Abstract
Shrimps are a very good source of protein; they contain small amount of fat and calories and are relished in many homes across the world. This study attempts to compare the proximate compositions and mineral contents of fresh Penaeus monodon and Penaeus notialis using standard analytical methods. Results of proximate composition revealed that percentage mean values for carbohydrate, crude protein, fat, crude fiber, ash and moisture levels in P. monodon were 13.15 ± 0.18, 9.21 ± 0.03, 4.67 ± 0.05, 1.21 ± 0.12, 3.53 ± 0.06 and 68.24 ± 0.11 respectively while values for P. notialis were 9.77 ± 0.04, 6.09 ± 0.05, 2.68 ± 0.06, 2.88 ± 0.06, 4.89 ± 0.03 and 73.71 ± 0.18, respectively. Both shrimp species were high in all the micro and macro minerals analyzed. However, values for calcium and zinc were significantly higher (p < 0.05) in P. monodon. It was concluded that although both shrimps are of different species and the nutrient levels in P. monodon were higher in some cases, nutritional differences between these two species in most cases are however not statistically different (p > 0.05).
Public Interest Statement
This research was designed to show how the comparative approach can increase understanding of the nutritional compositions of the two Penaeus shrimp species. We chose these two species because Penaeus notialis is the dominant native Penaeus species while Penaeus monodon is the dominant invasive Penaeus species in Nigeria.
Competing Interests
The authors declare no competing interest.
1. Introduction
Shrimps have become one of the major sources of animal protein to the low income earners. Its meat is high in protein, low in saturated fat and calories, and has a good flavor, and apart from supplying good quality proteins and vitamins A and D, it also contains several dietary minerals such as Fe, Ca, etc. which are beneficial to man and animals (Ravichandran, Rameshkumar, & Rosario Prince, Citation2009). Shrimps are also a rich source of vitamin B12, selenium, ω-3 highly unsaturated fatty acids (HUFA), and astaxanthin (Venugopal, Citation2009) which has been shown to provide antioxidant support to both the nervous system and musculoskeletal system. In addition, some animal studies have shown decreased risk of colon cancer to be associated with astaxanthin intake, as well as decreased risk of certain diabetes-related problems (The George Mateljan Foundation, Citation2015). Nigeria is among the tropical countries endowed with rich shrimp resources. Dublin-Green and Tobor (Citation1992), reported that the coastal waters of Nigeria are characterized by abundance of significant living resources including shrimps, predominantly members of the family Penaeidae. Economically, the importance of shrimp production in Nigeria and most especially along the coastal communities includes the provision of employment, source of food, source of income, source of tool to rural development and source of raw materials to manufactures. However, literature reports are scarce on the comparative nutritional chemical composition of the indigenous shrimp species (P. notialis) and the exotic species (P. monodon) in the southwestern part of Nigeria. Thus, the objective of this study is an attempt to compare the nutritional quality of these shrimp species.
2. Materials and methods
2.1. Sample collection and preparation
Samples of the pink shrimp (P. notialis) and tiger shrimp (P. monodon) were collected from local fishermen at Makoko Jetty along the Lagos lagoon environment, Southwest of Nigeria (6°29′-N and 3°23′-E). Samples were washed with de-ionized water to remove any adhering contamination and drained under folds of filter paper. Samples were then immediately preserved using iced packed cooler and transferred immediately to the Biochemistry laboratory of the College of Medicine, Lagos State University teaching Hospital. The drained samples were further wrapped in aluminum foil paper and frozen at a temperature of −4°C for 48 h before analysis.
2.2. Determination of proximate composition
Fresh samples of P. monodon and P. notialis were grounded using an electric grinding machine after crushing with mortar and pestle. The grounded portions were weighed individually and used for chemical analysis. The estimation of protein, carbohydrate and lipids were carried out by Bligh and Dyer (Citation1959), Lowry, Rosebrough, Farr, and Randall (Citation1951) and Morris (Citation1948), respectively. Moisture content was estimated by hot air oven method while minerals contents were analyzed by A.O.A.C. (Citation1990).
2.3. Statistical analysis
Biological data resulting from the experiment were analyzed for significant differences between groups by the two sample t-test using the SPSS (statistical Package Computer, Software 2004 version Chicago, Illinois, USA). Differences were regarded as significant at p < 0.05 (Zar, Citation1998).
3. Results and discussion
The proximate composition of fresh samples of P. monodon and P. notialis is presented in Table . According to Ockerman (Citation1992), proximate composition varies with species and is influenced by season, water temperature, and spawning cycle. In this study, the moisture content of P. monodon and P. notialis were 68.24 ± 0.11 and 73.71 ± 0.18%, respectively (p < 0.05). The crude protein percentage recorded for P. monodon (9.21%) and P. notialis (6.09%) were not significantly different (p > 0.05). This is similar to the findings of Bhavani and Karuppasamy (Citation2014). The high amount of protein content recorded for both shrimp species in this study may be attributed to their high protein dietary intake which included algae, diatoms, crustaceans, mollusks and partly digested fishes (Osibona, Citation2005). Similarly, carbohydrate content in P. monodon and P. notialis were 13.15 and 9.77% respectively, and differences were not significant (p > 0.05). Varadharajan and Soundarapandian (Citation2014) reported that in crustaceans, lipids are not only the main organic reserve and source of metabolic energy but also indispensable in maintaining cellular integrity. In this study, the lipid contents in both species were similar (p > 0.05), reaching in P. monodon a value of 4.67% while a value of 2.68% was recorded for P. notialis. The ash content values were 3.53 and 4.89% for P. monodon and P. notialis, respectively (p > 0.05). P. monodon had similar (p > 0.05) crude fiber content (1.21%) than that recorded for P. notialis (2.88%).
Table 1. Proximate composition of P. monodon and P. notialis (%)
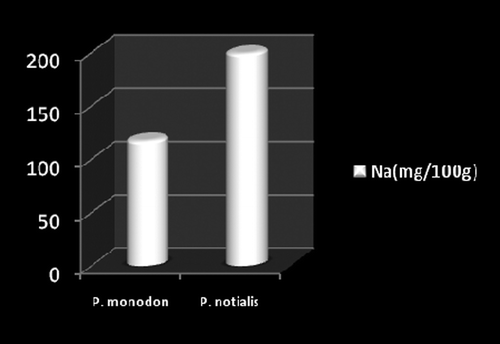
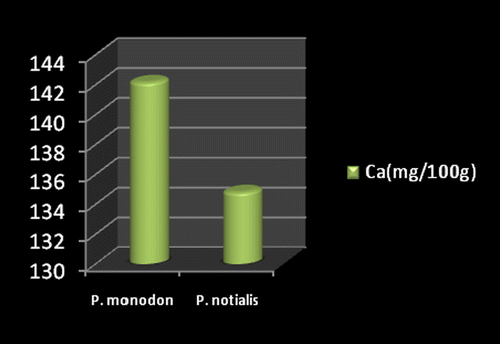
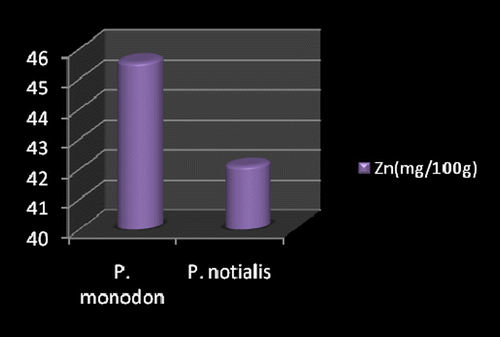
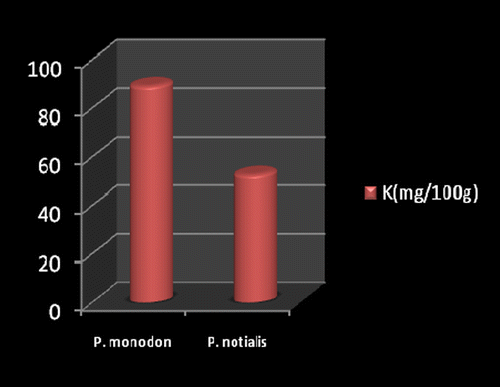
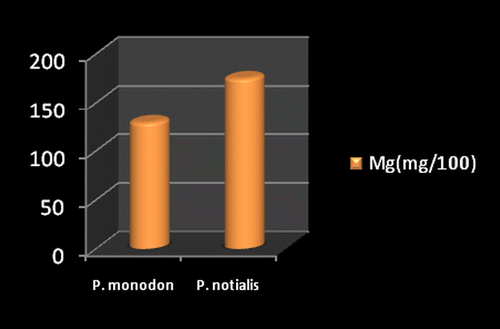
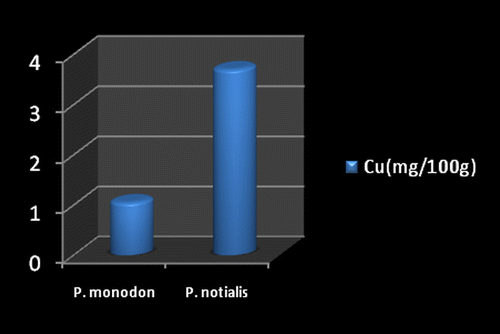
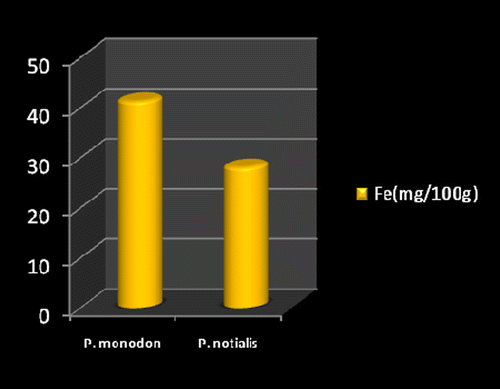
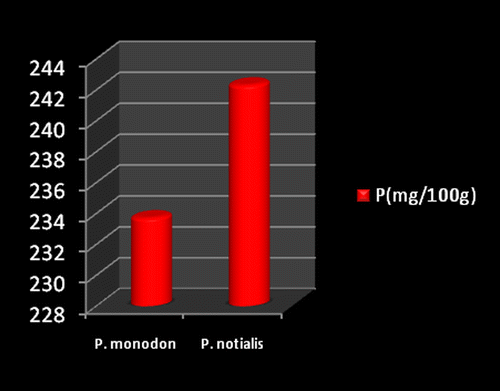
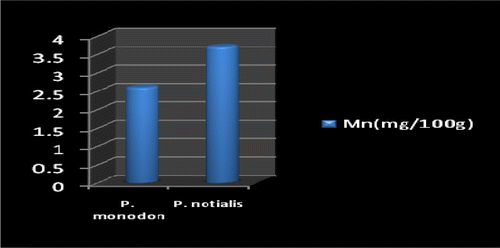
Marine foods are very rich sources of both macro and micro mineral components (Kumaran et al., Citation2012). Figures show the mineral composition of P. monodon and P. notialis. Among the nine nutrient elements investigated the most abundant was phosphorous (P) followed by magnesium (Mg), calcium (Ca), and sodium (Na). The phosphorous content for P. notialis was 242.3 mg/100 g and that recorded for P. monodon was 233.7 mg/100 g. The magnesium content in P. notialis was 174.8 mg/100 g while that recorded for P. monodon was 128.8 mg/100 g. The calcium content recorded for P. notialis was 134.8 mg/100 g while that for P. monodon was 142.2 mg/100 g. The sodium content recorded for P. notialis was 199.2 mg/100 g while that recorded for P. monodon was 117.3 mg/100 g. Values for K, Zn, Cu, Fe, and Mn recorded for P. notialis and P. monodon were 52.45 mg/100 g, 42.1 mg/100 g, 3.7 mg/100 g, 28.05 mg/100 g, 3.7 mg/100 g and 89.1 mg/100 g, 45.55 mg/100 g, 1.05 mg/100 g, 41.25 mg/100 g, 2.6 mg/100 g, respectively. The values of various minerals obtained from both shrimp species show a significant difference and samples examined in this study contained appreciable concentrations of Na, K, Ca, Mg, and P, suggesting that these shrimp species are a good source of nutrient minerals. The levels of micro minerals (Zn, Cu, Fe, and Mn) analyzed were also within tolerable limits (FAO/WHO, Citation1989).
The levels of K, Na, Mg, Cu, Fe, and Mn in P. notialis were higher than in P. monodon although differences were not significant (p > 0.05). This is similar to the report of Adeyeye (Citation2000) and Adeyeye, Habib, and Awodola (Citation2008). Furthermore, the levels of Ca, Zn, and P recorded for both species of shrimps in this study were significantly different (p < 0.05), showing P. monodon higher contents of Ca and Zn than P. notialis, while this latter species showed high contents of Syama Dayal et al. (Citation2013) reported values for Ca, Mg, P, K, Na, Cu, and Mn for P. monodon as 107.3 ± 1.96, 58.5 ± 1.38, 303.4 ± 3.22, 259.6 ± 3.25, 176.1 ± 3.04, 9.18 ± 4.62 and 50.5 ± 1.64 mg/100 g respectively which is also similar to the findings of this study.
The variations recorded in the concentration of the different nutritional components of both shrimp species examined in this study could have been as a result of the rate at which these components are available in the water body and the ability of the fish to absorb and convert the essential nutrients from the diet or the water bodies where they live (Adeniyi, Orikwe, Ehiagbonare, & Joshia, Citation2012; Yeannes & Almandos, Citation2003). These findings are in line with the reports of Adewoye, Fawole, and Omotosho (Citation2003) and Fawole, Ogundiran, Ayandiran, and Olagunju (Citation2007).
4. Conclusions
In conclusion, P. notialis and P. monodon examined in this study are good sources of proteins and mineral supply. Although both shrimps are of different species and the mineral levels in P. monodon are higher in some cases, differences between these two species are however not statistically different.
Additional information
Funding
Notes on contributors
Edah Bernard
Edah Bernard and Adeyemi Yewande Bolatito are both research scientists at the Department of Biotechnology/Fish Nutrition Unit of the Nigerian Institute for Oceanography and Marine Research, Lagos, Nigeria. Their passion for a better knowledge of these two Penaeus shrimp species in Nigeria spurred them to carry out this research as the collection of accurate date on species nutritional and mineral composition will in no small measure ensure their proper management.
References
- Adeyeye, E. I. (2000). Bio-concentration of mineral and trace minerals in four prawns living in Lagos Lagoon. Pakistan Journal of Scientific and Industrial Research, 43, 367–373.
- Adewoye, S. O., Fawole, O. O., & Omotosho, J. S. (2003). Concentrations of selected elements in some freshwater fishes in Nigeria. Science Focus, 4, 106–108.
- Adeyeye, E. I., Habib, O. A., & Awodola, O. J. (2008). Comparability of chemical composition and functional properties of shell and flesh of Penaeus notabilis. Pakistan Journal of Nutrition, 7, 741–747.10.3923/pjn.2008.741.747
- Adeniyi, S. A., Orikwe, C. L., Ehiagbonare, J. E., & Joshia, S. J. (2012). Nutritional composition of three different fishes (Clarias gariepinus, Malapterurus electricus and Tilapia guineenisis). Pakistan Journal of Nutrition, 8, 793–797.
- A.O.A.C. (1990). Official methods of analysis 15 edition (pp. 222–245). Washington, DC: Association of Official Analytical Chemists Washington.
- Bhavani, K., & Karuppasamy, R. (2014, January). Acute tsoxicity bioassay and behavioural changes on Zebra fish, Danio Rerio (Hamilton) under arsenic trioxide. International Journal of Modern Research and Reviews, 2, 40–46.
- Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.10.1139/o59-099
- Dublin-Green, C. O., & Tobor, J. G. (1992). Marine resources and activities in Nigeria (NIOMR Technical paper No. 84). Lagos: Nigerian Institute for Oceanography and Marine Research.
- FAO/WHO. (1989). National research council recommended dietary allowances (10th ed.). Washington, DC: National Academy Press.
- Fawole, O. O., Ogundiran, M. A., Ayandiran, T. A., & Olagunju, O. F. (2007). Proximate and mineral composition in some selected fresh water fishes in Nigeria. Internet Journal of Food Safety, 9, 52–55.
- Kumaran, R. S., Choi, Y., Lee, S., Jeon, H. J., Jung, H., & Kim, H. J. (2012). Isolation of taxol, an anticancer drug produced by the endophytic fungus, Phoma betae. African Journal of Biotechnology, 11, 950–960.
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin-Phenol reagents. Journal of Biology and Chemistry, 193, 265–275.
- Morris, D. L. (1948). Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science, 107, 254–255.10.1126/science.107.2775.254
- Ockerman, H. W. (1992). Fishery by-products. In G. M. Hall (Ed.), Fish processing technology (pp. 155–192). New York, NY: Chapman and Hall.
- Osibona, A. O. (2005). Comparative study of proximate composition, amino acids, fatty acids and aspects of the biology of some economic fish species in Lagos State, Nigeria (PhD thesis, p. 218). Department of Marine Sciences, University of Lagos, Lagos.
- Ravichandran, S., Rameshkumar, G., & Rosario Prince, A. (2009). Biochemical composition of shell and flesh of the Indian White Shrimp Penaeus indicus (H.milne Edwards 1837). American-Eurasian Journal of Scientific Research, 4, 191–194.
- Syama Dayal, J., Ponniah, A. G., Imran Khan, H., Madhu Babu, E. P., Ambasankar, K., & Kumarguru Vasagam, K. P. (2013). Shrimps—A nutritional perspective. General Articles, 104, 1487–1492.
- The George Mateljan Foundation. (2015, December 21–27). The worlds healthiest foods. Essential guide to your healthy way of eating.
- Varadharajan, D., & Soundarapandian, P. (2014). Proximate composition and mineral contents of freshwater crab Spiralothelphusa hydrodroma (Herbst, 1794) from Parangipettai, South East Coast of India. Journal of Aquatic Research and Development, 5(2), 1–6. doi:10.4172/2155-9546.1000217.
- Venugopal, V. (Ed.) (2009). Marine products for healthcare: Functional and bioactive nutraceutical compounds from the ocean (pp. 221–239). London: CRC Press.
- Yeannes, M. I., & Almandos, M. E. (2003). Estimation of fish proximate composition starting from water content. Journal of Food Composition and Analysis, 16, 81–92.10.1016/S0889-1575(02)00168-0
- Zar, J. H. (1998). Biostatistical analysis (4th ed., p. 662). London: Prentice-Hall International.