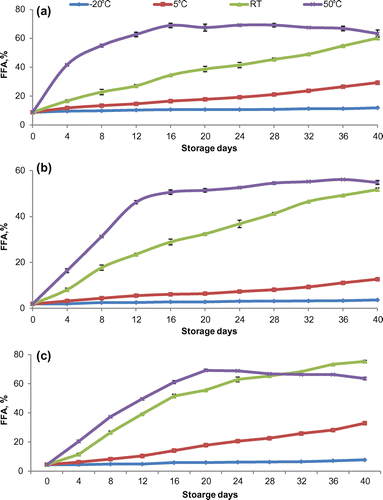
Under ambient condition, there was steady increase in FFA level till the end of study period. FFA content was the highest in superfine (75.3%) followed by coarse (60.1%) and fine (51.7%) after 40 days of storage. The rate of FFAs formation was the highest at 50°C temperature and the values reached to the maximum value of 69% in coarse rice bran after 28 storage days and 56 and 69% in fine and superfine rice bran after 36 and 20 days of storage, respectively.
Zullaikah et al. (Citation2005) studied the lipase activity at 5–70°C and reported that initially the activity of rice bran lipase was slightly higher at 50°C than 30°C and after 10 weeks activity was higher at 30°C. Present findings were similar as the trend indicated that at 30°C the lipase activity was close to that at 50°C at the end of 7 weeks of storage. Prabhu, Tambe, Gandhi, Sawant, and Joshi (Citation1999) determined the lipase activity in crude extract of rice bran at 10–45°C and found that there was non-significantly (p ≥ 0.05) difference in enzyme activity up to 30°C, and thereafter, it decreased. Present study indicated higher activity at 50°C which showed that the enzyme activity decreased immediately in crude extract, whereas in bran it occurred after some time.
3.3. Alkali treatment
Saturated solution of sodium hydroxide (40%) was used to control the lipase activity as it increased the pH value, thus making conditions unfavorable for the lipase enzyme. Results indicated that the control sample had the highest FFA content which leads to a higher enzymatic degradation (Figure ). Enzyme activity decreased with the increase in levels of alkali, but the effect was pronounced at 8 and 10%. Control sample showed increase in FFA values of fine, superfine, and coarse rice bran from 3.8 to 73.1%, 8.4 to 83.6%, and 11.4 to 85%, respectively, whereas alkali-treated samples at 10% revealed an increase from 0.19 to 25.3%, 0.3 to 27.2%, and 0.6 to 36.4%, respectively. The FFAs level decreased in alkali-treated rice bran due to the neutralization of oil. Alkali solution reacts readily with FFAs and form soap and water. Soap formation interferes with the extraction of oil from bran. The FFAs content varied significantly (p ≤ 0.05) in three types of bran with alkali concentration and storage period.
Figure 2. Effect of alkali concentration on FFA % development in bran obtained from (a) coarse, (b) fine, and (c) superfine rice cultivars.
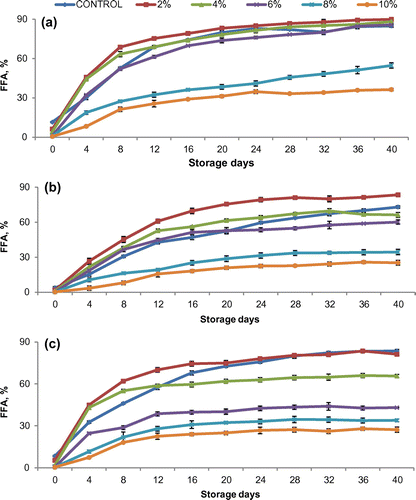
Alkali level in the bran was too high and its efficiency in controlling the lipase activity was insufficient. However, alkali-stabilized bran can be used for protein isolation directly. Similar study could not be traced from the literature, however, alkali has been used to extract protein from rice bran (Chandi & Sogi, Citation2006), neutralize the FFA in rice bran oil (Aryusuk, Puengtham, Lilitchan, Jeyashoke, & Krisnangkura, Citation2008), transesterification of rice bran oil (Akhtar et al., Citation2013), and increase the nickel-selective adsorption ability of rice bran (Zafar, Abbas, Nadeem, Sheikh, & Ghauri, Citation2009).
3.4. Acid treatment
FFA content of oil from untreated rice bran from coarse, fine, and superfine cultivars increased rapidly to 71–76% (Figure ).The lipase activity of rice bran reduced with the addition of hydrochloric acid which is due to decrease in pH. The pH value was 6.9% for control and was 5.8, 5.4, 4.5, and 3.0% when concentration of HCl was 1, 2, 4, and 5%, respectively. The FFA content of rice bran containing 1% HCl was slightly lower than the untreated bran. It indicated that the lipase of rice bran was not affected when HCl concentration was the lowest (1%). In all bran cultivars, FFAs level decreased with increase in acid concentration. Superfine rice bran showed a significant (p ≤ 0.05) decrease in FFAs from 72.33 to 69.02, 55.37, 1.82, and 6.02% when HCl concentration was increased 1, 2, 4, and 5%, respectively, after a storage period of 40 days. Similar trend was observed in fine and coarse cultivars. FFAs level in fine and coarse rice bran increased from 4.18 to 5.11% and 12.13 to 14.63%, respectively, after 40 storage days. Statistical analysis showed that there was significant difference (p ≤ 0.05) in FFA values with change in acid concentration and storage time for all the three cultivars.
Figure 3. Effect of acid concentration on FFA % development in bran obtained from (a) coarse, (b) fine, and (c) superfine rice cultivars.
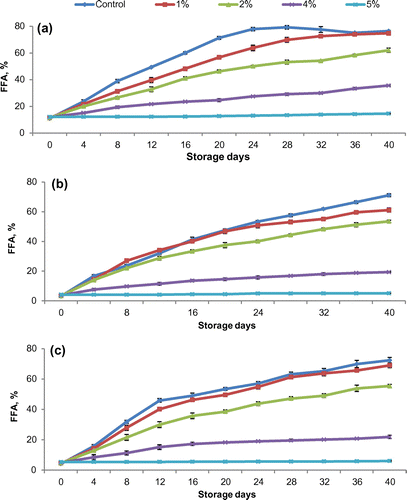
Prabhakar and Venkatesh (Citation1986) reported that lipase activity decreased by lowering the pH below 4.0. Champagne and Hron (Citation1992) revealed that inclusion of hydrochloric acid in ethanol extraction yielded product with very low residual lipase activity in rice kernel and flour.
3.5. Peroxide treatment
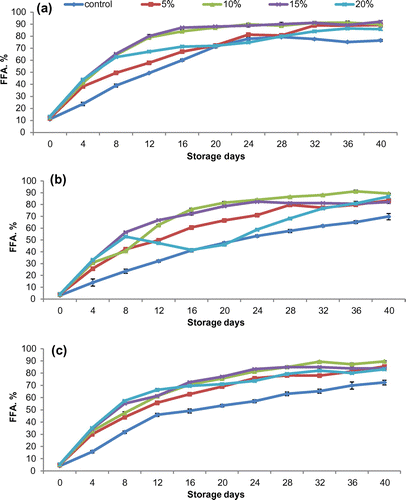
Hydrogen peroxide had negative effect on stability of rice bran oil (Figure ). In contrary, it increased the lipase activity resulting in higher FFAs content in treated samples. The FFAs content of control was the lowest which increased with the addition of hydrogen peroxide at all the levels. FFAs were increased with the addition of 5 and 10% hydrogen peroxide. However, further increase hydrogen peroxide level up to 15 and 20% did not change FFAs considerably. Statistical analysis revealed a significant (p ≤ 0.05) change in FFAs content as the concentration of the peroxide varied and storage period progressed in all the three cultivars. Shastry and Rao (Citation1976) reported that hydrogen peroxide at 1.0 mM or 3.4% concentration completely inhibited the lipase activity. However, present study got opposite results where lipase in bran degraded the oil into FFAs more than untreated sample at all the selected concentration of H2O2.
3.6. Peroxide value
The rice bran samples treated with HCl were analyzed for peroxide value (PV). Results (Table ) revealed that the initial PV ranged from 2.74 to 4.76 meq O2/kg of oil. The PV increased with storage period (40 days) in treated and without treated rice bran. After 40 storage days, PV was decreased with increase HCl concentration in bran of all three rice cultivars. The PV in untreated rice bran was varied from 10.19 to 14.15 meq O2/kg of oil which was higher than all treated rice bran after 40 days of storage. Mujahid, ulHaq, Asif, and Gilani (Citation2005) reported PV in raw rice bran increased to 4.6 and 32.8 meq/kg in 45 and 245 days, respectively, during storage. The PV in literature was lower than that of present study which might be due to the use of commercial samples exposed to heat and air during processing.
Table 2. PV of HCl-treated rice bran after 40 days (n = 3)
The initial PV of fine and superfine rice bran was higher as compared to coarse cultivar but after storage of 40 days, it was lower than coarse cultivar. This might be due to the presence of antioxidants and deviation in processing conditions. The PV was varied significantly (p ≤ 0.05) both with cultivar type and storage period. Statistical analysis indicated significantly (p ≤ 0.05) lower PV in HCl-treated bran as compared to untreated bran on storage for 40 days. It was further observed that the PV decreased as the amount of hydrochloric acid increased thereby indicating an inverse relation between lipase activity and acidity. Study revealed that the acid treatment decreased oxidation of fat in rice bran.
4. Conclusion
Coarse, fine, and superfine rice were milled commercially producing oil-rich bran as major byproduct. It can be utilized into various products but lipase degradation of oil need to be controlled during post-production storage. Physical and chemical techniques were employed to inhibit the lipase activity. Low-temperature storage at −20°C and HCl at 5% level inhibited the activity of the lipase in rice bran. Alkali and peroxide treatments did not hinder FFAs formation in rice bran during storage. Alkali reacts with FFAs, produce soap, and affect the oil extraction from the rice bran. Temperature and acid could be helpful in checking the lipase activity in bran of all the three classes of rice. The PV was higher in untreated sample than HCl-treated samples.
Additional information
Funding
Notes on contributors
Tajendra Pal Singh
Tajendra Pal Singh is a PhD scholar in Department of Food Science and Technology, Guru Nanak Dev University, Amritsar, India and working on the utilization of major rice by-product: Bran.
Research reported in the paper of Inhibition of lipase activity in commercial rice bran of coarse, fine, and superfine cultivars: Low economy level technique used to increase shelf life of rice bran without any negative effect on rice bran. So, we use treated rice bran for further processing such as oil and protein extraction.
Dalbir Singh Sogi
Tajendra Pal Singh is a PhD scholar in Department of Food Science and Technology, Guru Nanak Dev University, Amritsar, India and working on the utilization of major rice by-product: Bran.
Research reported in the paper of Inhibition of lipase activity in commercial rice bran of coarse, fine, and superfine cultivars: Low economy level technique used to increase shelf life of rice bran without any negative effect on rice bran. So, we use treated rice bran for further processing such as oil and protein extraction.
References
- AACC. (1995). Approved methods of the American association of cereal chemists (9th ed.). Minnesota: St Paul.
- Akhtar, F. H., Elsheikh, Y. A., Bassyouni, M., Kaukab, M., Muhammad, A., & Feroze, N. (2013). An alkali catalyzed trans-esterification of rice bran, cottonseed and waste cooking oil. HemijskaIndustrija, 1, 60–61. doi:10.2298/HEMIND130619061A
- Amarasinghe, B. M. W. P. K., Kumarasiri, M. P. M., & Gangodavilage, N. C. (2009). Effect of method of stabilization on aqueous extraction of rice bran oil. Food and Bioproducts Processing, 87, 108–114.10.1016/j.fbp.2008.08.002
- AOAC. (1990). Official methods of analysis of the association of the official analytical chemists. Virginia: Author.
- Aryusuk, K., Puengtham, J., Lilitchan, S., Jeyashoke, N., & Krisnangkura, K. (2008). Effects of crude rice bran oil components on alkali-refining loss. Journal of American Oil Chemist’s Society, 85, 475–479.10.1007/s11746-008-1215-0
- Barnes, P., & Galliard, T. (1991). Rancidity in cereal products. Lipid Technology, 3, 23–28.
- Champagne, E. T., & Hron, R. J. (1992). Stabilizing brown rice to lipolytic hydrolysis by ethanol vapors. Cereal Chemistry, 69, 152–156.
- Chandi, G. K., & Sogi, D. S. (2006). Functional properties of rice bran protein concentrates. Journal of Food Engineering, 79, 592–597.
- Cruz, N., & Khush, G. S. (2000). Rice grain quality evaluation procedures. In R. K. Singh, U. S. Singh, & G. S. Khush (Eds.), Aromatic rices (pp. 15–28). New Delhi: Oxford & IBH.
- Gangadhara, P. R. K., & Prakash, V. (2010). The structure functional catalytic activity of rice bran lipase in the presence of selenium and lithium. European Food Research and Technology, 230, 551–557.10.1007/s00217-009-1195-9
- Juliano, B. O. (1985). Production and utilization of rice. In B. O. Juliano (Ed.), Rice: Chemistry and technology (2nd ed.). (pp. 1–16). Minnesota: American Association Cereal Chemistry.
- Lakkakula, N. R., Lima, M., & Walker, T. (2004). Rice bran stabilization and rice bran oil extraction using ohmic heating. Bioresource Technology, 92, 157–161.10.1016/j.biortech.2003.08.010
- Malekian, F., Rao, R. M., Prinyawiwatkul, W., Marshall, W. E., Windhauser, M., & Ahmedna, M. (2000). Lipase and lipoxygenase activity, functionality and nutrient losses in rice bran during storage (Bull. No. 870, pp. 1–68). Baton Rouge, LA: LSU Agricultural Centre.
- Mujahid, A., ulHaq, I., Asif, M., & Gilani, A. H. (2005). Effect of various processing techniques and different levels of antioxidant on stability of rice bran during storage. Journal of the Science of Food and Agriculture, 85, 847–852.10.1002/(ISSN)1097-0010
- Munshi, S. K., Bhatia, N., Sekhon, B. S., & Sukhija, P. S. (1993). Inactivation of rice bran lipase with metal ions. Journal of Chemical Technology and Biotechnology, 57, 169–174.
- Prabhakar, J. V., & Venkatesh, K. V. L. (1986). A simple chemical method for stabilization of rice bran. Journal of the American Oil Chemists Society, 63, 644–646.10.1007/BF02638229
- Prabhu, V. A., Tambe, P. S., Gandhi, N. N., Sawant, B. S., & Joshi, B. J. (1999). Rice bran lipase: Extraction, activity and stability. Journal of Biotechnology, 15, 1083–1089.
- Prakash, J., & Ramanatham, G. (1995). Effect of stabilisation treatment of rice bran on functional properties of protein concentrates. Journal of the Science of Food and Agriculture, 67, 181–187.10.1002/(ISSN)1097-0010
- Rhee, J. S., & Yoon, N. H. (1984). Stabilization of rice bran by microwave energy. Journal of Food Science and Technology, 16, 113–117.
- Saunders, R. M. (1985). Rice bran: Composition and potential food uses. Food Reviews International, 1, 465–495.10.1080/87559128509540780
- Saunders, R. M. (1990). The properties of rice bran as a foodstuff. Cereal Food World, 35, 632–636.
- Shastry, B. S., & Rao, M. R. R. (1976). Chemical studies on rice bran lipase. Cereal Chemistry, 53, 190–200.
- Wu, M. T. (1990). Extending shelf life of fresh soybean curds by in-package microwave treatments. Journal of Food Science, 42, 1448–1450.
- Zafar, M. N., Abbas, I., Nadeem, R., Sheikh, M. A., & Ghauri, M. A. (2009). Removal of nickel onto alkali treated rice bran. Water, Air & Soil Pollution, 197, 361–370.
- Zullaikah, S., Lai, C. C., Vali, S. R., & Ju, Y. H. (2005). A two-step acid-catalyzed process for the production of biodiesel from rice bran oil. Bioresource Technology, 96, 1889–1896.10.1016/j.biortech.2005.01.028