Abstract
The major use of antibiotics is for the treatment and prevention of diseases. Antibiotics are used in the livestock industry to enhance growth rate, increase feed efficiency and reduce disease development. But unscrupulous use of antibiotic plays a major role in the emerging public health crisis of antibiotic resistance. The issue becomes more relevant on a future perspective because the global use of antimicrobials for food animal production is predicted to increase by 67% in 2030. This issue required timely intervention in India because India is a hotspot with higher consumption (30 kg/km2) for industrial poultry production, which is expected to grow 312% by 2030. But on same time complete ban on the use of antibiotic growth promoters (AGP) can create an economic loss of 44.1 billion USD in global meat production. Antibiotic residues in food have twofold concern; one is potential threat of direct toxicity in humans and the second is possible development of resistant strains and failure of antibiotic therapy in clinical cases. This review critically analyzes global trends in the use of AGP, its incidence, methods of detection and associated health concerns and suggests possible ways to reduce the residues in foods of animal origin.
Public interest statement
The review prepared in a holistic manner to provide the entire information regarding the use of antibiotics as growth promoters in animals and its related health risk to humans. The prime interests of the authors were to sensitize the consumers and policy makers regarding the ill effects of unscrupulous use of antibiotics in livestock rearing. The review also discusses about the present scenario of antibiotic use, its health effects and development of antimicrobial resistance in microbes and various methods of detection from food. As conclusion the authors provided different measures that can be adapted to minimize the level of residue in foods of animal origin.
Competing interests
The authors declare no competing interest.
1. Introduction
Antibiotics are substances either produced naturally by living organisms or produced synthetically in the laboratory and they are able to kill or inhibit the growth of other microorganisms. The major use of antibiotics in livestock sector was for the treatment and prevention of diseases. The history of antimicrobial use for better growth performance of food producing animals was accidental; it was found that by-products of antibiotic production (dried Streptomyces aureofaciens broth) which contain a high level of vitamin B12, when fed to poultry animals resulted in higher growth (Centre for Science & Environment Study (CSE, Citation2014). On due course, it was found that the traces of antibiotic present in these byproducts were responsible for the extra growth (Chapman & Johnson, Citation2002). From that point of time, antibiotic has been used widely in animal and poultry feed to enhance the growth performance (Figure ). Use of antimicrobial growth promoters (AGP) as feed additives may improve feed efficiency by 17% in beef cattle, 10% in lambs, 15% in poultry and 15% in swine (Nisha, Citation2008).
Figure 1. Classification of antibiotic families in food.
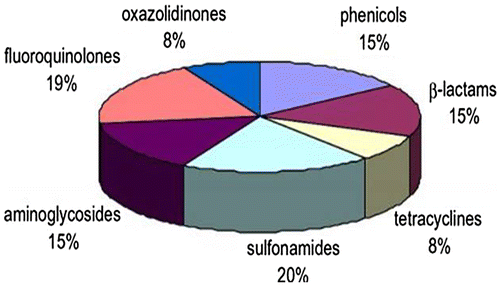
Overall health of the animal herd during any disease outbreak can be improved by use of appropriate antibiotics. Moreover, use of antibiotics as growth promoter especially in poultry helps in efficient production and which ultimately helps the consumers to have high quality meat and egg at reasonable price (Donoghue, Citation2003).However, the use of antibiotics in food animals poses a major risk for humans due to antibiotic resistance.
At present the global average annual consumption of antimicrobials per kilogram of animal produced is >100 mg/kg. India is a hot spot with respect to antibiotic use and present use of antibiotic in India for animal production is 30 kg/km2 and which is expected to grow 312% by 2030. Moreover, in a latest study conducted by the Pollution Monitoring Laboratory (PML) of New Delhi based research and advocacy think tank, Centre for Science and Environment (CSE) reported the poor condition of antibiotic use in India. In this study they reported the presence of one or more antibiotic in at least 40% of samples of chicken they studied. Antibiotics of the class Enrofloxacin and Ciprofloxacin were detected as high as 131.75 and 64.59 μg/kg from poultry liver samples (CSE, Citation2014).
In the global meat market, among the various high profile food trade disputes, the issue of drug residue especially antibiotic residue stands top. India used the dispute of antibiotic residue in chicken meat as an anti-dumping measure to prevent the dumping of low value chicken leg from USA to Indian market. One of the interesting finding is that 4/5th of all the antibiotics used in veterinary field is being utilized as growth promoters and most time it exceeds the total antibiotic use for medical care (Earth Times, Citation1998).One of the most elaborated studies of antimicrobial resistance in Salmonella Newport was carried out by Franco, Webb, and Taylor (Citation1990). They found that 80% of this infectious agent from ground beef were resistant to at least one antibiotic and 53% were resistant to at least three. Unexpectedly it showed resistance towards the tetracyclines and ampicillins, the two most widely used antibiotics for human treatments.
In the following sections, we first provide an overview into the global use of AGP in food producing animals. The second section reviews the public health hazards associated with AGP residues in food and the subsequent section details the methods of detection of antibiotics from foods of animal origin. Last section of this review highlights the recommendations to reduce or control the antibiotic residues in animal derived food.
2. Indian and international scenario of antibiotic use in livestock farming
Intensive livestock farming systems to cater the need for human consumption of animal proteins are invariably associated with regular and wide spread application of potentially significant antibiotics. So far no direct data is available regarding the global consumption of antibiotic in both extensive and intensive livestock farming system. In the latest study conducted by Van Boeckel et al. (Citation2015), they used the Bayesian statistical model to predict the consumption of antibiotic for livestock farming. Basically this method combines maps of livestock densities in various geographic regions, projected demand for meat and meat products along with the current consumption of antimicrobials. Using this model they succeeded in predicting the global trend of antibiotic use by 2030 following 2010 values. Similarly some other researchers derived the coefficient of antimicrobial use per kilogram of animal for each class of animal using the data from ESVAC (European Medicines Agency, Citation2013) and applied to high resolution maps of livestock densities to predict the geographic consumption of antibiotic by 2030.
Based on the study conducted by Van Boeckel et al. (Citation2015), it is found that the annual use of antimicrobials globally for cattle, chicken and pig production is 45,148 and 172 mg/kg of animal. This study also predicted a 67% increase in global consumption of antimicrobial in livestock production from 2010 to 2030. By 2030 the consumption will increase to 105,596 tonnes compared to 2010 consumption of 63,151 tonnes. It is reported that around one third increase in consumption of antimicrobials in livestock is pertaining to changes in livestock rearing practices in middle income countries. In BRICS countries this increase may be up to 99% along with a concomitant increase in animal proteins demand (Maron, Smith, & Nachman, Citation2013; Tilman, Balzer, Hill, & Befort, Citation2011).
In a developed country like USA, 80% of counties total antimicrobial consumption accounts for use in food animals (Food & Drug Administration (FDA, Citation2010). It is reported that global consumption of antibiotic in animals is twice that of humans (Aarestrup, Citation2012). The present consumption of antibiotic in India is estimated to be 30 kg/km2 and which is expected to grow 312% by 2030.
3. Public health hazards associated with antibiotic residues in foods of animal origin
Based on the report “Antibiotics in Chicken: From farm to fork” released on 30 July 2014 by PML of CSE it can be said that Indians are developing resistance to antibiotics and, hence, falling prey to a host of otherwise curable ailments. They are suggesting that some of this resistance might be due to large scale unregulated use of antibiotics in poultry farming (CSE, Citation2014).
A wide spectrum of antibiotics with different mechanisms of action are being using in livestock farming and mainly as therapeutic agents to treat diseases. Similarly it can be applied to animals as prophylactic agents when symptoms are not observed but infections are suspected. But the great concern of antimicrobial residue arises when a small dose of antimicrobial agent fed to animals or birds through food over the life time as growth promoters. Antibiotic growth promoters are known to suppress the gut bacteria leaving more nutrients for chicken to be absorbed for greater growth (CSE, Citation2014).
Khaniki (Citation2007) reported the public health significance of antibiotic residues in milk in relation to the wide consumption of milk by infants, youngsters and adults around the globe. In this regard the example suggested by Hameed, Sender, and Korwin-Kossakowska (Citation2006) is really valid, they told that mastitis pathogens in milk is having lower public health threat when milk is pasteurized compared to the hazards created by the improper use of antibiotics for treatments.
Unscrupulous use of antimicrobials in animal production leads to development of resistant bacteria and which later transmit to human through food, environment or by direct contact through affected meat. So it is mandatory to follow withdrawal periods for such compounds when using in animal production to safeguard public health (Figure ). Withdrawal period is a time between the last dose of antibiotic given to food animals and consumption of food animals or food derived from it. It needs to be mentioned on the antibiotics that are used for animals and if not mentioned properly it is considered to be as 28 days in Indian context (CSE, Citation2014).
Figure 2. Theoretical depletion of antibiotic residues in edible poultry tissue.
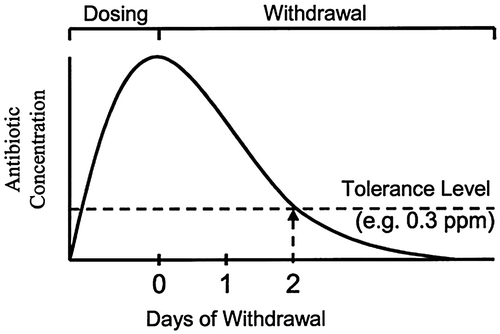
According to Nisha (Citation2008) the major pathological effects produced by antibiotic residues in food apart from transfer of antibiotic resistant strains of bacteria to the human beings includes immunological effects, autoimmunity disorders, arcinogenicity especially due to sulphamethazine, oxytetracyclines and furazilidone, mutagenecity, nephropathy due to gentamycin, hepatotoxicity, reproductive disorders, bone marrow toxicity due to chloramphenicol and allergy due to penicillin.
4. Quantitative and qualitative detection of antibiotic residue in foods of animal origin
In European Union the maximum residue limit (MRLs) for veterinary medicines in foodstuff of animal origin like meat, fish and eggs are regulated by the means of the Council Regulation (EEC) No. 470/2009. Whereas the measures to monitor certain substances and residues in live animals and animal products is governed by Council Directive No. 96/23/EC. In USA the food microbial residue reporting is carried out by the Food Safety and Inspection Service (FSIS) of USDA. In India, for domestic food market, Food Safety and Standards Authority of India (FSSAI, Citation2006) has set the tolerance limit for antibiotics only for fishery products and there is no such tolerance limit is available for meat and meat products under FSSAI. For export purpose, Export Inspection Council follows the residue monitoring plan from European Union (Table ).
Table 1. Maximum residue limit of antibiotics in poultry and fish meat as set by various regulatory agencies
The most widely used method for the detection of antibiotic residues in animal originated foodstuffs is the microbial inhibition method (Myers, Citation1964), this method is not only cost effective but also able to cover a large number of different antibiotics in a single test run (Mitchell, Griffiths, McEwen, McNab, & Yee, Citation1998). The two test formats of microbial inhibition tests are tube and plate, in which tube test with a viable bacterial culture along with a pH or redox indicator is commonly using for detection of residue in milk samples (Vermunt, Stadhouders, Loeffen, & Bakker, Citation1993). Similarly the plate test has been the main test format for screening of antibiotic residue in slaughter animals especially in Europe (Bogaerts & Wolf, Citation1980; Nouws, Schothorst, & Ziv, Citation1979). The pioneer workers in the field microbial inhibition test used Sarcina lutea (Nouws et al., Citation1979) and Bacillus subtilis (Schothorst, Citation1969) in Netherland and Germany respectively.
In due course EU established the four plates test (EU4pt) in which three plates are inoculated with Bacillus subtilis and one plate with Micrococcus luteus for screening meat for antibiotic residue (Tang & Gillevet, Citation2003). But due to the fact that the EU4pt is laborious and chances of getting false positive is higher with kidney samples, the test is replaced by an alternate one plate test (Nolan, Dooley, Nugent, & O’Keeffe, Citation2000). A novel screening method for the detection of antibiotic residues in chicken meat and poultry was reported by Johnston, Reamer, Harris, Fugate, and Schwab (Citation1981). This method involved inserting a cotton swab into meat or poultry tissue to absorb tissue fluid and kept in a test plate containing antibiotic medium No. 5 (BBL) and a seed layer of Bacillus subtilis ATCC 6,633 spores. For screening of antibiotics like chlortetracycline, oxytetracycline, tetracycline, erythromycin, neomycin, penicillin, streptomycin and tylosin, this test shown to have equal sensitivity compared to conventional test (Johnston et al., Citation1981).
A three plate test with three different species of bacteria viz Ecsherichia coli, Staphylococcus aureus and Bacillus subtilis were used to detect antibiotic residues in various organs of poultry (Elnasri, Salman, & El Rade, Citation2014). And in this study a highest occurrence of antibiotic residue was detected in muscle tissue compared to kidney. This finding was in agreement with the study of Nolan et al. (Citation2000), they recommended introduction of a membrane between the kidney sample and test plate to avoid aberrant results.
At present the regulatory agencies required methods with high throughput, fast, reliable and sensitive and which can even process solid samples for antibiotic residue detection. In this context of residue detection, immunoassays and biosensors are of great importance compared to microbial assays (Cháfer-Pericás, Maquieira, & Puchades, Citation2010). Biosensors always provide opportunity for automation, in situ analysis and development of large number of commercial detection kits. Any biosensor system comprise two main components namely, a transducing device and a recognition element. The advantage of biosensors includes, it can detect non polar molecules which are not receptive to most devices, high specificity, quick and real time application for industry (Koyun, Ahlatcolu, & Koca, Citation2012). But the drawbacks include chances of contamination of biosensor and it cannot be heat sterilized (Huet et al., Citation2010).
Enzyme linked immune sorbent assay (ELISA) is a widely used method for the detection of antibiotic residue in all kinds of tissue samples (Mungroo & Neethirajan, Citation2014). ELISA based techniques have the advantage of high sensitivity, broad specificity and ability to handle large number of small volume samples in short time (Wang, Xu, Zhang, & He, Citation2009). But the major drawbacks are this test is expensive and detection is not real time (Petz, Citation2009). Liquid Chromatography-Mass Spectrometry (LC-MS) coupling is another effective and sensitive system for the detection of antibiotic residue. Various methods of LC-MS include electro spray ionization source, direct injection methods and mobile phase. Mass spectrometry works on the principle of mass-to-charge ratio (Koyun et al., Citation2012). Shareef, Jamel, and Yonis (Citation2009) used Thin Layer Chromatography (TLC) for detection of antibiotic residue in stored poultry products and found that 52% of all the samples evaluated were positive for at least one antibiotic (Table ).
Table 2. Analytical methods for screening and quantification of antimicrobial residues under FSSAI guidelines, India
5. Measures to reduce antibiotic residue in livestock products
This section includes recommendations and measures to reduce antibiotic residues in foods of animal origin, which were adopted from the CSE report and other published literatures.
| (1) | Reduce antibiotics use in food animal rearing. Many developed countries have banned its use as growth promoters. | ||||
| (2) | Rapid screening methods should be developed for detecting and segregating samples contains above MRL levels of antibiotics. | ||||
| (3) | Appropriate MRLs need to be set by regulatory bodies and should enforce it. | ||||
| (4) | Appropriate withdrawal periods should be strictly followed and enforced to make the meat rendered safe for human consumption. | ||||
| (5) | Improve the individual and organizational aware by enhancing proper knowledge dissemination. | ||||
| (6) | Follow best hygiene practices during animal rearing and avoid unwanted use of antibiotics. | ||||
| (7) | Alternates to antibiotics like bio control measures and Ethno-veterinary practices should be developed and followed. | ||||
| (8) | Organic poultry farming may be encouraged by providing appropriate incentives to the farmers in form of subsidies. | ||||
| (9) | Use of proper processing techniques to inactivate the antibiotic residue, e.g. refrigeration causes inactivation of penicillin. | ||||
| (10) | Use of activated charcoal, resins and UV irradiation to inactivate residues. | ||||
6. Conclusions
Extensive use of antibiotics as growth promoters in animal feed poses the threat for development of new resistant strains of bacteria, which may not be susceptible to the most advanced antibiotics available nowadays. Stringent control over the unscrupulous use of these valuable tools should be elicited along with development of other bio control measures and organic farming systems to safeguard the public health and to protect the currently available antibiotics for future clinical uses. Ethno veterinary practices and good hygienic practices should also be promoted in animal production systems to reduce the development of antibiotic resistance in pathogens.
Funding
The authors received no direct funding.
Acknowledgements
The authors are thankful to the Director and Joint Director, ICAR-National Academy of Agricultural Research Management, Rajendranagar, Hyderabad, Telengana, India for the facilities provided. We are also thankful to Mr Ramakant Sahu, Ms Poornima Saxena, Prof (Dr) H.B. Mathur, Prof (Dr) H.C. Agarwal, Pollution Monitoring Laboratory, Centre for Science and Environment, New Delhi, India.
Additional information
Notes on contributors
M.R. Vishnuraj
M.R. Vishnuraj completed masters in veterinary science (MVSc) in Livestock Products Technology and presently working as scientist at ICAR-National Research Centre on Meat, Hyderabad, India. He has more than ten international publications in his credit.
G. Kandeepan
G. Kandeepan completed PhD degree in Livestock Products Technology and presently working as Scientist (senior scale) at ICAR-National Research Centre on Meat, Hyderabad, India. He has more than30 international research articles to his credit and also contributed to different books.
K.H. Rao
K.H. Rao is working as a principal scientist at ICAR-National Academy of Agricultural Research Management, Hyderabad, India. He had his PhD degree in Dairy science.
S. Chand
S. Chand completed his masters in veterinary science in Livestock Products Technology and presently he is working as scientist at ICAR-Indian Veterinary Research Institute, Uttar Pradesh, India.
V. Kumbhar
S. Kumbhar is presently doing his Masters in Livestock Products Technology from ICAR Indian Veterinary Research Institute, Uttar Pradesh, India.
References
- Aarestrup, F. (2012). Sustainable farming: Get pigs off antibiotics. Nature, 486, 465–466.10.1038/486465a
- Bogaerts, R., & Wolf, F. (1980). A standardised method for the detection of residues of antibacterial substances in fresh meat. Fleischwirtschaft, 60, 672–673.
- Centre for Science and Environment Study. (2014). Antibiotics in chicken meat (36 p.). New Delhi: Pollution Monitoring Laboratory/PR-48/2014.
- Cháfer-Pericás, C., Maquieira, A., & Puchades, R. (2010). Fast screening methods to detect antibiotic residues in food samples. TrAC Trends in Analytical Chemistry, 29, 1038–1049.10.1016/j.trac.2010.06.004
- Chapman, H. D., & Johnson, Z. B. (2002). Use of antibiotics and roxarsone in broiler chickens in the USA: Analysis for the years 1995 to 2000. Poultry Science, 81, 356–364.10.1093/ps/81.3.356
- Donoghue, D. J. (2003). Antibiotic residues in poultry tissues and eggs: human health concerns?. Poultry Science, 82, 618–621.10.1093/ps/82.4.618
- Earth Times. (1998). Benefits of grass fed beef (July Edition).
- Elnasri, H. A., Salman, A. M., & El Rade, S. A. (2014). Screening of Antibiotic Residues in Poultry Liver, Kidney and Muscle in Khartoum State, Sudan. Journal of Applied and Industrial Sciences, 2, 116–122.
- European Medicines Agency. (2013). Sales of veterinary antimicrobial agents in 25 EU/EEA countries in 2011: Third ESVAC report. London: Author.
- Food and Drug Administration. (2010). CVM updates-CVM reports on antimicrobials sold or distributed for food-producing animals. Silver Spring, MD: Author.
- Food Safety and Standards Authority of India. (2006). Food Safety and Standards Act. New Delhi: Author.
- Franco, D. A., Webb, J., & Taylor, C. E. (1990). Antibiotic and sulfonamide residues in meat: implications for human health. Journal of Food Protection, 53, 178–185.
- Hameed, K.G, A., Sender, G., & Korwin-Kossakowska, A. (2006). Public health hazard due to mastitis in dairy cows. Animal Science Papers and Reports, 25, 73–85.
- Huet, A., Delahaut, P., Fodey, T., Haughey, S. A., Elliott, C., & Weigel, S. (2010). Advances in biosensor-based analysis for antimicrobial residues in foods. TrAC Trends in Analytical Chemistry, 29, 1281–1294.10.1016/j.trac.2010.07.017
- Johnston, R. W., Reamer, R. H., Harris, E. W., Fugate, H. G., & Schwab, B. (1981). A new screening method for the detection of antibiotic residues in meat and poultry tissues. Journal of Food Protection, 828–831.
- Khaniki, R. J. (2007). Chemical contaminants in milk and public health concerns: a review. InternationalJournal of Dairy Science, 2, 104–115.
- Koyun, A., Ahlatcolu, E., & Koca, Y. (2012). Biosensors and their principles. In S. Kara (Ed.), A Roadmap of BiomedicalEngineers and Milestones. Rijeka: InTech.
- Maron, D.F. , Smith, T, J., & Nachman, K, E. (2013). Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Globalization and Health, 9, 48.10.1186/1744-8603-9-48
- Mitchell, J. M., Griffiths, M. W., McEwen, S. A., McNab, W. B., & Yee, A. J. (1998). Antimicrobial drug residues in milk and meat: Causes. Concerns, Prevalence, Regulations, Tests, and Test PerformanceJournal of Food Protection, 61, 742–756.
- Mungroo, N. A., & Neethirajan, S. (2014). Biosensors for the detection of antibiotics in poultry industry-A review. Biosensors, 4, 472–493.10.3390/bios4040472
- Myers, R. P. (1964). Antibiotics residues in milk. Reviews of Environmental Contamination and Toxicology, 7, 11–36.
- Nisha, A. R. (2008). Antibiotic residues-A global health hazard. Veterinary World, 1, 375–377.10.5455/vetworld.
- Nolan, M., Dooley, M., Nugent, A., & O’Keeffe, M. (2000). Residues of veterinary drugs in food (pp 781–785). Proceedings of Euroresidue IV conference, Veldhoven.
- Nouws, J. F. M., Schothorst, M., & Ziv, G. (1979). A Critical evaluation of several microbiological test methods for residues of antimicrobial drugs in ruminants. Archiv fur LebensmittelhygieneArch Lebensm Hyg, 30, 4–8.
- Petz, M. (2009). Recent applications of surface plasmon resonance biosensors for analyzing residues and contaminants in food. Monatshefte für Chemie-Chemical Monthly, 140, 953–964.10.1007/s00706-009-0142-6
- Schothorst, V. M. (1969). Antibiotic residues in slaughter animals ( Ph.D thesis). Utrecht: Utrecht University.
- Shareef, A. M., Jamel, Z. T., & Yonis, K. M. (2009). Detection of antibiotic residues in stored poultry products. Iraqi Journal of Veterinary Sciences, 23, 45–48.
- Tang, J. S., Gillevet, P. M. (2003). Reclassification of ATCC 9341 from Micrococcus luteus to Kocuria rhizophila. International Journal of Systematic and Evolutionary Microbiology, 53, 995–997.10.1099/ijs.0.02372-0
- Tilman, D., Balzer, C., Hill, J., & Befort, B. L. (2011). Global food demand and the sustainable intensification of agriculture. Proceedings of the National Academy of Sciences, 108, 20260–20264.10.1073/pnas.1116437108
- Van Boeckel, T. P., Brower, C., Gilbert, M., Grenfella, B. T., Levina, S. A., Robinsoni, T. P., ... Laxminarayan, R. (2015). Global trends in antimicrobial use in food animals. Proceedings of National Academy of Science, Washington, DC. Retrieved from www.pnas.org/cgi/doi/10.1073/pnas.1503141112
- Vermunt, A. E. M., Stadhouders, J., Loeffen, G. J. M., & Bakker, R. (1993). Improvements of the tube diffusion method for detection of antibiotics and sulfonamides in raw milk. Netherland milk and dairy journal, 47, 31–40.
- Wang, S., Xu, B., Zhang, Y., & He, J. X. (2009). Development of enzyme-linked immunosorbent assay (ELISA) for the detection of neomycin residues in pig muscle, chicken muscle, egg, fish, milk and kidney. Meat Science, 82, 53–58.10.1016/j.meatsci.2008.12.003
