Abstract
Edible components of Citrus aurantium (bitter orange) fruit i.e. whole fruit, separated peel and pulp, and processed preserved products, namely salt pickle, chilli pickle, and sweet preserve were analyzed for antioxidant potential by various in vitro assays. The antioxidants components were extracted in different media and freeze dried. Methanol and aqueous media were comparatively more effective in extracting the antioxidant components. The total phenol content of the extracts ranged from 2.5 to 22.5 mg/g and 5.0 to 45.0 mg/g of pulp and peel fragments, respectively. The fruit components exhibited proton radical, oxyradical, and hydroxyl radical scavenging abilities and were effective in preventing lipid peroxidation. Regression analysis showed positive association between total phenolics and different antioxidant assays. In processed products, there was an initial decrease in antioxidant capacity, which showed an increase on storage. In conclusion, bitter orange exhibited high antioxidant capacity which was retained even in processed and stored products.
Public Interest Statement
Citrus aurentium (Bitter orange) is a fruit known for its medicinal properties in traditional medicine. It is used in fresh and processed form in traditional diets and said to carry six basic tastes together. The paper investigates the antioxidant potential of fresh Citrus aurantium fruit as well as retention of its antioxidant properties in stored and processed products using different in vitro assays. The research throws light on the possible health benefits of this fruit on account of its antioxidant potential. Also, it justifies its use in traditional medicine as even after storage the products could retain and exhibit a very high antioxidant potential.
Competing interests
The authors declare no competing interests.
1. Introduction
C. aurantium (bitter orange), one of the citrus fruits belonging to the family Rutaceae, is known for its extremely bitter and sour taste. In traditional Indian Ayurvedic system of medicine, it is said to be an appetizer and digestive on account of its taste, which is said to be a mixture of all six basic tastes, namely, sweet, sour, salty, bitter, pungent, and astringent. C. aurantium is also called as Seville orange, sour orange, and Zhi shi. Common species of C. aurantium are Citrus aurantium amara, Citrus bergamia, Citrus bigaradia, and Citrus vulgaris. In general, citrus fruits contain sugar, pectin, vitamins (A, B1, and C), and carotenoid pigments; organic acids such as citric acid and ascorbic acid, minerals and a number of active phytochemicals such as coumarins, and flavonoids as naringin, naringenin, hesperidin, neohesperidin, rutin, hesperetin, nairutin, and tangeretin. Citrus peels are known to have high levels of phenolics which demonstrate strong antioxidant capability (Bocco, Cuvelier, Richard, & Berset, Citation1998). Polyphenols comprise a wide range of components including flavanols, phenols, tannins, etc. and extraction of these vary by the extracting solvent used. Many antioxidative phenolic compounds in plants are frequently present as a covalent bond formed with insoluble polymers (Umamaheswari, Asokkumar, Lalitha, Sivashanmugam, & Subhadradevi, Citation2011).
In some parts of the world, C. aurantium is consumed as a food and in other parts it is used as medicine to cure fungal and bacterial infections, human colon and breast cancer by alternative therapists (Poulose, Harrism, & Patil, Citation2005; Tian, Miller, Ahmad, Tang, & Patil, Citation2001). While some citrus fruits are consumed in native form, others with the characteristic taste and flavor are processed into products such as pickles, marmalades, juices, concentrates, both at industrial and household level for immediate use and for off-season consumption. Microbial safety, shelf life, storage structures for extended shelf life, organoleptic acceptability, and mineral content of the fruit preserves have been widely studied. However, information on the antioxidant potency of citrus fruits on processing and their retention on storage is scarce. The present investigation was undertaken to study the antioxidant potential of fresh C. aurantium fruit and retention of antioxidant activity in processed preserved products as a function of storage.
2. Methodology
2.1. Materials
The fruit samples needed for the study, C. aurantium were purchased from the local market of Mysore city, India in one lot and processed for the entire study. Chemicals such as β-Carotene, linoleic acid, 2,2-Diphenyl-1picryl hydrazyl (DPPH), Tween 40 (polyoxyethylene sorbitan monopalmitate), Folin-Ciocalteau reagent, deoxyribose were purchased from Sigma Chemical Co (St.Louis, USA). All other solvents and chemicals used were of analytical grade procured from E-Merk, Mumbai. All analysis was carried out in triplicate.
The study design consisted of determination of total phenol content and antioxidant activity of whole fresh fruit and separated out peel and pulp portions in different extracting media. Whole fruit was used for preparation of three preserved products, which were in turn analyzed for total phenol and antioxidant activity by different assay every month for a storage duration of 6 months.
2.2. Preparation of fruit products
2.2.1. Fresh fruit components
The fruits were washed, pulp and peel were manually separated, deseeded and freeze dried, and stored in airtight containers.
2.2.2. Pickle
Pickles are preserved products prepared with salt alone or in combination with chilli and other spices and stored at room temperature for off-season utilization. C. aurantium pickle was prepared by dicing the washed and cleaned fruit into 1.0 cm cubes, deseeding and mixing with salt (18.5%) alone in one variation and with salt, chilli powder (7.5%) and turmeric powder (0.5%) in another variation. It was mixed well and stored in airtight containers at room temperature for a period of six months.
2.2.3. Sweet preserve
A sugar-based fruit preserve was prepared by the conventional method. A 600 g portion of the fruit was cleaned, deseeded, and pressure-cooked for 30 min. The cooked fruit was ground to a fine paste using a mixer grinder (Kenstar Food Processor, India). Sugar syrup was prepared with 900 g sugar and the ground paste was added to the boiling syrup and cooked for 15–20 min on a low flame. The product was cooled and stored in airtight glass containers at room temperature for a period of six months (the way it is conventionally stored).
Antioxidant potency of all products was analyzed in three stages i.e. in fresh fruit components to check the antioxidant capacity of the fruit components and to identify the solvent for extraction, in prepared products to study the effect of processing, and in the stored products to check the stability.
2.3. Preparation of extract for antioxidant activity
The fruit components or the products were homogenized, freeze dried, and stored in the refrigerator until use. A known quantity of the dried fruit components/products was extracted in 25 ml of the solvent on a magnetic stirrer at room temperature for two hours, the supernatant was decanted and the residue was re-extracted under the same conditions using fresh solvent. The extracts were pooled and filtered through Whatman No.41 filter paper to obtain particle free extract. Yield of the components in different solvents was estimated by evaporating the organic solvents under vacuum using a Buchi rotary evaporator, followed by lyophilization.
2.4. Determination of total phenols and antioxidant activity
2.4.1. Total phenols
The concentration of total phenols in the extracts was determined by incubating 0.1 ml extract with 1.0 ml Folin–Ciocalteau reagent and 0.8 ml of 2% sodium carbonate solution, diluted to 10.0 ml with water–methanol mix at room temperature for 30 min (Matthäus, Citation2002). The absorbance of the reaction mixture was read at 765 nm and the results were expressed as tannic acid equivalents.
2.4.2. Total antioxidant activity
The total antioxidant activity was determined by phosphomolybdenum method wherein 0.1 ml extract was combined with 1.0 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate), incubated at 95°C for 90 min, and absorbance was read at 765 nm (Prieto, Pineda, & Aguilar, Citation1999). The antioxidant activity was expressed as equivalents of ascorbic acid (μmole/g extract).
2.4.3. β-carotene linoleate model system
Antioxidant activity of the fruit extracts was assayed in β-carotene linoleate model system by adding 0.1 ml of extract with 4.0 ml of emulsion (prepared by dissolving 0.2 mg of β-carotene in 0.2 ml chloroform and mixed with 20 mg linoleic acid, 200 mg tween 40, evaporating chloroform, and adding 50 ml oxygenated water) (Jayaprakasha, Singh, & Sakariah, Citation2001). The reaction mixture was incubated at 50°C in a water bath and absorbance was recorded at 470 nm at 20 min intervals for 180 min and antioxidant activity of the extracts was evaluated in terms of bleaching of β-carotene.
2.4.4. Reducing power
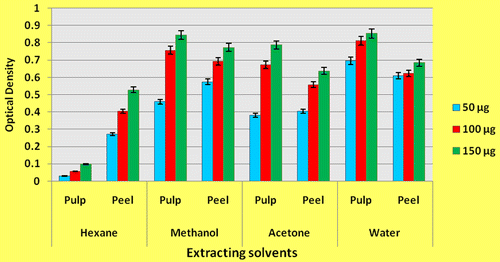
Reducing capacity of the extracts was determined by mixing extracts (50 μg, 100 μg and 150 μg,) with phosphate buffer (2.5 ml of 0.2 M of pH 6.6 and potassium ferricyanide (2.5 ml of 1% solution), incubated at 50°C for 20 min. Trichloroacetic acid (2.5 ml of 10%) was added and centrifuged at 3,000× g for 10 min. Upper layer of the solution (2.5 ml) was decanted and mixed with ferric chloride (0.5 ml of 0.1%) and the absorbance was read at 700 nm (Chu, Chang, & Hsu, Citation2000).
2.4.5. Free radical scavenging activity
This was determined by combining 0.1 ml of the extract with 0.3 ml of methanol and 0.6 ml of 0.1 mM methanolic solution of DPPH, incubated for 20 min, and absorbance was measured at 517 nm (Yamaguchi, Takamura, Matoba, & Terao, Citation1998). Butylated hydroxy anisole (BHA) was used as the positive control.
2.4.6. Hydroxyl radical scavenging activity
A 0.1 ml of sample extract was incubated with deoxyribose (0.3 ml of 30 mM,), hydrogen peroxide (0.3 ml of 20 mM), ferric chloride (0.3 ml of 1 mM), EDTA (0.3 ml of 1 mM), and ascorbic acid (0.3 ml of 1 mM) in potassium phosphate buffer (1.4 ml of 50 mM, pH 7.4) at 37°C for 60 min. The reaction was terminated by adding TCA (0.5 ml 5% solution) followed by addition of TBA (0.5 ml of 0.2% solution), and boiling in water bath for 15 min. The absorbance was measured at 535 nm. BHA was used as the positive control (Halliwell, Citation1995).
2.4.7. TBA assay
Inhibition of malondialdehyde formation in anti FeCl2-H2O2 stimulated linoleic acid peroxidation was assayed by incubating with 0.04 ml of extract to 0.04 ml of 0.1 M linoleic acid, 0.04 ml of 20 mM FeCl2, 0.04 ml of 20 mM H2O2 and 1.0 ml of 0.2 M phosphate buffer (pH 7.4) at 37°C for 24 h. To the reaction mixture 0.04 ml of BHA (20 mg/ml), 0.2 ml of 10% TCA and 0.2 ml of 1% TBA were added and boiled for 30 min on a boiling water bath. The solution was cooled and 1 ml of chloroform was added and centrifuged at 1,000× g for 10 min, the absorbance of the supernatant was measured at 532 nm against blank (Duh, Citation1998).
2.4.8. Metal ion chelating assay
The Fe2+-chelating ability of the extracts were determined by incubating 0.1 ml extract with FeCl2 (0.1 ml of 2 mM) and ferrozine (0.4 μl 5 mM), 0.4 ml of methanol at room temperature for 10 min. The absorbance was measured at 562 nm against blank (Dinis, Madeira, & Almeida, Citation1994). EDTA was used as the positive control.
Free radical scavenging activities by all protocols was done using a series of concentrations and IC50 values were derived from the data. IC50 values of different products by various assays were deduced to compare the efficiency against the standard. BHA was used as positive control.
2.5. Statistical analysis
The results presented represent the average of triplicate measurements. Analysis of variance was adopted to test the differences that existed in the free radical quenching ability of different extracts; multiple regression was applied to study the functional association between the assays using the statistical package Origin Pro version 7.0. The probability level was set to p < 0.05.
3. Results and discussion
The results of the study are compiled in Tables and Figure .
Table 1. Extract yield, total phenols, and total antioxidant activity of Citrus aurantium fruit components
Table 2. Antioxidant activity and IC50 values of the fruit components by in vitro assays in different media
Table 3. Regression analysis (functional association) between different antioxidant assays
Table 4. Effect of processing on the antioxidant activity of Citrus aurantium
Table 5. Effect of processing on the antioxidant activity of Citrus aurantium (expressed as percent retention in relation to fresh fruit)
3.1. Antioxidant components and total antioxidant activity
The yield of antioxidant components, total phenol content, and total antioxidant activity by phosphomolybdenum assay of the fruit extractives in different extracting media are presented in Table . Among all extracting media, the yield of the antioxidant components was highest in methanol with 40 g/100 g for pulp and 16.67 g/100 g for peel. Highest total phenol contents were estimated in methanolic media for pulp (22.5 mg/g) and water media for peel (45.0 mg/g). Others were at a much lower range. Other studies report similar results for citrus fruits. Shyamala and Prakash (Citation2016) studied antioxidant properties of orange pomace (residue left after juice extraction), and found that the yield of antioxidant components was 35.03 g and 34.4 g/100 g in methanol and water media, respectively. They also reported the polyphenol content of orange pomace as 1,032 mg (methanol extract) and 530 mg/100 g dry weight basis (water extract). Similar pattern was found in blood orange and citron fruits by Jayaprakasha and Patil (Citation2007). In another study the total phenolics were reported as 420 mg/100 g for whole lime in aqueous media by Adelpilerood and Prakash (Citation2015).
Total antioxidant activity of the pulp and peel extracts was estimated by phosphomolybdenum method, which is quantitative. It is based on the reduction of molybdenum (VI) to molybdenum (V) by the antioxidant compounds and subsequent formation of green molybdenum (V) complexes with the maximum absorption at 695 nm (Prieto et al., Citation1999). Peel extracts exhibited higher activity than pulp extracts in all the solvents used for extraction. A higher antioxidant activity of the peel than the pulp could be attributed to the higher concentration of phenolic acids and their derivatives in peel. Similar results are reported for sweet orange peel (Anagnostopoulou, Kefalas, Papageorgiou, Assimopoulou, & Boskou, Citation2006; Manthey & Grohmann, Citation1996). Certain limonoids present in the citrus fruit are reported to be responsible for the antioxidant activity exhibited by the citrus fruits (Patil, Jayaprakasha, & Padanad, Citation2004; Poulose et al., Citation2005). The antioxidant activity of the pulp extracts followed the order water > methanol > ethyl acetate > acetone. Among the peel extracts, methanol and aqueous extracts exhibited higher activity.
3.2. Reducing power
The antioxidant activity of peel and pulp of C. aurantium as measured by reducing power assay is presented in Figure . Research suggest that antioxidative effect is concomitant with the development of reductones. According to Gordon (Citation1990), antioxidant action of reductones is based on breaking the radical chain by donation of hydrogen atom. The antioxidants react with free radicals to convert them to more stable product that can terminate radical chain reaction. Reducing power of the different extracts of fruit components increased with increasing concentration in all solvents (Figure ). The pulp extracts exhibited higher reducing capacity than peel extracts in all except hexane; however the differences were not statistically significant (F = 1.77ns). Higher concentration of ascorbic acid in pulp could be attributed to higher reducing capacity than peel extracts.
3.3. Free radical (DPPH) scavenging activity
DPPH is a protonated radical that has characteristic absorption maxima at 517 nm, which decreases with the scavenging of the proton radical (Yamaguchi et al., Citation1998). Among the extracting solvents used, methanol and aqueous extracts exhibited higher proton radical scavenging activity with both pulp and peel (Table ). The differential activity exhibited by different solvent extracts can be attributed to the extracting ability of different antioxidant components as ascorbic acid, carotene, flavonoids, etc. These results are in agreement with those reported for citron and blood orange (Jayaprakasha & Patil, Citation2007). Antioxidants react with DPPH, (the nitrogen-centered free radical) that convert to 1,1, diphenyl-2-picryl hydrazine due to the hydrogen donating ability. This is known to intercept the propagation of free radical chain of oxidation and thereby form stable end products, which do not initiate further lipid oxidation. Peel extracts gave a comparatively higher activity than pulp in all media but the differences were found to be non-significant (F = 0.7061ns). A dose dependency was clearly evident in the proton radical scavenging activity of the fragments of C. aurantium in both the media. A comparatively lower IC50 value of methanol extract of pulp and aqueous extract of peel indicates a better potency. The differences in the extraction potential of the free radical scavenging ability of water and methanol were found to be almost similar in pulp (F = 2.980ns) and peel (F = 1.879ns).
3.4. Hydroxyl radical scavenging assay
Hydroxyl radicals are well known to abstract hydrogen atom from membrane lipids and bring about lipid peroxidation. Apparently, the ability to quench hydroxyl radical by the extracts seems to relate directly to the prevention of propogation of the process of lipid peroxidation (Halliwell & Gutteridge, Citation1984). In this reaction, Fe2+—EDTA chelate is incubated with deoxyribose in the presence of phosphate buffer at slightly alkaline pH to form the hydroxyl radicals. The scavenging ability of hydroxyl radicals by hexane extract of fruit components was negligible (Table ). Among the pulp extracts, acetone extract showed the least and methanol extract the highest activities. Peel extracts followed a similar trend with regard to the solvents used for extraction, but the scavenging potential was comparatively higher than that of pulp. Among the peel extracts, hexane and aqueous extracts showed 8 and 11% higher activity than pulp while others were comparable. On the whole, the scavenging potential of the fruit components were found to be similar (F = 0.089ns). Methanol extracts gave a low IC50 of 11.4 and 10.8 μg/ml in pulp and peel, respectively, 13–14 times higher quantity of aqueous extracts required to quench 50% of hydroxyl radical. The differences in the activity exhibited by the extracting media was found to be extremely significant in pulp (F = 207.77***) and peel (F = 137.69***).
3.5. Antioxidant activity by TBA assay
Iron salts react with hydrogen peroxide and liberate free radicals which effectively propagates lipid peroxidation (by Fenton reaction). These free radicals undergo some molecular rearrangement to produce conjugated dienes which readily react with oxygen molecule to give peroxy radical (Jadhav, Nimbalkar, Kulkarni, & Madhavi, Citation1996). These peroxy radical fragments are converted to aldehydes such as malondialdehyde (MDA) which is usually taken as the marker of lipid peroxidation (Janero, Citation1990). In the TBA assay, MDA formation is initiated and the antioxidants are introduced to see their efficacy in prevention of MDA. Unlike other assays, the pattern here was quite different, acetone extract of pulp showed the least inhibition. Aqueous extract exhibited the highest inhibition at 100 μg/ml of pulp followed by ethyl acetate, methanol, and hexane extracts. Similar trend was observed in the peel extracts except that ethyl acetate extract exhibited the highest activity (F = 0.039ns). In the peel extracts, methanol and ethyl acetate showed higher activity. The quantity of the fruit segments needed to cause 50% of inhibition of MDA was higher in case of methanol than aqueous extract but the differences in the extent of inhibition of MDA between the solvents were non-significant both in pulp (F = 4.608ns) and peel (0.981ns).
3.6. Metal ion chelating assay
Metal ion chelating capacity plays a significant role in antioxidant mechanisms since it reduces the concentration of the catalyzing transition metal in lipid peroxidation (Duh, Tu, & Yen, Citation1999). Except hexane extract all other extracts of peel showed lower activity. The metal binding ability of the fruit components were non-significant (F = 0.497ns). Gordon (Citation1990) noted that chelating agents which form œ bonds with a metal are effective as secondary antioxidants because they reduce the redox potential, thereby stabilizing the oxidized form of the metal ion. Methanol extract of pulp showed a significantly lower IC50 value than aqueous extract (F = 15.513**), such a trend was not exhibited in peel extracts (F = 0.183ns).
3.7. β-carotene linoleate model
In the β-carotene linoleate model, free radicals are generated by bringing in coupled oxidation between β-carotene and linoleic acid, the free radicals of linoleic acid are formed by abstracting the hydrogen atom from one of its diallylic methylene groups. The free radicals so formed attacks the unsaturated β-carotene and oxidizes it which is reflected in discoloration that can be spectrophotometrically monitored. The presence of antioxidants in the system prevents the bleaching of β-carotene. The extracts of the fruit components exhibited varied level of inhibition (with different extracting solvents) of bleaching of β-carotene by neutralizing the free radicals generated in the system (Table ). Hexane extract showed the highest activity in both the components tested. With the exception of hexane, in all other extraction media, pulp components showed higher inhibition of bleaching of β-carotene than peel components at 0.2 mg/ml level.
3.8. Functional association between antioxidant assays
An attempt was made to study the functional association between the assays involving different mechanisms and the reducing property. A multiple regression analysis was done between total antioxidant activity, total phenol content, reducing power, and the free radical scavenging assays (Table ). A high association was observed between total phenol content and the free radical scavenging and metal ion chelating assays. Similar significant association between total phenol content and free radical scavenging metal ion chelating assays was reported for citron and blood orange and orange juice (Jayaprakasha & Patil, Citation2007; Halliwell & Gutridge, Citation1990). However, the extent of association depends on the polyphenol and the transition metal concentration that enables the redox process. Further, the association between total phenol content and free radical scavenging assays were done individually and a higher association was found with DPPH assay.
3.9. Effect of processing on the antioxidant potential of C. aurantium
Processing of the fruit reduced the total phenol content but the extent varied by the product. Retention was higher in pickles than sweet preserve suggesting that thermal processing would have caused more destruction than mechanical destruction alone. The process of pickling reduced the antioxidant activity assessed by different assays except hydroxyl radical scavenging activity (Table ). The antioxidant activity of spice pickles was lower than that noticed for salt pickle in both the extracts in all assays. The antioxidant activity by phosphomolybdenum method, hydroxyl radical scavenging, and free radical scavenging of sweet preserve was comparatively better than pickles. Similar increase in the antioxidant activity on thermal processing of other varieties of citrus fruits has been reported which is attributed to liberation of low molecular weight antioxidants on thermal treatment (Gil-Izquierdo, Gil, Tomás-Barberán, & Ferreres, Citation2003). The losses that occurred in antioxidant components and the free radical scavenging ability in the products were found to be non-significant (F = 0.3485ns; p = 0.79). The insignificant decrease in antioxidant potential in salt pickle (F = 0.195ns; p = 0.66), and chilli pickle (0.279ns; p = 0.60) indicate that processing methods adopted in pickles does not cause deleterious effect. The differences in the antioxidant activity between salt and chilly added pickles suggest that the additives have no effect on the antioxidant activity (F = 0.050ns; p = 0.94). The differences between sweet preserve and the fresh fruit varied by the assay wherein a noticeable increase was noticed in the hydroxyl radical scavenging assay and decrease in the activity in other assays, however the differences were found to be non-significant (F = 0.001; p = 0.97).
3.10. Effect of storage on the antioxidant potential of the fruit products
The total phenol content of the processed products on storage is presented in Table . The data are computed as percent retention of the value in comparison to fresh fruit. The phenolic contents depended on the type of products as the trend was different in salt pickle, chilli pickle, and sweet preserve. Initially, salt pickle retained 85.4% of total phenolics in methanol, which exhibited a slow reduction to 77.7% at 6 months of storage. In water media, the value was 74.2%, which showed a gradual increase up to fifth month of storage and a slight decline later. For chilli pickle, initial values were 78.6 and 75.4% in methanol and water media, showed an increase through fifth month, and a slight decline at sixth month. Sweet preserve initially had only 50.4–57.6% of total phenols retained, however, it showed a consistent and a much higher increase during storage period, the trend being similar to chilli pickle. The results are in agreement with certain other minimally processed products citrus fruit segment and juices. Shamouti orange, Palazzelli, Mandarin, and Minneola tangelo showed an increase in total flavanoid content and hesperidin, in particular during storage (Del Caro, Piga, Vacca, & Agabbio, Citation2004). Certain phenolic acids as 5-hydroxylvaleric acid, 2, 3-diacetyl-1-phenylnaphthalene, vanillic acid, and ferulic acid were newly detected when citrus peel was heated at 130°C/min (Jeong et al., Citation2004). Several methods such as heat treatment for infrared radiation, fermentation and protease treatment have been reported to liberate and activate low molecular weight antioxidants (Duh et al., Citation1999). During the storage of citrus fruits certain phenolic compounds as p—cinnamic acid and isoferulic acid were known to be formed even without application of heat. However, it has been shown that the processing condition applied, species used, and media of extraction are known to influence the extent of liberation on storage. Phenolic compounds are known to act as antioxidants because of their stable radical intermediates which prevent the oxidation of various food ingredients, especially fatty acids (Cuvelier, Berset, & Richard, Citation1994).
The antioxidant potential of the fruit products on storage was computed in terms of percentage of activity in relation to the activity shown by the fresh fruit (Table ). The total antioxidant activity of all three products was low on initial day, the range being 41.5–53.1% for salt pickle, 38.7–43.5% for chilli pickle and 81.5–82.5% for sweet preserve. During storage, all products showed an increase in antioxidant activity to varying extent, least in salt pickle, and highest in sweet preserve. The results indicate that during storage, C. aurantium products exhibited enhanced antioxidant activity.
Free radical scavenging activity of the products reduced on processing and further on storage, but the trend varied by the processing adopted. Salt pickle showed a reducing trend in both the extracts on storage but the extent of losses was lower in the aqueous extractives. Similar reducing trend was observed in the aqueous extractives of chilli pickle. The methanol extractives of chilli pickle exhibited 61% of the activity observed in fresh fruit which increased by 8–16% during the storage period. In sweet preserve (methanol extract), an increase in the free radical scavenging activity was observed up to four months of storage beyond which a slight reduction was observed with both the extracts. A comparatively higher activity in the thermally processed sweet preserve can be attributed to disruption of the peel matrix on storage and leaching out of phytonutrients which are held intact in them. Similar results were also seen for the total phenol contents of sweet preserve. In different species of citrus fruits, phenolic acids were reported to increase on thermal treatments (Jeong et al., Citation2004). Similar change would have occurred in C. aurantium during preparation of sweet preserve.
The hydroxyl radical scavenging ability of methanol extracts of salted pickle was higher than fresh fruit and retained the activity up to four months and reduced by 7–10% beyond that. The aqueous extract showed an increase in the scavenging of hydroxyl radical up to three months and reduced thereon but the scavenging ability was higher than the fresh fruit during the entire storage period. Both the extracts of chilli pickle showed an increase in the activity up to fourth month but the activity was more than the fresh fruit. In sweet preserve, methanol extractives showed a 7–13% increase up to four months of storage, while aqueous extracts however, showed a twofold increase on preparation of the product and showed a gradual decrease in the activity during the storage period. Antioxidants of C. aurantium possess a good ability to scavenge hydroxyl radicals and are stable to processing and improve on storage.
Hydroxyl radical scavenging ability, total phenol content, and total antioxidant activity increased on storage more in chilli pickle and sweet pickle. The spices used in chilli pickle may also have contributed to the antioxidant activity and thermal processing would have enabled better release of antioxidants on storage.
4. Conclusion
From the above assays, it can be said that C. aurantium fruit with good antioxidant potential is effective both as a primary and a secondary antioxidant. The fruit components possess proton radical, oxyradical, and hydroxyl radical scavenging abilities. The ability of the fruit components to exhibit the antioxidant activities through different mechanisms varied among the fruit components, which can be attributed to compositional differences. Regression analysis infers that the multiple free radical scavenging activity of the fruit components was associated with their total phenol content. However, further confirmation is required for the free radical scavenging ability of C. aurantium phenols in in vivo models. Processing and storage resulted in reduced antioxidant activities compared to the fresh fruit in initial stages. During storage, enhanced activity was observed in all products. Hence, it can be said that C. aurantium is a good source of antioxidants and is stable to processing and storage. The common household processing and storage practices of the citrus fruits do not reduce the antioxidant potential of the fruits.
Acknowledgments
The authors are grateful to the Director CFTRI, for providing the necessary facilities for carrying out the work.
Additional information
Funding
Notes on contributors
Prakash J. Divya
Prakash J. Divya was a graduate student of the Department and this work represents her research project during the course of her studies. She is at present pursuing her doctoral degree at the Institution.
Prakash Jamuna
Prakash Jamuna was the research supervisor for the present work and is a senior faculty with extensive research and teaching experience. She is actively involved in research with many publications and presentations.
Lakshmi A. Jyothi
Lakshmi A. Jyothi is a senior scientist at the Institute with many years of research experience and many published papers in reputed journals. Her research interests are analytical studies on foods with reference to bioaccessibility of minerals. She is involved in formulation of protein-rich products to address undernutrition in children. She is also interested in understanding the factors that influence the glycemic index of food and the role of proteins in modulating the carbohydrate digestive profile of foods.
References
- Adelpilerood, S., & Prakash, J. (2015). Nutritional and antioxidant properties of dehydrated whole lime (Citrus latifolia) and shallot (Allium cepa var. aggregatum), two popular ingredients used in Iran. Malaysian Journal of Nutrition, 21, 93–103.
- Anagnostopoulou, M. A., Kefalas, P., Papageorgiou, V. P., Assimopoulou, A. N., & Boskou, D. (2006). Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chemistry, 94, 19–25.https://doi.org/10.1016/j.foodchem.2004.09.047
- Bocco, A., Cuvelier, M. E., Richard, H., & Berset, C. (1998). Antioxidant activity and phenolic composition of citrus peel and seed extracts. Journal of Agricultural and Food Chemistry, 46, 2123–2129.https://doi.org/10.1021/jf9709562
- Chu, Y. H., Chang, C. L., & Hsu, H. F. (2000). Flavonoid content of several vegetables and their antioxidant activity. Journal of the Science of Food and Agriculture, 80, 561–566.10.1002/(SICI)1097-0010(200004)80:5<>1.0.CO;2-Q
- Cuvelier, M. E., Berset, C., & Richard, H. (1994). Antioxidant constituents in sage (Salvia officianalis). Journal of Agricultural Food Chemistry, 42, 655–669.
- Del Caro, A., Piga, A., Vacca, V., & Agabbio, M. (2004). Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chemistry, 84, 99–105.10.1016/S0308-8146(03)00180-8
- Dinis, T. C. P., Madeira, V. M. C., & Almeida, L. M. (1994). Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archives of Biochemistry and Biophysics, 315, 161–169.10.1006/abbi.1994.1485
- Duh, P. D. (1998). Antioxidant activity of burdock (Arctium lappa Linné): Its scavenging effect on free-radical and active oxygen. Journal of the American Oil Chemists’ Society, 75, 455–461.10.1007/s11746-998-0248-8
- Duh, P. D., Tu, Y. Y., & Yen, G. C. (1999). Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT-Food Science and Technology, 32, 269–277.10.1006/fstl.1999.0548
- Gil-Izquierdo, A., Gil, M. L., Tomás-Barberán, F. A., & Ferreres, F. (2003). Influence of industrial processing on orange juice flavanone solubility and transformation to chalcones under gastrointestinal conditions. Journal of Agricultural Food Chemistry, 51, 3024–3028.10.1021/jf020986r
- Gordon, M. H. (1990). Food antioxidants. London: Elsevier Applied Science.10.1007/978-94-009-0753-9
- Halliwell, B. (1995). Antioxidant characterization. Biochemical Pharmacology, 49, 1341–1348.10.1016/0006-2952(95)00088-H
- Halliwell, B., & Gutridge, J. M. C. (1990). Role of free radicals and catalytic metal ions in human disease: An overview. Methods in Enzymology, 165, 215–219.
- Halliwell, B., & Gutteridge, J. M. C. (1984). Oxygen toxicity oxygen radicals transition metals and disease. Biochemical Journal, 219, 1–14.10.1042/bj2190001
- Jadhav, S. J., Nimbalkar, S. S., Kulkarni, A. D., & Madhavi, D. L. (1996). Lipid oxidation in biological and food system. In D. L. Madhavi, S. S. Deshpande, & D. K. Salunke (Eds.), Food antioxidants (pp. 5–64). New York, NY: Marcel Dekker.
- Janero, D. (1990). Malondialdehyde and thiobarbituric acid reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology and Medicine, 9, 515–540.10.1016/0891-5849(90)90131-2
- Jayaprakasha, G. K., & Patil, B. S. (2007). In vitro evaluation of the antioxidant activities in fruit extracts from citron and blood orange. Food Chemistry, 101, 410–418.10.1016/j.foodchem.2005.12.038
- Jayaprakasha, G. K., Singh, R. P., & Sakariah, K. K. (2001). Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chemistry, 73, 285–290.10.1016/S0308-8146(00)00298-3
- Jeong, S. M., Kim, S. Y., Kim, D. R., Jo, S. C., Nam, K. C., Ahn, D. U., & Lee, S. C. (2004). Effect of heat treatment on the antioxidant activity of extracts from citrus peels. Journal of Agricultural Food Chemistry, 52, 3389–3393.10.1021/jf049899k
- Manthey, J. A., & Grohmann, K. (1996). Concentrations of hesperidin and other orange peel flavonoids in citrus processing byproducts. Journal of Agricultural and Food Chemistry, 44, 811–814.10.1021/jf950572g
- Matthäus, B. (2002). Antioxidant activity of extracts obtained from residues of different oilseeds. Journal of Agricultural and Food Chemistry, 50, 3444–3452.10.1021/jf011440s
- Patil, B. S., Jayaprakasha, G. K., & Padanad, M. (2004, July 17–20). Isolation characterization and role of functional components in fruits and vegetables. Presented at American Society for Horticultural Science, Austin, TX.
- Poulose, S. M., Harrism, E. D., & Patil, B. S. (2005). Citrus limonoids induce apoptosis in human neuroblastoma cells and have radical scavenging activity. Journal of Nutrition, 135, 870–877.
- Prieto, P., Pineda, M., & Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Analytical Biochemistry, 269, 337–341.10.1006/abio.1999.4019
- Shyamala, B. N., & Prakash, J. (2016). Chemical composition and bioactivity of pomace from selected fruits. International Journal of Fruit Science. doi:10.1080/15538362.2016.1143433
- Tian, Q., Miller, E. G., Ahmad, H., Tang, L., & Patil, B. S. (2001). Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutrition and Cancer, 40, 180–184.10.1207/S15327914NC402_15
- Umamaheswari, M., Asokkumar, K., Lalitha, V., Sivashanmugam, T., & Subhadradevi, V. (2011). Anticataract and antioxidant activities of Citrus aurantium L. peel extract against naphthalene induced cataractogenesis in rats. Journal of Pharmacy Research, 4, 680–682.
- Yamaguchi, T., Takamura, H., Matoba, T., & Terao, J. (1998). HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Bioscience Biotechnology Biochemistry, 62, 1201–1204.10.1271/bbb.62.1201