Abstract
α-amylase was produced by Aspergillus terreus NCFT 4269.10 using both liquid static surface (LSSF) and solid-state fermentation using pearl millet residues as substrate. The maximum production of α-amylase was noticed at 30°C incubated for 96h. The crude α-amylase was purified to electrophoretic homogeneity and characterized. Characterization of amylase confirmed that the purified α-amylase was found to be most stable at pH 5.0, 60°C temperature, and a substrate concentration of 1.25%. The enzyme was active for 40 min at 70°C with an optimum enzyme–substrate reaction time of 60 min. Amylase was compatible with all detergents tested having highest activity with Surf excel followed by Henko and Ariel. SDS and Tween 20 reduced the activity. Among the metal ions tested, the maximum α-amylase activity was attained in the presence of Ca2+, followed by Mg2+ and Mn2+. The activity of α-amylase was not considerably affected in the presence of ethylenediaminetetraacetic acid and Triton X-100. Amylase activity was accelerated in the presence of sodium lauryl sulfate and phenylmethylsulfonyl fluoride did not significantly (or slightly) affect the activity and stability. Tween 20, urea (5%), and the reducing agent, β-mercaptoethanol significantly inhibited the activity of α-amylase. Owing to its noteworthy stability in the presence of detergents, additives, inhibitors, and metal ions, this α-amylase could be an impending enzyme for significant industrial exploitations.
Public Interest Statement
The present research will develop an interest to society/public in a way that agroindustrial wastes generating from domestic, agriculture, milling industries, etc. can also be effectively managed for the production of value-added products at low cost, as well as facilitating many industrial processes that are intended for the well-being of the society. These agroindustrial residues can be employed in many large-scale industrial fermentation processes as an effective renewable bioenergy source, further, it will serve as the suitable substrate for growth of micro-organisms and production of low-cost high-valued products.
Competing Interests
The authors declare no competing interest.
1. Introduction
The α-amylases (endo-1, 4-α-D-Glucan glucohydrolase; E.C. 3.2.1.1) catalyze the (α-1 → 4) O-linked glycosidic bond present in soluble starch (i.e. amylose) and hydrolyze in a random fashion to produce reducing sugars in the α-configuration (Kharkrang & Ambasht, Citation2012). Usually, upon the hydrolysis of starch by this enzyme, dextrin, oligosaccharides, maltose, and D-glucose are generated as the final products. There are mainly three kinds of amylases (α-amylases, β-amylases, and glucoamylase) reported till now basing on their hydrolysis of glycoside bond. The α-amylases are produced extracellularly that arbitrarily act on the 1,4-α-D-glycosidic linkages present between the neighboring glucose units in the linear amylose chain and are further categorized owing to their mode of action and characteristics. Sequence similarity analysis revealed that most the α-amylases are placed under the glycoside hydrolase (GH) family 13; whereas, few α-amylases of extremophiles origin belong to family GH57 (Henrissat, Citation1991; Henrissat & Bairoch, Citation1996) (refer CAZy website, http://www.cazy.org). The GH family has recently been further categorized into 35 subfamilies depending on a phylogenetic analysis of 1,691 different members of the GH13 family in which all of them are hydrolyzing the glycosidic bonds (Stam, Danchin, Rancurel, Coutinho, & Henrissat, Citation2006). Many of these subfamilies have α-amylase specificity along with other enzyme–substrate reaction specificities. The tertiary structure of these amylase reveals a (β/α)8 barrel having four highly conserved amino acid residues that are the parts of active site (MacGregor, Janeček, & Svensson, Citation2001). To reiterate, in 1894, the first industrial enzyme to be commercialized was an amylase of fungal origin which was employed as a pharmaceutical abet for alleviating the digestive disorders (Pandey, Nigam, Soccol, et al., Citation2000). Partial purification and characterization of this enzyme has also been documented in the literatures (Mohamed, Al-Malki, & Kumosani, Citation2009). Extensive studies on α-amylase has mostly been carried out using microbes (Sidkey, Abo-Shadi, Balahmar, Sabry, & Badrany, Citation2011), though, it has also been reported from few plants like finger millet (Nirmala & Muralikrishna, Citation2003), mung bean (Tripathi, Lo Leggio, Mansfeld, Ulbrich-Hofmann, & Kayastha, Citation2007), Korean pine seed (Azad et al., Citation2009), and soybean (Kumari, Singh, Fitter, Polen, & Kayastha, Citation2010).
However, enzymes of microbial origin have immensely exploited in many industries owing to their low production cost, higher yield, chemical and thermal stability, eco-friendly nature, flexibility, and gigantic accessibility (Mishra & Behera, Citation2008). Nonetheless, amylases are among the most imperative group of enzymes having enormous importance in biotechnology for such novel properties. Amylases only constitute approximately 25–30% of the total world enzyme trading (Zaferanloo, Bhattacharjee, Ghorbani, Mahon, & Palombo, Citation2014). In contemporary, amylases have occupied a pivotal place in many biotechnological applications that includes textile, paper, detergents, fermentation, brewing, distilling industry, pharmaceuticals, food, animal nutrition, bioconversion of solid waste, etc. With the advancement in biotechnology research, the employment of amylase has enhanced in the field of starch analytical chemistry, medical chemistry, and clinical research. Due to such huge demand of amylase, an enhanced amylase production, the search for more efficient fungus and processes are obligatory (Wolfgang, Citation2007).
Many amylases are reported to be metalloenzymes and displayed enhanced activity in the presence of divalent ions like Ca2+, Mg2+, Mn2+, and Zn2+ (Polaina & MacCabe, Citation2007). Hence, for characterization and stability study, purification of α-amylase from the crude enzyme is essential (Vengadaramana, Balakumar, & Vasanthy, Citation2013) as reduced enzyme stability results in low yield of the end product (Riaz, Perveen, Javed, Nadeem, & Rashid, Citation2007). To achieve an effective enzyme production, activity and kinetic properties, many filamentous fungi have been isolated, identified, and studied as the source of novel enzymes with valuable novel properties (Bakri, Magali, & Thonart, Citation2009). Further, among microbial production of amylases, fungal amylases are superior over the bacterial amylases mainly because of their easy cultivation, low nutritional need, facilitated product recovery, higher yield, and stability (Prakash, Vidyasagar, Madhukumar, Muralikrishna, & Sreeramulu, Citation2009).
Keeping in view the noteworthy applications, the α-amylase was produced from a fungal isolate Aspergillus terreus cultivated on pearl millet (PM) as substrates under both liquid static surface fermentation (LSSF) and solid-state fermentation (SSF) conditions. Further, the crude enzyme was purified and characterized. This novel approach may facilitate in developing an eco-friendly, industrially suitable protocol for ease extraction, purification, and characterization of low-cost high-valued biotechnologically relevant amylase for numerous industrial applications.
2. Materials and methods
2.1. Substrate and chemicals
For the fermentation and production of amylase, PM was purchased from the local market of Bhubaneswar, Odisha, India. The substrate was collected in a new sterilized steel container (5 kg capacity) and transported to the laboratory. The sample was dried at 60°C for 48 h inside the oven, then powdered and kept in sterile container until required. Analytical reagent (AR)-grade chemicals, like, soluble starch, β-mercaptoethanol, phenylmethylsulfonyl fluoride (PMSF), ethylenediaminetetraacetic acid, sodium lauryl sulfate (SLS), Triton X-100, Tween 20, urea, etc. were purchased from HiMedia Limited, SRL Pvt. Limited, and Merck India Limited, Mumbai, India for this study.
2.2. Micro-organism and inoculum
For the present investigation, an amylolytic fungus, A. terreus NCFT 4269.10 (GenBank accession Number: KT222271) was used. The fresh culture of A. terreus was mixed in 5.0 ml of sterile deionized water and 1.0 ml of the suspension was used as the inoculum for pre-fermentation culture. The culture broth was incubated at 30 ± 1°C for a week to develop a fungal density of about 5.0 × 108 spores ml−1. The spore suspension was used as the inoculum for all the subsequent experiments to give an initial cell density of 1 × 107 cells ml−1 (Sethi, Rout, Das, Nanda, & Sahoo, Citation2013).
2.3. Fermentation for amylase production
In this investigation, 10 g of PM powder as substrate was boiled with 100 ml double-distilled water for at least 15 min. Boiled substrate was filtered after cooling; filtrate was transferred to 150-ml Erlenmeyer flasks and autoclaved at 121°C for 15 min to carry out LSSF. Sterilized and cooled fermentation broth medium was inoculated with 1 × 107 cells ml−1 from 7 days old culture and incubated at 30 ± 1°C static conditions. After 96 h of incubation, the fermented sample was analyzed for amylase activity. Ten ml of culture was centrifuged at 10,000× g, at 4°C, for 10 min to obtain a cell-free supernatant. The clear supernatant thus obtained was filtered through Whatman No. 1 filter paper before amylase activity assay.
For conducting the SSF, the powered PM was thrice washed with double-distilled water and then oven dried at 80°C for 48 h. Erlenmeyer flasks (250 ml) having 5 g (w/w) of the PM as substrate was mixed properly with 8 ml of minimal salt solution (MSS) solution to adjust the moisture content up to 80% and autoclaved under standard conditions for 15 min (at 121°C and 15 psi pressure). This sterilized fermentation medium was inoculated aseptically with optimum number of spore inoculums and incubated for 4 days at 30 ± 1°C with intermittent observation.
2.3.1. Enzyme recovery
The enzyme was extracted from the fermented media (SSF) after fermentation using the method described by Alva et al. (Citation2007). Solid substrate fermented matter was mixed with 50 ml cold 0.1 M phosphate buffer (pH 6.5) and the mixtures were mixed properly for 30 min at 30°C manually. The mixture was filtered out using cheese cloth and subjected to centrifugation (Remi Compufuge) at 8,000× g at 4°C for 15 min to separate the spores and other insolubles.
The clear supernatant achieved after this centrifugation was filtered thrice through Whatman No. 1 filter paper. The filtrate was thereafter used as crude amylase for enzyme assay. The enzyme obtained from SSF was kept in the deep freezer at −20°C for long-term preservation and to protect the enzyme from all sorts of damage. The preserved enzyme was used for further experiments. Likewise, the fermented submerged broth was centrifuged and passed through the filter paper to remove all cell debris and insolubles to get the clear fluid and finally used as the crude enzyme.
2.4. Purification of amylase
The crude culture filtrate (~500 ml) was precipitated by gradual addition of 40–80% ammonium sulfate [(NH4)2SO4] with constant stirring by a magnetic stirrer at 4°C up to 24 h. Each precipitate was separated from the supernatant by centrifugation at 10,000× g for 15 min at 4°C. After centrifugation, the supernatant was decanted and the solid precipitate was dissolved in phosphate buffer (pH 6.5) at a ratio of 0.1 g ml−1 so as to obtain 10 times more concentrated enzyme solution. Thereafter, ammonium sulfate precipitated enzyme solution was dialyzed for 24 h at 4°C with incessant stirring against a large volume (1 L capacity) of phosphate buffer (pH 6.5) for the complete removal of lower molecular weight metabolites and ammonium sulfate from the dialysate (Jana et al., Citation2013). To improve solute exchange, the dialysis buffer was replaced after every 2 h of incubation so as to ascertain a new concentration gradient. Then, the dialyzed enzyme was loaded into the Sephadex G-100 column (2.5 × 70 cm) and eluted with 50 mM phosphate buffer (pH 6.5) with the flow rate of 1 ml min−1. Fractions of 2 ml each were subsequently collected for estimation of protein (Lowry, Rosebrough, Farr, & Randall, Citation1951). The fractions showing maximum absorption at 750 nm were collected and evaluated for its enzyme activity. The enzyme positive fractions with higher enzyme activity were combined together, lyophilized and stored at −20°C for further characterization.
2.5. Assay methods
2.5.1. Amylase assay
Amylase activity was measured at 540 nm as per Bernfeld (Citation1955) method. One unit of enzyme activity was defined as the amount of enzyme that releases 1 μmol of maltose at 1 min under the assay conditions.
2.6. Enzyme characterization
2.6.1. Effect of pH on enzyme activity
Rate of hydrolysis of starch solution with purified enzyme was evaluated at various pH values to perceive the effect of pH on amylase activity. In case of amylase, 0.5 ml of starch solution was taken as substrate and reaction volume was adjusted with 0.5 ml buffer of different pH (3.0–10.0) in order to acquire different pH conditions and activity was determined by amylolytic assay (Bernfeld, Citation1955).
2.6.2. Effect of temperature on enzyme activity
Effect of temperature was evaluated by incubating 100 μl of amylase with 0.5 ml of starch solution and 0.4 ml phosphate buffer, pH 7.0 (50 mM) at temperature ranging from 30 to 90°C for 15 min and activities of amylase at different temperature were measured.
2.6.3. Determination of thermostability of enzyme
Thermal stability of the amylase was determined by incubating the enzyme for 120 min at various temperatures ranging from 30 to 80°C. Enzyme activity was determined at regular time intervals of 30 min.
2.6.4. Effect of substrate concentration
The purified amylase was incubated with its substrate at different concentrations (0.25–2.0%) under the optimum conditions of the enzyme. Then the amylolytic activity was determined.
2.6.5. Effect of enzyme- substrate reaction time
The purified amylase and its substrate were incubated at time intervals of 10, 20, 30, 40, 50, 60, 70, 80, and 90 min at the optimum conditions, and then the amylolytic activity was measured.
2.6.6. Effect of commercial detergents on amylase stability
Different detergents [sodium dodecyl sulfate (SDS), Tween 20 (0.05%), Ariel, Henko, Surf excel, and Rin (0.1% w/v) were purchased from the local dealer, Bhubaneswar, Odisha, India] were added to the enzyme solution at a concentration of 1 mg/ml and incubated for 30 min at 30°C to investigate the stability of amylase in detergents. Samples were taken after 30 min and assayed for enzyme activity.
2.6.7. Effects of metal ions on enzyme behavior
To study the influence of various metal ions like K+, Na+, Ca2+, Mn2+, Hg2+, Mg2+, Fe2+, Cu2+, Ag+, Zn2+, and Co2+ on the enzyme activity, sample of 100 μl amylase containing 1 mM metal ion was incubated at 50°C for 60 min. Thereafter, each sample was drawn for amylase assay.
2.6.8. Effects of additives on enzyme activity
Enzyme was incubated in different surfactants [Triton X-100, Tween-20, and sodium lauryl sulfate (SLS)] solutions of 0.03% (w/v) in appropriate buffer for 30 min to evaluate the influence of various additives such as surfactants, reducing agents on the enzyme. Inhibitors such as (EDTA), PMSF were used to characterize the active sites of enzymes in order to classify them. For this, purified amylase was incubated in 1 mM solution of the inhibitors (PMSF, EDTA) for 1 h and their effect on the activity of amylase was assayed. Influence of strong reducing agents like β-mercaptoethanol (β-merc) on amylase was studied by incubating the enzyme with corresponding substrates in the presence of β-mercaptoethenol [0.5% (v/v)].
2.7. Statistical analysis
All experiments were carried out in triplicates (n = 3) and repeated three times. The samples collected from each replicate were analyzed for characterization of the enzyme. Each value is an average of three parallel replicates. The ± and error bars indicate standard deviation among the replicates.
3. Results and discussion
Enzyme characterization and specificity studies have always been indispensable for exploitation of enzymes and the endurance of respective fungi in their environmental niche can also be precious to industry. For instance, the manufacturing of pulp, paper, and textiles demand the enzymes with pH and thermostability. Dairy, beverage, food manufacturing processes, and detergent formulations generally require enzymes with optimum activity at low temperature and pH. Another fascinating but seldom studied topic is the exploration of different biochemical properties existing in enzymes produced under two kinds of fermentations (SmF and SSF).
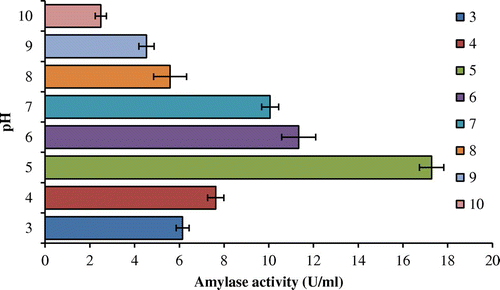
To appraise the effect of different pH on amylase activity, rate of digestion of starch with purified enzyme was evaluated at various pH values. Enzyme characterization study revealed that purified amylase was found stable in a broad range of pH (3–9) with maximum amylase stability and activity in pH 5.0 (Figure ). α-Amylases from fungi were known to have pH optima in the acidic to neutral range (Gupta, Gigras, Mohapatra, Goswami, & Chauhan, Citation2003) which is akin with the present findings.
The optimal pH for A. terreus amylase activity was 5.0, which is similar to the α-amylase from M. cinnamomea S168 (Han et al., Citation2013). Generally, most of the fungal α-amylases are stable in a narrow pH range, but the range was pH 3.0–10.0 for A. terreus. Whereas glucoamylase was produced by two lineages of A. terreus, that executed pH optima of 4.0 and 5.0 (Ghosh, Saxena, Gupta, Yadav, & Davidson, Citation1996), respectively. The broad range of pH i.e. acidic to alkaline can make the amylase of A. terreus to be utilized by confectionary and food industries or textile and detergent industries, respectively, since 34% of the applications of microbial amylase are in detergents and the second most important application (14%) is in dairy (Zaferanloo, Virkar, Mahon, & Palombo, Citation2012), A. terreus NCFT4269.10 could be a new contender for the upcoming era.
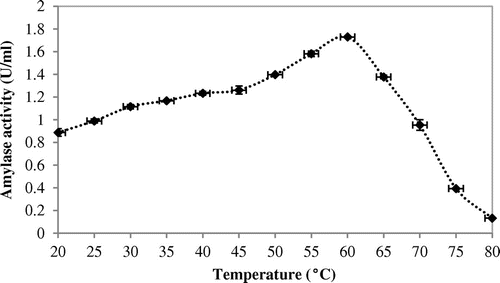
Effect of temperature and its characterization study revealed that when purified amylase was exposed to a broad range of temperature i.e. 20–80°C for 30 min, it remained active from 30 to 70°C temperature optima at 60°C (Figure ).
Thermal stability was determined by incubating the enzyme for 120 min at 30–80°C. Thermostability study revealed that the threshold temperature of the enzyme was up to 70°C for 40 min. Half of the activity was degraded beyond 80 min at 70°C (Figure ). Working temperature optima (60°C) and good thermostability (70°C) of amylase from A. terreus were both substantially superior to the enzyme of Aspergillus sp AS-2 (50°C) (Soni, Kaur, & Gupta, Citation2003). This result reinforces the capability of this enzyme for applications in those processes that demand elevated temperature regimen (Figures and ).
Figure 3. Thermostability of the amylase produced by A. terreus (amy30: amylase activity at 30°C; amy40: amylase activity at 40°C; amy50: amylase activity at 50°C; amy60: amylase activity at 60°C; amy70: amylase activity at 70°C; amy80: amylase activity at 80°C).
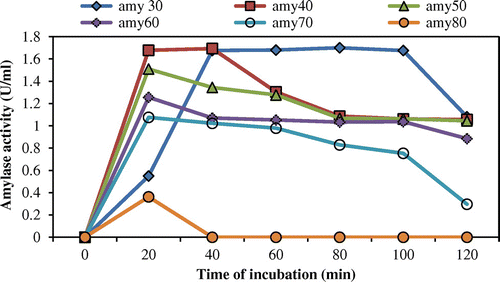
The optimal temperature (60°C) of A. terreus amylase is significantly higher than that of most fungal α-amylases (Li et al., Citation2011; Michelin et al., Citation2010). Moreover, amylase from A. terreus showed excellent thermostability, whereas most fungal α-amylases were stable up to 50°C (Hostinová, Janeček, & Gašperík, Citation2010). Owing to its low pH and high thermal stability, a new aspect of use of α-amylase is in detergent formulations at laundry industries can be feasible.
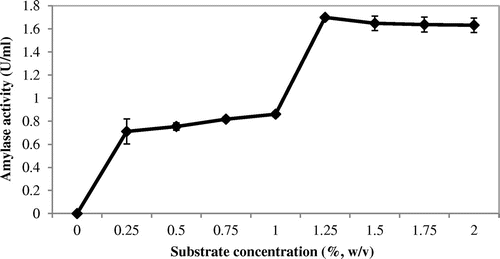
The purified amylase was incubated with its substrate at different concentrations (0.25–2.0%). Maximum amylolytic activity was obtained at 1.25% of starch concentration (Figure ).
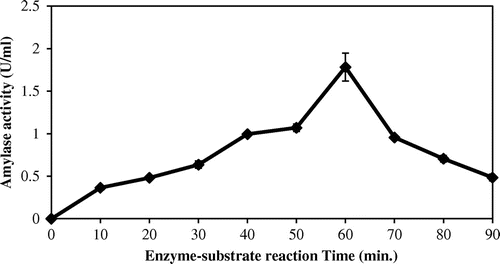
The effect of enzyme–substrate reaction time on purified enzymes was evaluated at time interval of 10, 20, 30, 40, 50, 60, 70, 80, and 90 min at the optimum conditions. Maximum amylase activity was recorded at 60 min (Figure ).
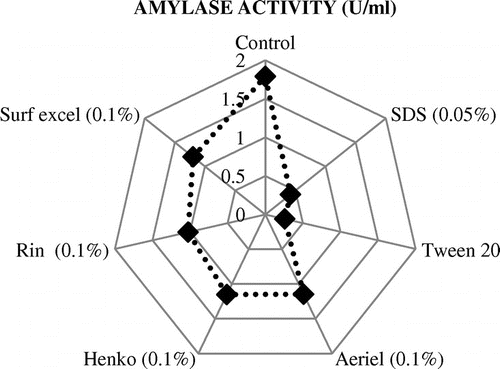
Different commercial detergents were added to the purified enzyme solution at a concentration of 1 mg/ml to analyze the stability of amylase in detergents. Results showed that the enzyme was compatible with all detergents having highest activity with Surf excel, followed by Henko and Ariel. SDS and Tween 20 reduced the activity as shown in the figure (Figure ). In this study, the objective of using Tween 20 was to compare the activity of amylase with the activity produced by commercial detergents.
Amylase in detergents removes starchy stains/residues from clothes. Permanence of an enzyme with metal salts implies a pivotal function in industrial applications. To investigate the stability of amylase in detergents, different detergents were tried and amylase from A. terreus NCFT4269.10 executed excellent stability and compatibility in the presence of commercial detergents like Surf Excel, Henko, and Ariel (Figure ). Beside pH and temperature stability, amylase activity was reasonable in the presence of commercial detergents.
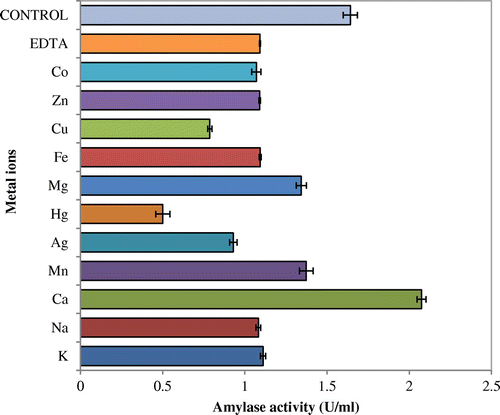
Influences of various metal ions on the enzyme activity were studied by incubating purified amylase containing 1 mM metal ion at 50°C for 60 min. This study confirmed that enzyme was most stable in Ca2+ followed by Mn2+where its activity was almost inhibited by Hg2+ and Cu2+ (Figure ).
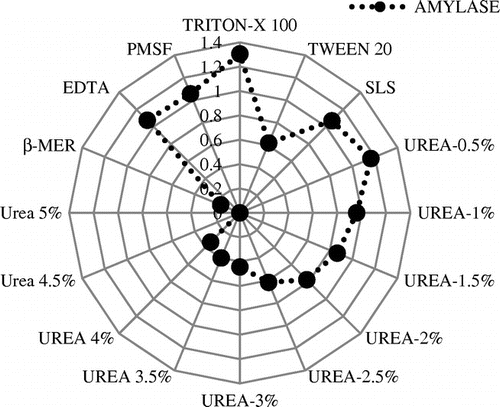
Similarly, the influence of various additives such as surfactants, reducing agents, and denaturant on the amylase activity was evaluated. It was concluded that the purified enzyme was stable with all the three surfactants giving maximum activity with Triton-X 100 and lowest activity with Tween 20 (Figure ). In this part of study, the effect of Tween 20 was compared with the other surfactants and denaturants.
Highest amylase activity was obtained at 0.5% concentration of urea and reduced activity was obtained as the concentration increased thereafter. β-mercaptoethenol reduced the activity to a greater extent. No significant effect of EDTA and PMSF were observed on amylase activity and stability. Many fungal amylases narrated in the literatures are activated by metal ions and the amylase from A. terreus showed a slightly enhanced activity in the presence of low concentrations of most of the metallic ions tested, with the exception of Ag2+, Hg+, and Cu2+, which inhibited the activity (Figure ). da Silva et al. (Citation2009) reported that amylase of A. niveus was inhibited by Ag+. Most α-amylases are reported to be metalloenzymes that demand divalent ions such as Ca2+, Mn2+, Zn2+, and Fe2+ for its enhanced activity which corroborates with this finding. Traditionally, Ca2+ has been considered obligatory for maintenance of the structure of the active site of α-amylases, and thus in maintaining the activity and stability of α-amylases (Bai et al., Citation2012). In case of amylase obtained from A. terreus, it was most stable with Ca2+, whereas Han et al. (Citation2013) reported that McAmyA was strongly activated by Mn2+ and Co2+. In this study, Cu2+, Co2+, Mn2+, Ca2+, Mg2+ Na+, and K+ did not hamper the amylolytic activity, and in the presence of Ca2+ and Mn2+ the activity was enhanced. Hg2+ was having inhibitory action on it. This might be due to the non-specific binding and aggregation of the enzyme. Similar findings were reported by Sumantha, Sandhya, Szakacs, Soccol, and Pandey (Citation2005) while working with fungal amylase. Amylase activity was accelerated in the presence of SLS and Triton X-100, while greatly inhibited by Tween 20. This could possibly be attributed to the effect of surfactants on the folding of the substrate moiety (Vita & Dalzoppo, Citation1985), as well as positively influencing the hydrophobic interactions that plays an imperative role in stabilizing the tertiary structure and unswervingly intermingling with the protein molecule (Creighton, Citation1989). Urea has a significant role in unfolding and refolding of proteins. As seen in Figure , amylase activity increased in the presence of 0.5% urea. But beyond this, a gradual diminished activity was observed with complete inhibition at 4.5%. The rationale for this finding could be established as at lower concentration of urea, it unfolds the tertiary structure to such a degree that the hidden active sites present inside the enzyme get exposed to substrate, thus, enhancing the activity. At higher concentrations, urea denatures the entire enzyme structure and function. Similar types of results were also reported by Negi and Banerjee (Citation2009). The thiol activator, β-mercaptoethanol (0.5%, v/v) greatly inhibited the activity. Negi and Banerjee (Citation2009) reported that lower concentration of β-mercaptoethanol facilitates the exposure of hidden active sites and restabilizes disulfied bonds, thereby, increases the activity of enzymes, while at higher concentration enzymes get denatured. The effect of group-specific reagents and inhibitors has been evaluated in order to explain the presence of amino acid at or near the active site of enzymes. In the present work, amylase was inhibited at higher concentrations of urea and stayed unaffected or slightly inhibited by PMSF and EDTA, hence, predicted to be an amylase. The present results correlate with the findings of Negi and Banerjee (Citation2009).
4. Conclusions
In the present study, A. terreus was found to be a potent amylolytic fungus. Further, the amylase produced by this fungus was found to be highly stable at high temperature (60°C), acidic pH (5.0) with an optimum enzyme–substrate reaction time of 60 min. The purified amylase displayed high thermostability and remarkable stability in the presence of commercial detergents (Surf excel, Henko, and Ariel), additives like, ethylenediaminetetraacetic acid, SLS and Triton X-100, Tween 20, urea, and metal ions. These unique properties of amylase make it suitable for numerous biotechnological applications.
Acknowledgments
This work was financially supported by UGC-RGNF Scheme, New Delhi, India under grant number F.14-2(SC)/2008 (SA-III) dated 31 March 2009. We also thank Dr Pradeep Kumar Das Mohapatra and Prof Keshab C. Mondal, Department of Microbiology, Vidyasagar University, Midnapore, West Bengal, for providing necessary laboratory facilities to BKS.
Additional information
Funding
Notes on contributors
Bijay Kumar Sethi
Bijay Kumar Sethi, Prativa Kumari Nanda, Santilata Sahoo and Sangeeta Sena have been working on the production of extracellular fungal enzymes through solid state and liquid static surface fermentation using agrowastes and their suitable applications in biotechnological industries since last seven years. Numerous agroresidues from agricultural activities are generated annually and dumped in the environment. Agroindustrial wastes provide an alternative source of carbon as substrates and may help in solving some pollution problems, which otherwise might be caused by their dumping in developing country like India. These agrowastes have gained significant interest as an abundant renewable bioenergy resource and economic substrates for fermentation processes in the production of enzymes, food, fuel, and chemicals. In the study, amylase was produced from pearl millet residues. The crude enzyme was purified and characterized for suitable biotechnological applications. Further, effective utilization of agrowastes, decrease in the cost of enzyme production, energy conservation, and recycling is stated.
References
- Alva, S., Anupama, J., Salva, J., Chiu, Y. Y., Vyashali, P., Shruti, M., …, Varalakshmi, K. N. (2007). Production and characterization of fungal amylase enzyme isolated from Aspergillus sp. JGI 12 in solid state culture. African Journal of Biotechnology, 6, 576–581.
- Azad, M. A. K., Bae, J.-.H., Kim, J.-S., Lim, J.-K., Song, K.-S. Shin, B.-S., & Kim, H.-R. (2009). Isolation and characterization of a novel thermostable α-amylase from Korean pine seeds. New Biotechnology, 26, 143–149. doi:10.1016/j.nbt.2009.09.006
- Bai, Y., Huang, H., Meng, K., Shi, P., Yang, P., & Luo, H., … Yao, B.. (2012). Identification of an acidic α-amylase from Alicyclobacillus sp. A4 and assessment of its application in the starch industry. Food Chemistry, 131, 1473–1478. doi:10.1016/j.foodchem.2011.10.036
- Bakri, Y., Magali, M., & Thonart, P. (2009). Isolation and identification of a new fungal strain for amylase biosynthesis. Polish Journal of Microbiology, 58, 269–73.
- Bernfeld, P. (1955). Amylase α and β. In S. P. Colowick & N. O. Kaplan (Eds.), Methods in enzymology. New York, NY: Academic Press.
- Creighton, T. E. (1989). Protein functions: A practical approach. Oxford: IRL Press.
- da Silva, T. M., Maller, A., de Lima Damásio, A. R. L., Michelin, M., Ward, R. J., Hirata, I. Y., …, de Polizeli, M. L. T. M. (2009). Properties of a purified thermostable glucoamylase from Aspergillus niveus. Journal of Industrial Microbiology and Biotechnology, 36, 1439–1446. doi:10.1007/s10295-009-0630-z.10.1007/s10295-009-0630-z
- Ghosh, P. K., Saxena, R. K., Gupta, R., Yadav, R. P., & Davidson, S. (1996). Microbial lipases: Production and applications. Science Progress, 79, 119–157.
- Gupta, R., Gigras, P., Mohapatra, H., Goswami, V. K., & Chauhan, B. (2003). Microbial α-amylases: A biotechnological perspective. Process Biochemistry, 38, 1599–1616. doi:10.1016/S0032-9592(03)00053-010.1016/S0032-9592(03)00053-0
- Han, P., Zhou, P., Hu, S., Yang, S., Yan, Q., & Jiang, Z. (2013). A Novel multifunctional α-amylase from the thermophilic fungus Malbranchea cinnamomea: Biochemical characterization and three-dimensional structure. Applied Biochemistry and Biotechnology, 170, 420–435. doi:10.1007/s12010-013-0198-y
- Henrissat, B. (1991). A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochemical Journal, 280, 309–316.10.1042/bj2800309
- Henrissat, B., & Bairoch, A. (1996). Updating the sequence-based classification of glycosyl hydrolases. Biochemical Journal, 316, 695–696.10.1042/bj3160695
- Hostinová, E., Janeček, Š., & Gašperík, J. (2010). Gene sequence, bioinformatics and enzymatic characterization of α-amylase from Saccharomycopsis fibuligera KZ. The Protein Journal, 29, 355–364. doi:10.1007/s10930-010-9260-6
- Jana, A., Maity, C., Halder, S. K., Das, A., Pati, B. R., Mondal, K .C., & Das Mohapatra, P. K. (2013). Structural characterization of thermostable, solvent tolerant, cytosafe tannase from Bacillus subtilis PAB2. Biochemical Engineering Journal, 77, 161–170. doi:10.1016/j.bej.2013.06.00210.1016/j.bej.2013.06.002
- Kharkrang, K., & Ambasht, P. K. (2012). Purification and characterization of alpha amylase from seeds of pearl millet (Pennisetum typhoides). Journal of Proteins and Proteomics, 3, 47–60.
- Kumari, A., Singh, V. K., Fitter, J., Polen T., & Kayastha, A. M. (2010). α-Amylase from germinating soybean (Glycine max) seeds - Purification, characterization and sequential similarity of conserved and catalytic amino acid residues. Phytochemistry, 71, 1657–1666. doi:10.1016/j.phytochem.2010.06.012
- Li, S., Zuo, Z., Niu, D., Singh, S., Permaul, K., & Prior, B. A., … Wang, Z.. (2011). Gene cloning, heterologous expression, and characterization of a high maltose-producing α-amylase of Rhizopus oryzae. Applied Biochemistry and Biotechnology, 164, 581–592. doi:10.1007/s12010-011-9159-510.1007/s12010-011-9159-5
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurements with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
- MacGregor, E. A., Janeček, Š., & Svensson, B. (2001). Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochimica et Biophysica Acta (BBA)—Protein Structure and Molecular Enzymology, 1546, 1–20.10.1016/S0167-4838(00)00302-2
- Michelin, M., Silva, T. M., Benassi, V. M., Peixoto-Nogueira, S. C., Moraes, L. A. B., Leão, J. M. … Polizeli Mde, P.. (2010). Purification and characterization of a thermostable α-amylase produced by the fungus Paecilomyces variotii. Carbohydrate Research, 345, 2348–2353. doi:10.1016/j.carres.2010.08.01310.1016/j.carres.2010.08.013
- Mishra, S., & Behera, N. (2008). Amylase activity of starch degrading bacteria is isolated from soil receiving kitchen wastes. African Journal of Biotechnology, 7, 3326–3331.
- Mohamed, S. A., Al-Malki, A. L., & Kumosani, T. A. (2009). Partial purification and characterization of five α-amylases from wheat local variety (Balady) during germination. Australian Journal of Basic and Applied Science, 3, 1740–1748.
- Negi, S., & Banerjee, R. (2009). Optimization of extraction and purification of glucoamylase produced by Aspergillus awamori in solid-state fermentation. Biotechnology and Bioprocess Engineering, 14, 60–66. doi:10.1007/s12257-008-0107-310.1007/s12257-008-0107-3
- Nirmala, M., & Muralikrishna, G. (2003). Three α-amylases from malted finger millet (Ragi, Eleusine coracana, Indaf-15)—Purification and partial characterization. Phytochemistry, 62, 21–30. doi:10.1016/S0031-9422(02)00443-010.1016/S0031-9422(02)00443-0
- Pandey, A., Nigam, P., Soccol, C. R., Soccol, V. T., Singh, D., & Mohan, R. (2000). Advances in microbial amylases. Biotechnology and Applied Biochemistry, 31, 135–152.10.1042/BA19990073
- Polaina, J., & MacCabe, A. P. (2007). Industrial enzymes. Dortrecht: Springer.10.1007/1-4020-5377-0
- Prakash, B., Vidyasagar, M., Madhukumar, M. S., Muralikrishna, G., & Sreeramulu, K. (2009). Production, purification, and characterization of two extremely halotolerant, thermostable, and alkali-stable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochemistry, 44, 210–215. doi:10.1016/j.procbio.2008.10.013
- Riaz, M., Perveen, R., Javed, M. R., Nadeem, H., & Rashid, M. H. (2007). Kinetic and thermodynamic properties of novel glucoamylase from Humicola sp. Enzyme and Microbial Technology, 41, 558–564. doi:10.1016/j.enzmictec.2007.05.010
- Sethi, B. K., Rout, J. R., Das, R., Nanda, P. K., & Sahoo S. L. (2013). Lipase production by Aspergillus terreus using mustard seed oil cake as a carbon source. Annals of Microbiology, 63, 241–252. doi:10.1007/s13213-012-0467-y
- Sidkey, N. M., Abo-Shadi, M. A., Balahmar, R., Sabry, R., & Badrany, G. (2011). Purification and characterization of α-amylase from a newly isolated Aspergillus flavus F2Mbb. International Research Journal of Microbiology, 2, 96–103.
- Soni, S. K., Kaur, A., & Gupta, J. K. (2003). A solid state fermentation based bacterial α-amylase and fungal glucoamylase system and its suitability for the hydrolysis of wheat starch. Process Biochemistry, 39, 185–192. doi:10.1016/S0032-9592(03)00058-X10.1016/S0032-9592(03)00058-X
- Stam, M. R., Danchin, E. G., Rancurel, C., Coutinho, P. M., & Henrissat, B. (2006). Dividing the large glycoside hydrolase family 13 into subfamilies: Towards improved functional annotations of -amylase-related proteins. Protein Engineering Design and Selection, 19, 555–562.10.1093/protein/gzl044
- Sumantha, A., Sandhya, C., Szakacs, G., Soccol, C. R., & Pandey, A. (2005). Production and partial purification of a neutral metalloprotease by fungal mixed substrate fermentation. Food Technology and Biotechnology, 43, 313–319.
- Tripathi, P., Lo Leggio, L. L., Mansfeld, J., Ulbrich-Hofmann, R. U., & Kayastha, A. M. (2007). α-Amylase from mung beans (Vigna radiata)—Correlation of biochemical properties and tertiary structure by homology modelling. Phytochemistry, 68, 1623–1631. doi:10.1016/j.phytochem.2007.04.006
- Vengadaramana, A., Balakumar, S., & Vasanthy, A. (2013). Supplementation of carbohydrate to enhance the α-amylase production by Bacillus licheniformis ATCC 6346 in presence of seed cakes. Malaysian Journal of Microbiology, 8, 242–247.
- Vita, C. D. D., & Dalzoppo, D.e (1985). Limited proteolysis of thermolysin by subtilisin, isolation and characterization of a partially active enzyme derivative. Biochemistry, 24, 1798–1806.10.1021/bi00328a034
- Wolfgang, A. (2007). Enzyme in industry: Production and applications. Weinheim: Wiley-VCH.
- Zaferanloo, B., Bhattacharjee, S., Ghorbani, M. M., Mahon, P. J., & Palombo, E. A. (2014). Amylase production by Preussia minima, a fungus of endophytic origin: optimization of fermentation conditions and analysis of fungal secretome by LC-MS. BMC Microbiology, 14, 55. doi:10.1186/1471-2180-14-5510.1186/1471-2180-14-55
- Zaferanloo, B., Virkar, A., Mahon, P. J., & Palombo, E. A. (2012). Endophytes from an Australian native plant are a promising source of industrially useful enzymes. World Journal of Microbiology and Biotechnology, 29, 335–45. doi:10.1007/s11274-012-1187-y