Abstract
In Nigeria, fodder plants are important components of ruminant feeding systems. The wide arrays of secondary metabolites in them are potential source of antibacterial materials; if harnessed, could serve as alternative to conventional antibiotics. This study was designed to evaluate the phytochemical and antibacterial properties of acetone and ethanol extracts of Ficus polita, Azadirachta indica, and Vernonia amygdalina leaves against selected bacterial species by Agar well diffusion and broth micro dilution methods. Phytochemical screening showed that concentration of phytochemicals differed significantly (p < 0.05) among the leaf extracts. Alkaloids (13.04–15.74 mg/100 g) were highest in all the fodder plants. All the leaf extracts were more effective against Staphylococcus aureus and Pseudomonas aeruginosa with zone of inhibition ranging from 12.06–28.00 mm and 10.50–23.00 mm respectively. In terms of sensitivity, the reference antibiotic (gentamycin) showed more activity against Escherichia coli and Salmonella typhi than leaf extracts. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values ranged from 0.14 to 0.35 mg/mL and 0.60 to 1.90 mg/mL respectively. The lower range of MIC and MBC values translates to high antibacterial potency of the plant extracts; an indication of broad spectrum of activity against all the tested bacterial species.
Public Interest Statement
Indiscriminate use of synthetic antibiotics in livestock industry has been reported to be responsible for treatment failure of infectious animal diseases due to development of resistant bacteria strains; while antibiotic residues in animal products are issues of risks among health conscious consumers. There is need to research on fodder plants as alternative antibacterial agents without residual effect on animals and consumers. Ficus polita, Azadirachta indica and Vernonia amygdalina was evaluated for phytochemical and antibacterial properties against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella typhi which causes economic losses to livestock farmers. Findings revealed higher concentration of alkaloids than other phytochemicals. Acetone and ethanol leaf extracts were more effective against S. aureus, and P. aeruginosa than gentamycin except for E. coli and S. typhi. Nonetheless, all the leaf extracts inhibited bacterial growth. These fodder plants are readily available, hence, would serve as are potential antibacterial agents against bacterial pathogens of ruminant diseases.
Competing Interests
The authors declare no competing interest.
1. Introduction
In Nigeria, ruminants are a major provider of animal protein (meat and milk) that is utilized for human consumption. Among the Fulani herdsmen, it represents a reserve of family wealth and mark of respectability. Apart from being a source of income and insurance to small holder livestock producers in times of financial crisis, it also contributes to the Gross Domestic Products of the country. However, increased ruminant production in Nigeria is constrained amongst others by: inadequate nutrition, poor management practices and health challenges. The consequences of these problems include production and economic losses, while production of unhealthy products (meat and milk) from infected animal causes serious health risk to consumers. This is not far-fetched, because many of the illnesses and death of humans has been reported to be attributed to foodborne diseases associated with pathogenic bacterial and fungal species (Zare et al., Citation2014).
Intervention measures at the farm levels against these challenges have been through improved nutrition by the use of non-conventional feedstuffs with high crude protein and total digestible nutrients. Recently, herbs or plant extracts are included in animal feed, either as growth promoters or antibiotics (Tipu, Akhtar, Anjum, & Raja, Citation2006) because frequent and indiscriminate use of synthetic antibiotics in livestock industry have resulted to development of resistant bacteria strains to commonly used antimicrobials (Owens, Ray, Watts, & Yancey, Citation1997) which is assumed to be responsible for treatment failure of infectious diseases. In addition, the residues of antibiotics in meat and milk products are issues of health concern among the consumers due to emergence of multiple drug resistance pathogens in human beings (Virdis et al., Citation2010).
Since the production and management of ruminants is largely concentrated in traditional hands in rural communities (Ajala, Citation1995), the concept of Ethnoveterinary Medicine (EVM) have become a common traditional practice among the smallholder livestock farmers where veterinary services is expensive (Nnadi, Umunakwe, Nnadi, & Okafor, Citation2012). Ethnoveterinary survey of some medicinal plants that are used in Nigeria and sub-Saharan Africa for treatment of ruminant diseases have been reported (McGaw & Eloff, Citation2008; Olanipekun & Tedela, Citation2013). Phytochemical screening of plant secondary metabolites and detection of antimicrobial properties of medicinal plants have also been documented (Compean & Ynalez, Citation2014); while the active principles, mechanism of actions and quantities required of some of these plant extracts for ruminant health care have also been reported (Alawa, Jokthan, & Akut, Citation2002; Rochfort, Parker, & Dunshea, Citation2008).
Ficus polita, also called Fig tree, is one of the Ficus species belonging to the family Moraceae. They are often distinguished by their characteristic root growing from the branches (Keay, Onochie, & Standfied, Citation1964) and a common homestead tree in Nigeria grown to provide shade around houses. The leaves are consumed by small ruminants during the dry season to supplement low quality roughages (Ndamitso, Jacob, Idris, & Jimoh, Citation2010).
Azadirachta indica, commonly known as Neem tree belongs to the family Meliaceae. It is widely cultivated and well adapted in drier and humid ecological zones with an estimation of four million trees in North-West Zone of Nigeria (Anon, Citation2010). They are used in afforestation programme for wind breaks and in agro-pastoral system to provide shade and fodder for livestock. Calves fed neem leaf supplement showed increased total feed intake and weight gain (Kudke, Kalaskar, & Nimbalka, Citation1999).
Vernonia amygdalina (family; compositaea), is a shrub of the savannah and forest areas in tropical Africa (Burkill, Citation1985). In Nigeria, it is a homestead vegetable plant called bitter leaf, and a fodder often browsed by small ruminants. The leaf meal improved weight gain in sheep (Firisa, Adunga, & Diriba, Citation2013) and have been used against parasitic infections of the gastrointestinal tract (Nalule, Mbaria, & Kimenju, Citation2011). Studies have also shown that extracts of these plants contain array of active biochemical compounds which has found relevance in animal nutrition and ethno veterinary medicines (Herre, Jandér, & Machado, Citation2008; Kausik, Ishita, Ranajit, & Uday, Citation2002; Nwanjo, Citation2005).
As a result of various findings from antibiotic resistance to bacterial infections, its use in livestock health management in the future is still uncertain. Hence, this study was conducted to evaluate quantitatively the phytochemical and antibacterial properties of F. polita, A. indica, and V. amygdalina against selected bacterial species of ruminant diseases.
2. Materials and methods
2.1. Collection of plant materials
Leaves from the trees of F. polita, A. indica, and V. amygdalina shrub were collected in the paddock of Institute of Agricultural Research and Training, Moor Plantation, Ibadan (Nigeria). The leaves were plucked from healthy and uninfected plants at 8–10 weeks of regrowth and washed thoroughly under a running tap water to remove dust and other foreign particles. Leaves were finally rinsed with sterile distilled water and thereafter, air dried under shade.
2.2. Phytochemical analysis
Phytochemical screening and identification of bioactive constituents in the plants under study were carried out according to standard procedures as described in Talukdar, Choudhary, Chakraborty, and Dutta (Citation2010). One hundred (100) grams of fresh leaves (F. polita, A. indica, and V. amygdalina) were boiled with 200 ml of solvents (n-hexane) for 1 h. The extracts obtained were filtered through Whatman filter paper No. 1, vacuum dried (on water bath at 40–50°C) using a rotary evaporator and stored at 0–4°C in air-tight containers for further use.
2.3. Quantitative determination of phytochemicals
Phytochemicals in the crude extracts were quantified according to the following methods: Phenols (McDonald, Prenzler, Antolovich, & Robards, Citation2001), Alkaloids (Harbone, Citation1973), Flavonoids (Chang, Yang, Wen, & Chem, Citation2002), Steroids (Edeoga, Okwu, & Mbaebie, Citation2005), Terpenenoids (Ferguson, Citation1956), Tannins (Dawra, Makkar, & Singh, Citation1988), Saponin (Brunner, Citation1984), Glycosides (Ferguson, Citation1956), Trypsin Inhibitor (Liener, Citation1979), Oxalates (Fasset, Citation1996), and Phytates (Maga, Citation1983).
2.4. Preparation of leaf extracts (acetone and ethanol)
One hundred (100) grams each of F. polita, A. indica, and V. amygdalina leaves were ground separately into fine powder using a stainless steel grinder. Two hundred (200) ml each of 100% ethanol and acetone was added to each leaf sample and kept for 24 h with periodic shaking (Puri, Citation1999). Ethanol and acetone fractions were separated by using sterile muslin cloth and then filtered through sterile Whatman filter paper (No. 2). The filtrates were later concentrated using Rotary film evaporator, collected and pooled.
2.5. Source of test organisms
The test organisms (Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Salmonella typhi) were obtained from the stock culture of Microbiology Laboratory, Institute of Agricultural Research and Training, Moor Plantation Ibadan, Nigeria.
2.6. Standardization of microorganisms
Culture was standardized as described by NCCLS (Citation2002). Zero point two (0.2) ml of an 18 h old culture of each bacterium was suspended into sterile universal bottles containing 20 ml nutrient broth and incubated for 5 h at 37°C to obtain a logarithm growth phase. Normal saline was gradually added to compare its turbidity with McFarland Standard of 0.5 which correspond to approximately 1.0 × 108 CFU/ml as recommended by World Health Organization.
2.7. Antibacterial activity
Antibacterial activity of leaf extracts was determined by the standard agar well diffusion method against E. coli, S. aureus, P. aeruginosa and S. typhi. Mueller Hinton agar was prepared to obtain a standard bacterial stock suspension by mixing with crude extract (30 mg) of acetone and ethanol dissolved in 1 ml of DMSO (Di-methyl Sulphoxide). Wells were made in each Mueller Hinton agar plates using sterile cork borer (5.0 mm diameter). Thereafter, 1 ml of each plant extracts was introduced into the different wells; and same volume of the respective medium was mixed with Gentamycin (control) and filled into the wells with micropipette. The plates were kept for 6 h after which they were incubated for 24 h at 37°C. After incubation, the antibacterial activity of each plant extract and the control were assessed by measuring the zone of inhibition using a meter rule.
2.8. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The minimum inhibitory concentration (MIC) of the leaf extract against the test organisms was determined by broth micro dilution method. Twenty percent (20%) of the extracts were prepared. Zero point one (0.1) ml of each extract was put in test tubes and 2 ml of the nutrient broth was added. Zero point one (0.1) ml of the standard test organisms was also set up by using solvents and test organisms without extracts (Gentamycin). The tubes containing bacteria cultures were incubated at 37°C for 24 h. Thereafter, the tubes were examined for microbial growth by observing the turbidity. The tube with the least concentration of extract which inhibited the growth of the respective organism after incubation was recorded as the MIC. Minimum bactericidal concentration (MBC) was determined from each of the test tubes in the MIC determination. A loopful of broth was collected from those tubes that did not show any growth and inoculated onto a sterile nutrient agar by streaking. Nutrient agar plates were also streaked with the respective test organism to serve as controls. All the plates were incubated as previously stated and the concentration with no visible growth was inferred to be the MBC.
2.9. Statistical analysis
Data obtained for phytochemical constituents were analyzed using analysis of variance procedures of SAS (SAS, Citation1998) and descriptive statistics for antibacterial properties of leaf extracts on all the test organisms. Means, where significant, were separated by Duncan Multiple range Tests at level of 5%.
3. Results and discussion
3.1. Phytochemicals and concentrations of leaf extract of plants
The phytochemicals and concentrations of F. polita, A. indica and V. amygdalina (Table ) was similar with documented reports (Abdel-Hameed, Citation2009; Aslam, Rehman, Asghar, & Sarwar, Citation2009; Eleyinmi, Sporns, & Bressler, Citation2008) demonstrating that plants contain a plethora of secondary metabolites. The levels of tannin (0.345–0.414 mg/100 g) in this study were below the range (5–9%) that may affect rumen function and gastrointestinal tract utilization of nitrogen (Okoli, Anunobi, Obua, & Enemuo, Citation2003). Tannin interferes with the digestive processes by binding with enzymes or feed components such as proteins or minerals (Liener, Citation1989). Saponins are foam-producing compounds occurring in wide range of plants, which when ingested produce some toxic effects, alter cell wall permeability and affect absorption of nutrients across the intestinal wall (Belmar, Nava-Montero, Sandoval-Castro, & McNab, Citation1999). The range of saponin (0.674–0.682 mg/100 g) obtained in these leaves is lower than the 7.94% (F. polita) reported not to be harmful to ruminants (Ndamitso et al., Citation2010).
Table 1. Phytochemical constituents of leaf extract of Ficus polita, Azadirachta indica, and Vernonia amygdalina
Quantitatively, concentrations of each phytochemical in this study differed significantly (p < 0.05) among the leaf extracts as reported (Bamikole, Ikhatua, Arigbede, Babayemi, & Etela, Citation2004). The values for alkaloids (15.740 mg/100 g), saponin (0.682 mg/100 g), glycosides (0.573 mg/100 g), tannin (0.414 mg/100 g) and phenol (0.320 mg/100 g) in A. indica was highest compared to those of F. polita and V. amygdalina; which is similar with the study of Aslam et al. (Citation2009) in leaf extracts of A. indica. Higher proportion of alkaloid (13.04–15.74 mg/100 g) recorded in all the fodder plants in this study is in contrast to the alkaloid contents (2.83 mg/100 g and 6.32 mg/100 g) reported (Onyeonagu, Obute, & Eze, Citation2013) for grasses and legumes respectively.
Glycosides are cytochrome oxidase inhibitors which interferes with aerobic respiratory system. The mean values of glycosides (0.365–0.573 mg/100 g) recorded in this study was low compared to the 5.0% reported (Aslam et al., Citation2009) for neem. The concentrations of Oxalate (0.138–0.185 mg/100 g) and Phytate (0.118–0.213 mg/100 g) in this study were low. The values of oxalate were lower than 0.23–0.71 g/100 g reported for five species of Ficus (Bamikole et al., Citation2004); 95.5 mg/100 g for V. amygdalina (Alabi, Oyero, Jimoh, & Amusa, Citation2005) and 5.02–8.15 mg/100 g for some semi-arid browses in North Eastern Nigeria (Njidda, Citation2010). The values of phytate (0.118–0.213 mg/100 g) obtained in this study falls below the range (0.13–0.39 g/100 g) reported for Ficus species (Bamikole et al., Citation2004). None of these Phytochemicals could pose any threat to ruminants.
3.2. Antibacterial activity of acetone and ethanol extracts (F. polita, A. indica, and V. amygdalina)
The results of antibacterial activities of F. polita, A. indica, and V. amygdalina (Figures ) showed the responses of selected bacterial species to varying solvent of extraction compared with the control (gentamycin) as recorded from the zones of inhibition (mm). Acetone and ethanol extracts of F. polita, A. indica, and V. amygdalina leaves had considerable antibacterial activities (p < 0.05) with zones of inhibition (4.50–8.00 mm) against E. coli (Figure ). Acetone extract of V. amygdalina exhibited good activity while ethanol extract of F. polita showed the least activity against E. coli compared to the control (gentamycin) with highest zone of inhibition (9.96 mm). Acetone and ethanol extracts of A. indica demonstrated greater activity (p < 0.05) with zones of inhibition of 28.00 and 21.00 mm against S. aureus (Figure ). The zones of inhibition of leaf extracts (acetone and ethanol) against S. aureus ranged from 15.00–28.00 and 12.06–21.00 mm respectively. Leaf extracts of A. indica and V. amygdalina also showed significant (p ˂ 0.05) activity against P. aeruginosa (23.00 and 21.50 mm and 20.00 and 17.00 mm, respectively), compared to gentamycin (9.97 mm) as presented in Figure . Gentamycin showed maximum zone of inhibition (9.98 mm) against S. typhi (Figure ), while acetone and ethanol extracts of F. polita and A. indica were similar (p > 0.05) with minimum zones of inhibition (4.8 and 5.05 mm; 3.00 and 4.1 mm, respectively).
Figure 1. Antibacterial activity of leaf against Escherichia coli AC = acetone, ET = ethanol, and GE = gentamycin.
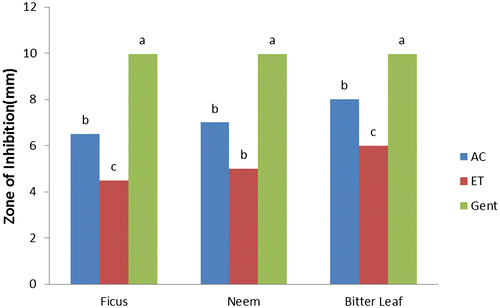
Figure 2. Antibacterial activity of leaf against Staphylococcus aureus AC = acetone, ET = ethanol, and GE = gentamycin.
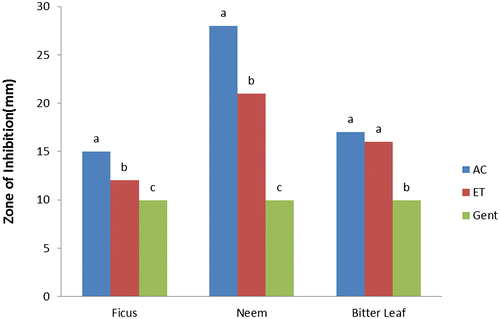
Figure 3. Antibacterial activity of leaf against Pseudomonas aeruginosa AC = acetone, ET = ethanol, and GE = gentamycin.
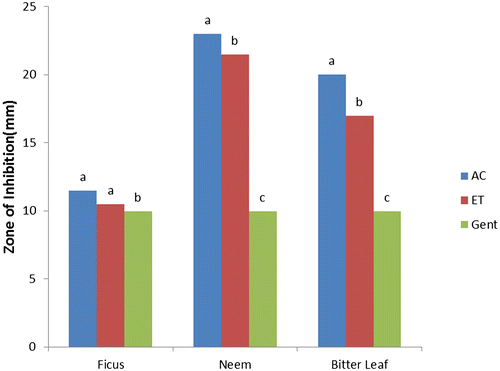
Figure 4. Antibacterial activity of leaf against Salmonella typhi AC = acetone, ET = ethanol, and GE = gentamycin.
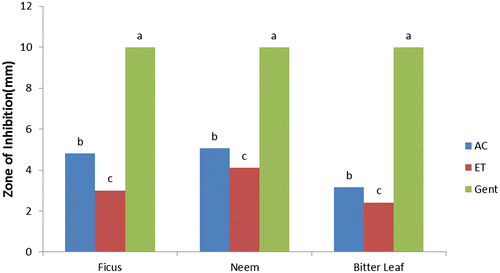
It is evident from this study that acetone and ethanol extracts of F. polita, A. indica and V. amygdalina produced strong antibacterial activities against all the tested bacterial species. This is similar with the findings of several authors (Koona & Budida, Citation2011; Opara, Egbuobi, Ndudim, Onyewuchi, & Nnodim, Citation2014; Shamila, Jeeva, Sheela, Brindha, & Leksinmi, Citation2012) for leaf extracts of A. indica, Ficus spp. and V. amygdalina, respectively, against some gram positive and gram negative bacterial species. From the trend of antibacterial properties, leaf extracts of these plants were more effective against S. aureus and P. aeruginosa in the following trend: A. indica > V. amygdalina > F. polita compared with the control. In terms of sensitivity, the reference antibiotic (gentamycin) showed more sensitivity against E. coli and S. typhi than the plant extracts.
The remarkable activities of leaf extracts having higher values of zones of inhibition against S. aureus and P. aeruginosa than gentamycin conforms with Koona and Budida (Citation2011) as reported for some medicinal plants and antibiotics against bacteria species. Comparatively, antibacterial activity of acetone extracts of A. indica, and V. amygdalina is “strong” against S. aureus and P. aeruginosa, “moderate” against E. coli but “weak” against S. typhi. While ethanol extract of F. polita is “moderate” against S. aureus and P. aeruginosa but “weak” against E. coli and S. typhi. However, both acetone and ethanol extracts of V. amygdalina and F. polita exhibited weak activity against S. typhi compared with the control (gentamycin). The differences in zones of inhibition exhibited by the leaf extracts against the tested bacteria species could be attributed to; varying concentrations of plant bioactive components and intrinsic tolerance of the bacterial as reported (Suree & Pana, Citation2005); differences in polarity of solvent of extraction (Ncube, Afolayan, & Okoh, Citation2008), which invariably could influence the solubility of the antimicrobial bioactive compounds in the plants.
3.3. MIC and MBC of leaf extracts
Tables and show the MIC and MBC of F. polita, A. indica and V. amygdalina for E. coli, S. aureus, P. aeruginosa and S. typhi. The MIC values for all the organisms tested ranged from 0.14 to 0.35 mg/mL. E. coli has MIC of 0.20–0.27 mg/mL and 0.22–0.35 mg/mL; others are S. aureus (0.20–0.25 and 0.21–0.29 mg/mL), P. aeruginosa (0.25–0.32 mg/mL) and S. typhi (0.18 and 0.14–0.25 mg/mL) for acetone and ethanol extracts of F. polita, A. indica and V. amygdalina respectively. The lowest MIC values (0.18 and 0.14 mg/mL) were obtained in acetone and ethanol extract of V. amygdalina against S. typhi. However, the range of MIC values recorded for all leaf extracts against all the tested bacteria were higher than MIC values (0.01–0.02 mg/mL) obtained for the reference antibiotic (gentamycin, 10 μg/mL). Since extracts with MIC values below 1 mg/mL from natural products (Ríos & Recio, Citation2005) were considered to exhibit antimicrobial activity, hence, this results implies a strong antibacterial activity of the plant extracts as reported (Diaz et al., Citation2010) that a range of MIC values (0.05–0.50 mg/mL) indicated a strong activity.
Table 2. MICs of F. polita, A. indica, and V. amygdalina
Table 3. MBCs of F. polita, A. indica, and V. amygdalina
Acetone and ethanol extracts of F. polita, A. indica and V. amygdalina leaves showed remarkable activities at MBC with values ranging from 0.60 to 1.90 mg/mL compared with the reference antibiotic, gentamycin (0.01–0.02 mg/mL) against all the tested bacterial species. The bactericidal effect of F. polita leaf extracts was strong against S. aureus and S. typhi, with MBCs of 0.82 and 0.88 mg/mL and 0.70 and 0.90 mg/mL respectively, ditto, V. amygdalina with MBC of 0.60 and 0.92 mg/mL for S. typhi. However, S. aureus has MBC of 0.90 mg/mL for acetone extract of V. amygdalina. Acetone and ethanol extracts of A. indica exhibited a stronger bactericidal activity against all the organisms tested in which S. aureus has MBC of 0.64 and 0.65 mg/mL and E. coli of 0.66 and 0.70 mg/mL, while acetone extracts has MBCs of 0.62 and 0.60 mg/mL for P. aeruginosa and S. typhi respectively.
The bactericidal activity of leaf extracts is suggestive of membrane disruption and inhibition of nucleic acids, protein and phospholipid membrane biosynthesis (Arvind, Reg, & Ezno, Citation2004; Franklin, Snow, Barrett-Bee, & Nolan, Citation1987). Based on the MBC values, leaf extracts of A. indica can be considered as a stronger anti bactericidal agent compared with F. polita, and V. amygdalina; as the MBC values is no more than four times of the MIC values that is required to inhibit growth of the tested bacterial species as reported (Yamamoto, Citation2003).The lower range of MIC and MBC values (<100 mg/mL) of the plant extracts in this study translates to high antibacterial potency on all the bacterial species tested while the inhibitory activities is an indication of broad spectrum activity against gram positive and gram negative bacteria; which implies that the phytochemicals found in the plant extracts possessed broad spectrum antibiotic compounds.
Similar research findings have reported that alkaloids, even in small quantity, produced strong antimicrobial activity (Sibi, Chatly, Adhikari, & Ravikumar, Citation2012). Flavonoids displayed strong antimicrobial activity and inhibits Klebsiella pneumonia (Özçelik, Orhan, Özgen, & Ergun, Citation2008), as they form complexes with cell wall components and adhesins to prevent microbial growth (Rojas, Hernandez, Pereda-Miranda, & Mata, Citation1992). Tannins (Abdulhamid, Fakai, Sani, Argungu, & Bello, Citation2014) have been demonstrated to have antibacterial activities against S. aureus and P. aeruginosa; ditto saponin (Oboh, Citation2010) from ethanol extract of Ocimum gratissimum. The varying degree of sensitivity of the bacterial species in this study could have resulted from several contributing factors: the nature, concentrations and combined activities of secondary metabolites present in the leaf extracts, variations in plant species, organic solvent and methods of extraction and the intrinsic tolerance of the bacterial species to the various antibacterial agents being tested.
4. Conclusion
From this study, inhibition of bacterial growth is a demonstration of the antibacterial potency of acetone and ethanol extracts of F. polita, A. indica and V. amygdalina. Although A. indica demonstrated a stronger antibacterial activity compared to F. polita and V. amygdalina, however, any of these plant have the potential to be considered as an effective antibacterial agent to replace synthetic antibiotics (gentamycin) to combat bacterial infections in ruminants. More importantly, mastitis and foot and mouth diseases in dairy ruminants caused by specific bacteria species such as S. aureus and P. aeruginosa. Also, E. coli (an opportunistic pathogen) which causes bovine diarrhea and other food borne related diseases in livestock farm environment.
Funding
The authors received no direct funding for this research.
Additional information
Notes on contributors
Festus Temitope Ajayi
Festus Temitope Ajayi is a Senior Research Fellow in the Institute of Agricultural Research and Training, Ibadan, Nigeria and holds a PhD in ruminant nutrition. His research focused on nutritional evaluation of herbaceous legumes and grasses for sustainable ruminant feeding programmes, livestock health, survey and validation of ethnoveterinary practices.
Sunday Oloruntoba Omotoso
Sunday Oloruntoba Omotoso is a Junior Research Fellow in the Institute of Agricultural Research and Training, Ibadan, Nigeria. His research interest includes forage production and nutritional evaluation of fodder plants, crop residues and agro-industrial by products as feed for ruminants and antimicrobial activity of lactic acid bacteria in silage production.
Joseph Oluwafemi Odejide
Joseph Oluwafemi Odejide is a Senior Lecturer in a Department of Animal Production, Federal College of Agriculture, Ibadan, Nigeria. His research interest includes livestock health and management. Increasing demand for animal protein across Nigeria has led to rapid growth of the livestock sector; with greater reliance on synthetic antibiotics as drug or growth promoter. Therefore, there is need to ensure that safe animal products are produced for human consumption from a healthy livestock in a safe environment.
References
- Abdel-Hameed, E. S. S. (2009). Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chemistry, 114, 1271–1277.10.1016/j.foodchem.2008.11.005
- Abdulhamid, A., Fakai, I. M., Sani, I., Argungu, A. U., & Bello, F. (2014). Preliminary phytochemical and antibacterial activity of ethanolic and aqueous stem bark of extracts of Psidium guajava. American Journal Drug Discovery Development, 4, 85–89.
- Ajala, A. A. (1995). Women’s tasks in the management of goats in Southern Nigeria. Small Ruminant Research, 15, 203–208.10.1016/0921-4488(94)00031-2
- Alabi, D. A., Oyero, L. A., Jimoh, & Amusa, N. A. (2005). Fungitoxic and phytotoxic effect of Vernonia amygdalina Del., Bryophylum pinnatus Kurtz. Ocimum gratissimum (closium) L. Eucalypta globules (Caliptus) Labill water extracts on cowpea and cowpea seedlings pathogens in Ago Iwoye, South West Nigeria. World Journal Agricultural Science, 1, 70–75.
- Alawa, J. P., Jokthan, G. E., & Akut, K. (2002). Ethnoveterinary medical practice for ruminants in the subhumid zone of northern Nigeria. Preventive Veterinary Medicine, 54, 79–90.10.1016/S0167-5877(01)00273-2
- Anon. (2010). The Neem Tree. Retrieved from http://www.Theneem.co.uk/actalog/TheNeemTree.html
- Arvind, S., Reg, F. C., & Ezno, A. P. (2004). Identification of antimicrobial components of an ethanolic extract of Australian medicinal plant. Eremophila duttonii Phytother Research, 18, 615–618.
- Aslam, F., Rehman, K., Asghar, M., & Sarwar, M. (2009). Antibacterial constituent of various Phytoconstituents of Neem. Pakistan Journal of Agricultural Sciences, 46, 456–463.
- Bamikole, M. A., Ikhatua, U. J., Arigbede, O. M., Babayemi, O. J., & Etela, I. (2004). An evaluation of the acceptability as forage of some nutritive and antinutritive components and of the dry matter degradation profiles of five species of Ficus. Tropical Animal Health and Production, 36, 157–167.10.1023/B:TROP.0000012104.47814.78
- Belmar, R., Nava-Montero, R., Sandoval-Castro, C., & McNab, J. M. (1999). Jack bean (Canavalia ensiformis L. DC) in poultry diets: Antinutritional factors and detoxification studies—A review. World's Poultry Science Journal, 55, 37–59.10.1079/WPS19990004
- Brunner, J. H. (1984). Direct spectrophotometric determination of saponin. Analytical Chemistry, 42, 1752–1754.
- Burkill, H. M. (1985). The useful plant of West Tropical Africa (2nd ed., Vol. 2, p. 960). London: Royal Botanic Gardens, Kew.
- Chang, C., Yang, M., Wen, H., & Chem, J. (2002). Estimation of total flavonoids contents in plants by two complementary colorimetric methods. Journal of Food Drug Analysis, 10, 178–182.
- Compean, K. L., & Ynalez, R. A. (2014). Antimicrobial activities of plant secondary metabolites: A review. Research Journal of Medicinal Plants, 8, 204–213.
- Dawra, R. K., Makkar, H. S. P., & Singh, B. (1988). Protein-binding capacity of microquantities of tannins. Analytical Biochemistry, 170, 50–53.10.1016/0003-2697(88)90088-7
- Diaz, M. A. N., Rossi, C. C., Mendonça, V. R., Silva, D. M., Ribon, A. D. O. B., Aguilar, A. P., & Munoz, G. D. (2010). Screening of medicinal plants for antimicrobial activities on Staphylococcus aureus strains isolated from bovine mastitis. Brazilian Journal of Pharmacognosy, 20, 724–728.
- Edeoga, H. O., Okwu, D. E., & Mbaebie, B. O. (2005). Phytochemical constituents of some Nigerian medicinal plants. African Journal of Biotechnology, 4, 685–688.10.5897/AJB
- Eleyinmi, A. F., Sporns, P., & Bressler, D. C. (2008). Nutritional composition of Congronema latifolium and Vernonia amygdalina. Nutrition, Food and Science, 38, 99–109.
- Fasset, D. W. (1996). Oxalates. In Toxicants occurring naturally in foods. Washington, DC: National Academy of Science Research Council.
- Ferguson, N. M. A. (1956). Textbook of pharmacognosy (p. 191). New Delhi: McMillan Company.
- Firisa, W., Adunga, T., & Diriba, D. (2013). Feed intake, digestibility, growth performance of Horo lambs fed natural pasture hay supplemented graded levels of Vernonia amygdalina leaves and sorghum grain mixture. Science Technology and Arts Research Journal, 2, 30–37.
- Franklin, T. L., Snow, G. A., Barrett-Bee, K. J., & Nolan, R. D. (1987). Biochemistry of antimicrobial action (4th ed., Vol. 112, pp. 71–73). London, New York: Chapman and Hall.
- Harbone, J. B. (1973). Phytochemical methods: A guide to modern techniques of plant analysis (pp. 267–270). New York, NY: Chapman and Hall.
- Herre, E. A., Jandér, K. C., & Machado, C. A. (2008). Evolutionary ecology of figs and their associates: Recent progress and outstanding puzzles. Annual Review of Ecology, Evolution and Systematics, 39, 439–458.10.1146/annurev.ecolsys.37.091305.110232
- Kausik, B., Ishita, C., Ranajit, K. B., & Uday, B. (2002). Biological activity and medicinal properties of Neem (Azardirachta indica). Current Science, 82, 1336–1345.
- Keay, R. W. J., Onochie, C. F. A., & Standfied, D. P. (1964). Nigerian Trees (Vol. 11). Department of Forestry Research Ibadan: Ibadan.
- Koona, S., & Budida, S. (2011). Antibacterial potential of the extract of leaves of neem (Azardirachta indica Linn.). Nature, Science and Biology, 3, 65–69.
- Kudke, R. J., Kalaskar, S. R., & Nimbalka, R. V. (1999). Neem leaves as feed supplement for livestock. Pushudhn, 14, 12.
- Liener, I. E. (1979). Determination of anti-tryptic activity of soyabeans. Journal of Agricultural Science, 16, 602–609.
- Liener, I. E. (1989). Anti-nutritional factors in legume seeds. In J. Huisman, A. F. B. Vander Poel, & I. E. Liener (Eds.), Recent advances of research in anti-nutritional factors in legume seeds (pp. 6–14). Wageningen: Pudoc.
- Maga, J. A. (1983). Phytate: Its chemistry, occurrence, food interaction, nutritional significance and method of analysis. Journal of Agriculture Food and Chemistry, 30, 1–9.
- McDonald, S., Prenzler, P. D., Antolovich, M., & Robards, K. (2001). Phenolic content and antioxidant activity of olive extracts. Food Chemistry, 73, 73–84.10.1016/S0308-8146(00)00288-0
- McGaw, L. J. & Eloff, J. N. (2008). Ethnoveterinary use of southern African plants and scientific evaluation of their medicinal properties. Journal of Ethnopharmacology, 119, 559–574.10.1016/j.jep.2008.06.013
- Nalule, A. S., Mbaria, J. M., Olila, D., & Kimenju, J. W. (2011). Ethnopharmacological practices in management of livestock helminthes by pastoral communities in the dry lands of Uganda. Livestock Research for Rural Development, 23. Retrieved from http://www.lrrd.org/lrrd23/nalu23036.htm
- NCCLS. (2002). Performance standard for antimicrobial disk and dilution susceptibility tests for bacterial isolated from animals: Approved standard M31–A2 (2nd ed.). Wayne, PA: National Committee for Clinical Laboratory Standards.
- Ncube, N. S., Afolayan, A. J., & Okoh, A. I. (2008). Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. African Journal of Biotechnology, 7, 1797–1806.10.5897/AJB
- Ndamitso, M. M., Jacob, J. O., Idris, S., & Jimoh, T. (2010). Prospect in the use of Ficus polita as a local ruminant feed. African Journal of Biotechnology, 9, 3116–3121.
- Njidda, A. A. (2010). Chemical composition, fibre fraction, and anti-nutritional substances of semi-arid browse forages of North–Eastern Nigeria. Nigerian Journal of Basic and Applied Science, 18, 181–188.
- Nnadi, F. N., Umunakwe, P. C., Nnadi, C. D., & Okafor, A. E. (2012). Socio-economic determinants of farmers use of ethnoveterinary medicine in Mbaitoli LGA of Imo State, Nigeria. Research Journal of Agriculture, Environment and Management, 1, 25–33.
- Nwanjo, H. U. (2005). Efficiency of aqueous leaf extracts of Vernonia amygdalina on plasma lipoprotein and oxidative status of diabetic rat model. Nigerian Journal of Physiology and Science, 20, 39–42.
- Oboh, G. (2010). Antioxidant and antimicrobial properties of ethanolic extract of Ocimum gratissimum leaves. Journal of Pharmacology and Toxicology, 5, 396–402.
- Okoli, I. C., Anunobi, M. O., Obua, B. E., & Enemuo, V. (2003). Studies on selected browses of South Eastern Nigeria with particular reference to their proximate and some endogenous anti-nutritional constituents. Retrieved from http://www.irrd.org/irrd15/9/Oko1159.htm
- Olanipekun, M. K., & Tedela, P. O. (2013). Ethno-medicinal importance of some selected plants used for the treatment of ruminant animal diseases in Ekiti State, Nigeria. Journal of Agriculture and Veterinary Services, 5, 16–22.
- Onyeonagu, C. C., Obute, P. N., & Eze, S. M. (2013). Seasonal variation in the anti-nutrient and mineral components of some forage legumes and grasses. African Journal of Biotechnology, 12, 142–149.
- Opara, A. U., Egbuobi, R. C., Ndudim, J. N., Onyewuchi, C. E., & Nnodim, J. K. (2014). Antibacterial activity of Ocimum gratissimum (Nchu-Anwu) and Vernonia amygdalina (Bitter-Leaf) antibacterial activity of Ocimum gratissimum (Nchu-Anwu) and Vernonia amygdalina (Bitter-Leaf). British Biotechnology Journal, 4, 1115–1122.10.9734/BBJ
- Owens, W. A., Ray, C. H., Watts, J. L., & Yancey, R. J. (1997). Comparison of success of antibiotic therapy during lactation and results of antimicrobial susceptibility tests for bovine mastitis. Journal of Dairy Science, 80, 313–317.10.3168/jds.S0022-0302(97)75940-X
- Özçelik, B., Orhan, DD, Özgen, S., & Ergun, F. (2008). Antimicrobial activity of flavonoids against extended-spectrum β-lactamase producing (ESβL) Klebsiella pneumonia. Tropical Journal of Pharmaceutical Research, 7, 1151–1157.10.4314/tjpr.v7i4.14701
- Puri, H. S. (1999). Neem (Azardirachta Indica), the divine tree. The Netherlands: Hardwood Academic Pub.10.4324/9780203304310
- Ríos, J. L., & Recio, M. S. (2005). Medicinal plants and antimicrobial activity. Journal of Ethnopharmacology, 100, 80–84.10.1016/j.jep.2005.04.025
- Rochfort, S., Parker, A. J., & Dunshea, F. R. (2008). Plant bioactives for ruminant health and productivity. Phytochemistry, 69, 299–322.10.1016/j.phytochem.2007.08.017
- Rojas, A., Hernandez, L., Pereda-Miranda, R., & Mata, R. (1992). Screening for antimicrobial activity of crude drug extracts and pure natural products from Mexican medicinal plants. Journal of Ethnopharmacology, 35, 275–283.10.1016/0378-8741(92)90025-M
- SAS. (1998). Statistical Analytical System. SAS/STAT User’s Guide Statistical Analysis Institute Inc. Version 6 (3rd ed., p. 943). Cary, NC.
- Shamila, I. M. R., Jeeva, S., Sheela, D. J., Brindha, J. R., & Leksinmi, N. C. J. P. (2012). Antimicrobial spectrum and phytochemical study of Ficus tslela L. (Moraceae). Drug Invention Today, 4, 337–339.
- Sibi, G., Chatly, P., Adhikari, S., & Ravikumar, K. R. (2012). Phytoconstituents and their influence on antimicrobial properties of Morinda citrifolia L. Research Journal of Medicinal Plant, 6, 441–448.10.3923/rjmp.2012.441.448
- Suree, N., & Pana, L. (2005). Antibacterial activity of crude ethanolic extracts and essential oils of spices against Salmonellae and other Enterobacteriaceae. KMITL Science and Technology Journal, 5, 527–538.
- Talukdar, A. D., Choudhary, M. D., Chakraborty, M., & Dutta, B. K. (2010). Phytochemical screening and TLC profiling of plant extracts, Cyathea gigantean (Wall. Ex. Hook) Haltt. Cyathea brunoniana. Wall.ex.Hook. (Cl. and Bak.). Assam University Science and Technology Journal, 5, 70–74.
- Tipu, M. A., Akhtar, M. S., Anjum, M. I., & Raja, M. A. (2006). New dimension of medicinal plant as animal feed. Pakistan Veterinary Journal, 26, 144–148.
- Virdis, S., Scarano, C., Cossu, F., Spanu, V., Spanu, C., & Santis, E. P. L. (2010). Antibiotic resistance in Staphylococcus aureus and coagulase negative Staphylococci isolated from goats with sub-clinical mastitis. Veterinary Medicine International, doi:10.4061/2010/517060
- Yamamoto, L. G. (2003). Inhibitory and bactericidal principles (MIC and MBC). Case based pediatrics for medical students and Residents. Retrieved from www.hawaii.edu/medicine/pediatrics/pedtext/s06c04.html
- Zare, P., Ghorbani-Choboghlo, H., Jaberi, S., Razzhaqi, S., Mirzae, M., & Mafuni, K. (2014). Occurrence and antimicrobial resistance of Salmonella spp. and Escherichia coli isolates in apparently healthy slaughtered cattle, sheep and goats in East Azarbaijan province. International Journal of Enteric Pathogen, 2, 1–4, e15451.
