Abstract
Biodeterioration of poultry feed by fungal load due to improper storage is a major concern. The present investigation was carried out to determine the effect of relative humidity (RH) on biodeterioration of poultry feed, biochemical changes and ochratoxin A (OTA) production by Penicillium species examined during September 2009 to July 2010. All the relative humidities studied were supported 16 species of Penicillium with varying percentage of prevalence. Penicillium species produced different mycotoxins with ochratoxin A (OTA) predominating. The OTA production in feed, increased with increasing RH with increasing storage period. The feed stored under RH at 90 and 100% reached maximum OTA production 16.19 and 20.45 μg/g respectively on fourth month of storage period, while RH at 50 and 70% the amount of OTA increased gradually and reached maximum 11.37 and 14.48 μg/g respectively on sixth month of storage. The highest phenol and reducing sugar content was observed under all RH on fourth month of storage and maximum with RH at 70 and 90%, while protein, free amino acid content of feed initially decreased and increased on end of the storage period. In conclusion, relative humidity also plays a major role on biodeterioration of poultry feed and OTA production by Penicillium species.
Public Interest Statement
The present investigation reveals the importance of maintaining relative humidity of poultry houses is of crucial importance. Humidity plays a critical role in maintaining hygienic poultry feed by preventing mould growth specially mycotoxigenic fungi and mycotoxin contamination which certainly promote better utilization of nutrients by the poults. On the other hand, low humidity conditions of poultry houses are also not desirable as it may result deterioration of feed and loss of nutrients to be available for poults. The preservation also reveals that species of the same fungal genus differ in their tolerance to relative humidity. Thus by controlling humidity of poultry, it is possible to present mould infestation and contamination poultry feed and protect the poults from mycotoxicosis which inturn boost poultry production and save the common man from consumption of health hazardous unavoidable mycotoxins.
Competing Interests
The authors declare no competing interest.
1. Introduction
Cereals can be colonized by fungal communities at harvest and during faulty storage (Nesci, Ferrari, & Etcheverry, Citation2008). Mould infestation reduces the quality and quantity of grain produce. The contamination of foods and feeds with toxigenic fungi and mycotoxins is a problem of great agro-economic importance and adversely affects both humans and animals (Hussein & Brasel, Citation2001). Humidity and temperature are key environmental determinants for the safety of feed ingredients and stored finished products; inappropriate storage of feeds inevitably leads to fungal contamination of final mixed feed and mycotoxins production (Fareed, Hassan Khan, Anjum Ashraf, & Naveed, Citation2014). Mycotoxins are often found as natural contaminants in raw ingredients of poultry feed (Khan, Shamsul, Rozina, & Muhammad, Citation2011; Koteswara Rao, Shilpa, Girisham, & Reddy, Citation2011). Poultry are highly susceptible to mycotoxicoses caused by aflatoxins (AF) and ochratoxin A (OTA) (Anjum, Sahota, Akram, & Ali, Citation2011). The AF and OTA are the major components of a group of secondary metabolites produced by several fungi. Aspergillus, Penicillium, Fusarium and Alternaria are the main genera of fungal contaminants of foods and feeds (Council for Agricultural Science & Technology, Citation2003). Among these fungi, species of Penicillium, Penicillium chrysogenum, Penicillium citrinum, Penicillium expansum, Penicillium verrucosum and Penicillium rubrum are the most prevalent species and produce variety of mycotoxins such as OTA, citrinin, patulin, mycophenolic acid and griseofulvin which effects on animal and human health (Girisham, Koteswara Rao, & Reddy, Citation2016; Walker, Citation1999). Two species of Penicillium, P. verrucosum and Penicillium nordicum are reported to produce OTA.
In recent times OTA producing Penicillium species present in feeds has gained the importance due to adverse effect on poultry industry. Ochratoxin A is receiving major attention because of its nephrotoxic, carcinogenic, teratogenic and immunosuppressive properties (Creppy, Citation1999). Ochratoxin A in cereals and cereal based foods was reported to be associated with porcine nephropathy (Krogh, Hald, & Pedersen, Citation1973). Hence, there is need for OTA detection in foods and feeds before their utilization. The toxicity of Penicillium mycotoxins necessitates low detection limits (5 ng/g). In spite of the importance of commercial feedstuffs in modern animal husbandry, only few studies on the incidence of Penicillium species and OTA contamination of poultry feeds are available in India (Koteswara Rao et al., Citation2011). Therefore, the present study was carried out to determine the biodeterioration of poultry feed stored at different relative humidities, as well to evaluate OTA production by Penicillium species.
2. Materials and methods
2.1. Sampling
The compounded poultry feed (5 kg) collected from poultry industry of Warangal, Telangana, India were used to study the effect of different relative humidities on biodeterioration of poultry feed by different fungal species with special reference to Penicillium species, biochemical composition of the feed and OTA production was analysed for September 2009 to July 2010. From this feed 1 kg of poultry feed stored under different relative humidity condition viz., 30, 50, 70, 90 and 100%. At the end of incubation period, 10 g used for mycological examination of Penicillium species, 1.0 g of poultry feed was used for biochemical analysis and 25 g stored feed used for quantification of ochratoxin A under different humidity conditions. This compounded poultry feed is a mixture of corn cereals (60%), soybean meal (20%), sunflower meal (5%), broken rice (5%), wheat bran (5%), rice bran (3%) and fish meal (2%).
2.2. Reagents and standards
Standard OTA, acetonitrile, acetic acid, and HPLC grade water were obtained from Sigma Aldrich (Mumbai, India). Thin layer chromatography plates, oxalic acid, toluene, ethyl acetate, formic acid, o-phosphoric acid, sodium hydroxide and all media were obtained from Merck (Mumbai, India).
2.3. Application of relative humidity
Appropriate quantities of sterile water (226, 420, 510 and 712 ml) were added to appropriate quantities of sulphuric acid (686, 514, 348 and 161 ml) to adjust different relative humidity conditions to 30, 50, 70, 90 and 100%. Relative humidity of 100% was achieved by taking pure sterile water. One kg of poultry bird feed was taken individually and incubated in each humid chamber for creating atmosphere with different RH (Buxton & Mellanby, Citation1934). The prepared solutions were aseptically poured, sealed and incubated at 27 ± 2°C under dark condition for September 2009 to July 2010. Regular monitoring of feed sample, mycoflora and OTA was analysed by bimonthly interval (i.e. September 2009, November 2009, January 2010, March 2010, May 2010 and July 2010) qualitative and quantitatively by taking 25 g of feed, with special emphasis on Penicillium species under different humid atmosphere.
2.4. Isolation and identification of Penicillium species
The feed sample was analyzed for the presence of fungal propagules. This was made on solid nutrient media using pour-plate method by blending 10 g sample with 90 ml peptone solution (1.0%). Serial dilution 1 × 10−5 was made for each sample and 500 μl aliquots were poured in a sterile petri-plate in triplicates (n = 3) containing Czapek yeast autolysate (CYA) agar medium and incubated for 7 days at 27 ± 2°C under dark. At the end of incubation period, fungal colonies appearing on the petri plates were scored, were picked up and transferred to Malt Extract Agar (MEA) slants for further identification with special emphasis to Penicillium species. Identifications were made based on morphology, colony characters, branching of the conidiophores, shape, ornamentation, color of conidium and diffusing pigment with the help of standard keys (Frisvad & Samson, Citation2004; Frisvad, Smedsgaard, Larsen, & Samson, Citation2004; Samson & Pitt, Citation2000). The percentages of occurance of individual fungus were calculated following the method outlined by Koteswara Rao et al. (Citation2011).
2.5. Sample extraction and cleanup
Seven day old monosporic cultures of Penicillium species were inoculated into CYA broth. At the end of 6 days incubation period, cultures were harvested and 1 ml of spore suspension (1 × 10–5) containing ~80 spores were inoculated in 100 ml of broth in 250 ml Ehrlenmeyer conical flask and incubated at 27 ± 2°C on rotary shaker (Yihder LM-450 D) at 120 rpm for 12 days. At the end of the incubation period, the cultures were harvested on Whatman filter paper No. 42 and centrifuged at 12,000 × g to get cell-free filtrates. Thirty milliliter of culture filtrate was acidified with 0.1 M o-phosphoric acid and extracted twice with chloroform (1:1, v/v) and evaporated by rotary evaporator. The extract was eluted in 500 μl of methanol used for TLC/HPTLC analysis.
2.6. TLC/HPTLC analysis
Culture extracts were applied onto precoated silica gel plates obtained from Merck, Millipore (Mumbai, India) using CMAG sample auto applicator (CMAG, Germany, Gamb). The TLC plate activated by immersing in 10% oxalic acid solution in methanol for 5–10 min and heated at 110°C for 2 min and allowed to cool and developed in a solvent system, toluene: ethyl acetate: formic acid (6:3:1, v/v/v). After separation, the compounds were examined for fluorescence under long wavelength ultraviolet light and compared against the Rf value of standard OTA and further confirmed by chemical tests.
2.7. Extraction and cleanup of OTA in feed samples
For the extraction of OTA, 25 g well grounded feed sample was taken from sample stored under different RH condition and analysed for the OTA detection. The extraction of OTA was carried out by using immuno affinity columns (LC-TECH, USA) and extracted with 250 ml of methanol: water (60:40) using high speed blending for 10 min. The extracts were filtered through Whatman filter paper No.42 and an equal volume of chloroform (1:1, v/v) was added to the filtrates. The filtrates were passed through OTA cleanup immuno affinity columns for recovery of OTA. One ml of final elute was collected in methanol and used for HPLC analysis.
2.8. Quantification of ochratoxin A by RP-HPLC
Liquid chromatographic (LC) analysis of OTA, using JASCO-975 (Japan), Luna C-18 isocratic reverse phase column (250 × 4.6 mm internal diameter, 5 μM particle size) by injecting 20 μl of extract was run in acetonitrile:water:acetic acid (99:99:2) as mobile phase at flow rate of 1 ml/min under UV detection at 333 nm. Calibration curve made using standard OTA was used to estimate the amount of OTA by plotting the values on to a standard curve.
2.9. Biochemical changes in poultry feed
The biochemical change in feed was estimated by standard method of protein (Lowry, Rosebrough, Farr, & Randall, Citation1951), reducing sugars (Plummer, Citation1971), total phenol (Bray & Thorpe, Citation1954) and free amino acids (More & Stein, Citation1948).
3. Results and discussion
3.1. Isolation of Penicillium species from poultry feed
The collected poultry fed sample supported Aspergillus, Penicillium, Fusarium and other genera. In the present study all the relative humidity container during entire period of storage revealed the presence of 16 Penicillium species with different percentage of incidence (Table ). Relative humidity at 30% supported ten species of Penicillium namely Penicillium brevicompactum, Penicillium camemberti, Penicillium caseifulvum, P. chrysogenum, P. citrinum, Penicillium discolor, P. expansum, Penicillium nalgiovense, P. rubrum and P. verrucosum could be detected. The highest percentage of incidence was recorded with P. chrysogenum followed by P. citrinum, P. expansum and P. rubrum. The least percentage of incidence was obtained with P. discolor, P. nalgiovense, Penicillium olsonii and Penicillium italicum. The rest of the species of Penicillium found to be intermediate in their prevalence. Fungal count is an indicator of quality of feeds and should not exceed 1 × 105 CFU/g (Good Manufacturing Practice, Citation2008). P. verrucosum contamination of more than 103 CFU/g indicates a high probability of OTA biosynthesis (Lindblad, Johnsson, Jonsson, Lindqvist, & Olsen, Citation2004). It was found that fungal propagulates were helpful indicators to determine a feed hygienic quality. Mixed poultry feed was infested by variety of moulds which may be attributed to raw materials used (Okoli, Nweke, Okoli, & Opara, Citation2006).
Table 1. Effect of relative humidity on biodeterioration of poultry feed by Penicillium species
In relation to Penicillium species, P. chrysogenum followed by P. citrinum, Penicillium commune, P. rubrum, P. verrucosum and Penicillium roqueforti were the most frequent fungi (Accensi, Abarca, & Cabañes, Citation2004; Mariana, María, Silvia, Alejandro, & Graciela, Citation2014). The feed samples supports A. flavus and P. citrinum showed the highest isolation frequency (Pitt & Hocking, Citation1997). Various factors influence the bio-deterioration of foods and feeds under storage. Moisture content, temperature and relative humidity of storage place are of primary importance in mould infestation. Humid conditions stimulate the sporulation and increase the inoculum of the fungus and moisture content of the seed which are ultimately responsible for the deterioration of feeds and mycotoxin production (Egal et al., Citation2005). The OTA producing species of Penicillium was not only increased with increasing relative humidity but also toxigenic strains, at this condition, produce high amount of OTA. Biodeterioration of feed occurred with increasing fungal load and also increased RH storage atmosphere. Interestingly, humidity of 30 and 50% not supported the nearly one-third of the species, but with increasing the humidity conditions supported the increasing the number of species with varying percentage of prevalence. In relative humidity 90 and 100% studied were not only supported 15 species of Penicillium, but also their occurrence (frequency) was high as compared with other humidities and were collectively contributed 48–50%. The rest of the 50% was occupied with other fungal genera and complete deterioration of feed was observed at RH 100% (Lozada, Citation1995). Many studies have proved that most poultry feeds have Aspergillus and Penicillium genera as predominant flora (Al-Fifi, Abd El-Ghany, Al Abboud, Alawlaqi, & Shater, Citation2016; Bragulat, Abarca, Castellá, & Cabañes, Citation1995; Zimmerli & Dick, Citation1996).
3.2. Biochemical changes in poultry feed
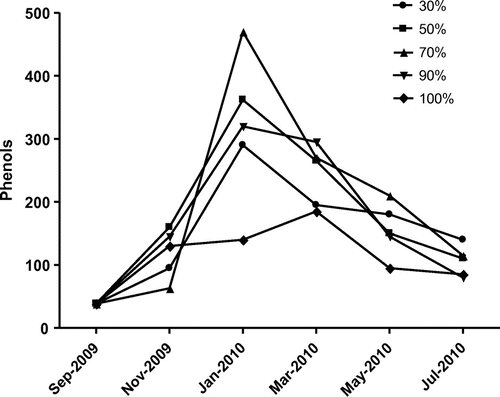
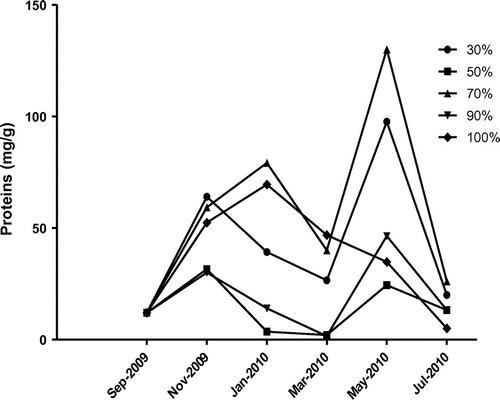
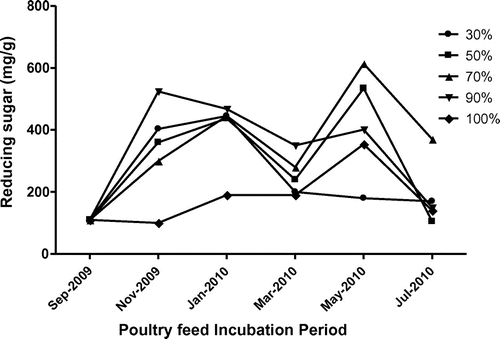
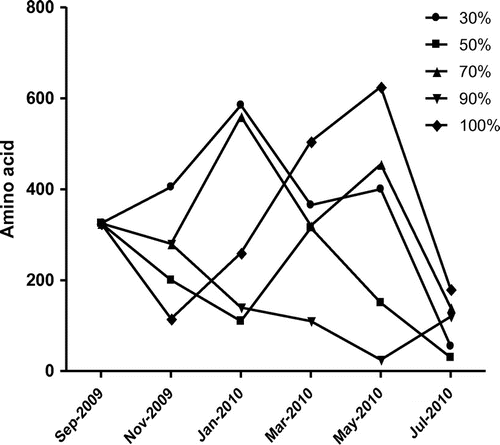
Analysis of biochemical content of the poultry feed such as phenols, proteins, reducing sugars and free amino acid were also analysed and presented in Figure –. The collected poultry feed contained 38.5 μg/g of phenols and showed increasing trend up to fourth month and decreased subsequently further storage period. The highest phenol content was observed at feed stored under RH at 70% (Figure ). The highest protein content of poultry feed was observed at RH 30 and 50% in November 2009, decreased further incubation period and again decreased (Figure ). The rest of the humidity conditions showed increasing trend up to fourth month, decreasing subsequently. The reducing sugars increased and sharply decreased with advance of storage and resulted maximum at RH 70 and 90% on second month of storage period, decreased due to the complete deterioration of feed by fungi with increasing humidity (Figure ). The highest free amino acid content at RH 90 and 100%, gradually increased and decreased with advances of storage under humidity (Figure ).
The deterioration of feed by fungus and a significant change in nutritive values were observed. This consisted in an increase in free amino acids and reducing sugars and decrease in protein content due to deteriorative activity of fungus present in feed. A positive correlation between decreased germinability and increased free amino acid (FAA) with increasing moisture content was observed (Iqbal, Shahzad basra, & Rahman, Citation2002). Reducing sugars in feed was maximum at RH 70, 90 and 100%, when compared to feeds incubated at other relative humidity. The protein content of infested feeds changed marginally. However, at RH 90%, the protein content of the feed increased significantly. The increase in protein content under the influence of feed-borne fungi may be attributed to the accumulation of fungal proteins or due to increased protein synthesis during host-pathogen interaction (Amusa, Ashaye, Amadi, & Oladapo, Citation2006; Onifade & Jeff-Agboola, Citation2002).
3.3. Mycotoxins analysis by TLC/HPTLC
Toxin chemotyping and chemical analysis by TLC/HPLC revealed that Penicillium species had the ability to produce one or other type of mycotoxins such as OTA, citrinin, mycophenolic acid, patulin, penitremA, rubratoxinB, cyclopiazonic and roquefortine previously reported (Koteswara Rao et al., Citation2011; Koteswara Rao, Girisham, & Reddy, Citation2016). The characterization of OTA showed blue colour band under UV fluorescence, Rf value compared with the standard OTA and chemical tests previously reported (Koteswara Rao, Ramana, Girisham, & Reddy, Citation2013).
3.4. Evaluation of ochratoxin A production in feed sample
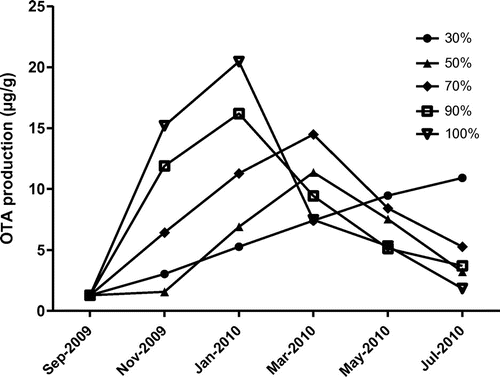
The collected poultry feed sample had 1.29 μg/g OTA quantified by HPLC at the time of sample collection without going any prior incubation. The OTA production not only increased with increasing storage period, but also with increasing humid atmosphere (Figure ). However, in feed stored under RH at 50 and 70% the amount of OTA increased gradually and reached maximum of 11.37 and 14.48 μg/g respectively on sixth month of storage and gradually decreased with further incremental storage period. The feed stored under RH 90 and 100% reached maximum amount of 16.19 and 20.45 μg/g on fourth month of storage period and decreased at final storage period. The relative humidity not only supported fungal load but also OTA production in the feed. The biodegradation of poultry feed by the fungi was observed, it may be due to some antimycotic compounds produced by fungi (Abrunhosa, Serra, & Venâncio, Citation2002). Significant differences were observed in production not only between occurrences of different species but also among relative humidities (Milani, Citation2013). Ochratoxin A concentration in feed, once reached the maximum level, decrease slowly with further incubation period due to metabolites produced by the co-existing fungi (Bejaoui, Mathieu, Taillandier, & Lebrihi, Citation2006).
4. Conclusion
In this study we conclude that the influence of different relative humidity conditions on incidence of mycotoxigenic Penicillium and biochemical changes favor the OTA production. The other environmental factors such as temperature, water activity and prolonged incubation periods will also be needed in order to access real level of OTA production and quantified in regular interval, and to evaluate the P. verrucosum and P. nordicum contamination in broiler feeds and co-occurrence of OTA and other mycotoxins in feed samples and their biodeterioration.
Acknowledgement
This Research has been supported by the Head Department of Microbiology, Kakatiya University for which authors are grateful.
Additional information
Funding
Notes on contributors
V. Koteswara Rao
Dr V. Koteswara Rao, Post Doctoral Research Fellow at the Department of Microbiology. His research field is mycotoxins. He worked as JRF during 2009–2011 and SRF during 2011–2012. He Awarded the Doctor of Philosophy in Department of Microbiology, Kakatiya University in 2012. He has published more than 22 peer-reviewed research papers in reputed National and International Journals. He is editorial member and reviewer in many journals including International Journal of Pharma and Biosciences. He has expertise in fungal biology, biotechnology, toxicology, molecular biology, bio-nanotechnology, and bio-informatics. He is a co-author of book Taxonomy of Mycotoxigenic Fungi.
References
- Abrunhosa, L., Serra, R., & Venâncio, A. (2002). Biodegradation of ochratoxin A by fungi isolated from grapes. Journal of Agricultural and Food Chemistry, 50, 7493–7496.10.1021/jf025747i
- Accensi, F., Abarca, M. L., & Cabañes, F. J. (2004). Occurrence of Aspergillus species in mixed feeds and component raw materials and their ability to produce ochratoxin A. Food Microbiology, 21, 623–627.10.1016/j.fm.2003.12.003
- Al-Fifi, Z. E., Abd El-Ghany, T. M., Al Abboud, M. A., Alawlaqi, M. M., & Shater, A.-R. M. (2016). Save Strategies for Controlling of Fungal Growth and their Mycotoxins in Poultry Feeds. Journal of Biological and Chemical Research, 33, 72–84.
- Amusa, N. A., Ashaye, O. A., Amadi, J., & Oladapo, O. (2006). Guava fruit anthracnose and the effects on its nutritional and market values in Ibadan, Nigeria. Journal of Applied Sciences, 6, 539–542.
- Anjum, M. A., Sahota, A. W., Akram, M., & Ali, I. (2011). Prevalence of mycotoxins in poultry feeds and feed ingredients in Punjab (Pakistan). The Journal of Animal and Plant Sciences, 2, 117–120.
- Bejaoui, H., Mathieu, F., Taillandier, P., & Lebrihi, A. (2006). Biodegradation of ochratoxin A by Aspergillus section Nigri species isolated from French grapes: A potential means of ochratoxin A decontamination in grape juices and musts. FEMS Microbiology Letters, 255, 203–208.10.1111/fml.2006.255.issue-2
- Bragulat, M. R., Abarca, M. L., Castellá, G., & Cabañes, F. J. (1995). A mycological survey on mixed poultry feeds and mixed rabbit feeds. Journal of the Science of Food and Agriculture, 67, 215–220.10.1002/(ISSN)1097-0010
- Bray, H. G., & Thorpe, W. V. (1954). Analysis of phenolic compounds of interest in metabolism. Methods of Biochemical Analysis, 1, 27–52.
- Buxton, P. A., & Mellanby, K. (1934). The measurement and control of humidity. Bulletin of Entomological Research, 25, 171–175.10.1017/S0007485300012608
- Council for Agricultural Science and Technology. (2003). Mycotoxins: Risks in plant, animal and human systems (Tech. Rep. 139). Ames, IA: Task Force.
- Creppy, E. E. (1999). Human ochratoxicosis. Journal of Toxicology: Toxin Reviews, 18, 277–293.
- Egal, S., Hounsa, A., Gong, Y. Y., Turner, P. C., Wild, C. P., Hall, A. J., ... Cardwell, K. F. (2005). Dietary exposure to aflatoxin from maize and groundnut in young children from Benin and Togo, West Africa. International Journal of Food Microbiology, 104, 215–224.10.1016/j.ijfoodmicro.2005.03.004
- Fareed, G., Hassan Khan, S., Anjum Ashraf, M., & Naveed, A. (2014). Determination of aflatoxin and ochratoxin in poultry feed ingredients and finished feed in humid semi-tropical environment. Journal of Advanced Veterinary and Animal Research, 1, 201–207.
- Frisvad, J. C., & Samson, R. A. (2004). Polyphasic taxonomy of Penicillium subgenus Penicillium A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Studies in Mycology, 49, 1–173.
- Frisvad, J. C., Smedsgaard, J., Larsen, T. O., & Samson, R. A. (2004). Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Studies in Mycology, 49, 201–242.
- Girisham, S., Koteswara Rao, V., & Reddy S. M. (2016). Taxonomy of mycotoxigenic fungi. Jodhpur: Scientific Publishers. ISBN: 978-81-7233.
- Good Manufacturing Practice. (2008). Certification scheme animal feed sector 2006, Appendix 1. Product standards regulations on product standards in the animal feed sector. GMP, 14, 1–39.
- Hussein, H., & Brasel, J. M. (2001). Toxicity, metabolism and impact of mycotoxins on humans and animals. Toxicology, 167, 101–134.10.1016/S0300-483X(01)00471-1
- Iqbal, N., Shahzad basra, M. A., & Rahman, K. (2002). Evaluation of vigour and oil quality in cotton seed during accelerated ageing. International Journal of Agriculture and Biology, 4, 310–322.
- Khan, S. H., Shamsul, H., Rozina, S., & Muhammad, A. A. (2011). Occurrence of aflatoxin B1 in poultry feed and feed ingredients in Pakistan. International Journal for Agro Veterinary and Medical Sciences, 5, 30–42.10.5455/ijavms.
- Koteswara Rao, V., Girisham, S., & Reddy, S. M. (2016). Prevalence of toxigenic Penicillium species associated with poultry house in Telangana, India. Archives of Environmental and Occupational Health, 71, 1–9.10.1080/19338244.2016.1140627
- Koteswara Rao, V., Ramana, M., Girisham, S., & Reddy, S. M. (2013). Culture media and factors influencing ochratoxin A production by two species of Penicillium isolated from poultry feeds. National Academy Science Letters, 36, 101–110.10.1007/s40009-012-0096-9
- Koteswara Rao, V., Shilpa, P., Girisham, S., & Reddy, S. M. (2011). Incidence of mycotoxigenic Penicillia in feeds of Andhra Pradesh, India. International Journal of Biotechnology and Molecular biology Research, 2(289), 46–50.
- Krogh, P., Hald, B., & Pedersen, E. J. (1973). Occurrence of ochratoxin A and citrinin in cereals associated with mycotoxic porcine nephropathy. Acta Pathologica Microbiologica Scandinavica Section B Microbiology and Immunology, 81, 689–695.
- Lindblad, M., Johnsson, P., Jonsson, N., Lindqvist, R., & Olsen, M. (2004). Predicting noncompliant levels of ochratoxin A in cereal grain from Penicillium verrucosum counts. Journal of Applied Microbiology, 97, 609–616.10.1111/jam.2004.97.issue-3
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin-phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
- Lozada, A. F. (1995). Isolation and identification of mycotoxigenic fungi in selected foods and feeds. Food Additives & Contaminants, 3, 509–514.
- Mariana, V. G., María, L. F., Silvia, L. R. G., Alejandro, G. P., & Graciela, N. P. (2014). Mycotoxins and mycotoxigenic fungi in poultry feed for food-producing animals. The Scientific World Journal, 1–9. doi:10.1155/2014/968215
- Milani, J. M. (2013). Ecological conditions affecting mycotoxin production in cereals: A review. Veterinarni Medicina, 58, 405–411.
- More, S., & Stein, W. H. (1948). Photometric method for use in the chromatography of amino acids. The Journal of Biological Chemistry, 176, 367–388.
- Nesci, A., Ferrari, L., & Etcheverry, M. (2008). Effect of synthetic antioxidants on stored maize grain mycoflora in situ. Journal of the Science of Food and Agriculture, 88, 797–804.10.1002/(ISSN)1097-0010
- Okoli, I. C., Nweke, C. U., Okoli, C. G., & Opara, M. N. (2006). Assessment of the mycoflora of commercial poultry feeds sold in the humid tropical environment of Imo State, Nigeria. International Journal of Environmental Science and Technology., 3, 9–14.10.1007/BF03325902
- Onifade, A. K., & Jeff-Agboola, Y. A. (2002). Effect of fungal infection on proximate nutrient composition of coconut (Cocos nucifera Linn) fruit. Journal of Agricultural and Environmental, 2, 141–142.
- Pitt, J. I., & Hocking, A. D. (Eds.). (1997). Fungi and food spoilage (2nd ed.). London: Blackie Academic & Professional.
- Plummer, D. T. (1971). An introduction to practical biochemistry. New Delhi: Tata Mc Graw-Hill.
- Samson, R. A., & Pitt, J. I. (2000). Integration of modern methods for Penicillium and Aspergillus (p. 510). Amsterdam: Harwood Academic Publishers.
- Walker, R. (1999). Risk analysis of mycotoxins by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Presented at the 3rd Joint FAO/UNEP International Conference on Mycotoxins, Tunis, March 3-6, 1999.
- Zimmerli, B., & Dick, R. (1996). Ochratoxin A in table wines and grape juices occurrence and risk assessment. Food Additives and Contaminants, 13, 655–668.10.1080/02652039609374451