Abstract
This review aims to review hormones mechanisms that affect fuel metabolism and are involved in regulation of blood glucose, dealing insulin and glucagon hormones, and includes other related hormones, which increase the blood glucose level: growth hormone, thyroxine, cortisol and adrenaline. However, this review focuses on insulin and glucagon hormones as widely, and on other related hormones as briefly. Insulin plays an important role in a decrease blood glucose concentration in hyperglycemic response to emergencies or stress by an increasing rate of glucose transport into the muscle cell of animals and facilitating glucose utilization and by maintaining normal blood glucose concentrations. Insulin is a hypoglycemic hormone, promoting the storage of metabolites in peripheral stores. While, glucagon is a hyperglycemic hormone, stimulates gluconeogenesis—at the expense of peripheral stores by enhancing the hepatic removal of certain glucose precursors and stimulates lipolysis; however, it has not influence on peripheral protein stores directly. Insulin, glucagon and other related hormones regulate blood glucose concentrations and act on movement of glucose, amino acids and possibly volatile fatty acids between the liver and peripheral tissues directly. In another way, glucagon may be considered catabolic and insulin anabolic. In conclusion, insulin promotes body gain by stimulating protein and fat synthesis, growth hormone increases protein retention and decrease fat deposition. Growth hormone can alter the sensitivity of tissues to insulin. In contrast, catabolic hormones such as glucagon, epinephrine and glucocorticoids are provided for mobilization of energy reserves to allow the animal to deal with adverse situations.
Public Interest Statement
The review prepared in a holistic manner to provide the entire information regarding the role of insulin and other related hormones in metabolic processes involved in the synthesis and degradation of body tissues in the animals. In addition, this review discusses the role of these hormones in blood glucose regulation. This review article is significant because elucidates role and mechanisms of insulin, glucagon and other related hormones in regulations of carbohydrate, protein and lipid metabolism for provide energy and growth. As conclusion, the authors provided insulin as anabolic promotes body weight gain, through deposition of muscle and fats by stimulating protein and fat synthesis. Growth hormone increases protein retention and decrease fat deposition. On the other hand, glucagon, epinephrine and glucocorticoids as catabolic hormones provided for mobilization of energy reserves to allow the animal to deal with adverse situations.
Competing Interests
The authors declare no competing interest.
1. Introduction and background
The stimulation of metabolic enzymes partially relies on hormonal balance. Thus, a comprehension of the roles of hormones in metabolic regulation is essential to evaluate of any metabolic action or disorders. Metabolism in all living organisms includes anabolic and catabolic processes. Glycolysis, glycogenesis, glycogenolysis gluconeogenesis, lipolysis, lipogenesis, protein synthesis, proteolysis are important metabolic processes in the body of living organisms. In addition, there are some related hormones that play an important role in these metabolic processes. Glycogenesis is the process of glycogen synthesis. Glycogenolysis is the process of glycogen degradation. Glycolysis is a cytoplasmic non-oxidative reaction for glucose degradation Gluconeogenesis is the process in which non-carbohydrate molecules such as pyruvate, lactate, glycerol, alanine and glutamine are converted to glucose in the tissues of liver, kidneys, brain, testes and in the erythrocytes (Dashty, Citation2013).
In the ruminant, as a result of microbial activity (chiefly bacteria) in the rumen most of the dietary carbohydrate is fermented to volatile fatty acids (VFAs) (Annison & Armstrong, Citation1970). As little dietary carbohydrate is absorbed as glucose (by pass starch), its needs must be met by glucose synthesis from non-carbohydrate sources, that is, gluconeogenesis (Bergman, Citation1973; Leng, Citation1970). In ruminant animals hepatic glucose output (primarily gluconeogenesis) is greatest in the recently fed (post-absorptive) animal (Bergman, Katz, & Kaufman, Citation1970), whereas in nonruminants gluconeogenesis is greatest during starvation (Shoemaker, Yanof, Turk, & Wilson, Citation1963). Acetate is the major substrate for lipogenesis in ruminants (Ballard, Hanson, & Kronfeld, Citation1969; Hanson & Ballard, Citation1967). In ruminant gluconeogenesis and lipogenesis are maximal at the same time, while in non-ruminants gluconeogenesis is greatest when lipolysis is greatest. Major substrates for gluconeogenesis and lipogenesis are absorbed from the intestinal tract, substrate supply may play an important role in the regulation of these processes. In ruminants, the major glucogenic precursors are propionate, lactate/pyruvate, amino acids and glycerol (Bergman, Citation1973). In the absence of insulin, proteolysis and lipolysis is promoted, thus providing substrate for gluconeogenesis and energy production. Insulin’s direct effect on the liver, particularly glucose production, appears to be marginal in ruminants. Insulin’s inhibition of gluconeogenesis appears to depend upon substrate availability (Bloom, Edwards, Hardy, Malinowska, & Silver, Citation1975; Brockman, Citation1977). The major glucose precursors are absorbed propionate and amino acids during the fed animal. However, during starvation, the major substrates for gluconeogenesis are small amount of glycerol from lipid stores (glucose derived from glycerol is about 15%) (Bergman, Starr, & Reulein, Citation1968), amino acids and, hence peripheral protein. Therefore, a balance must be maintained in order to avoid excessive gluconeogenesis at the expense of peripheral protein by sparing glucose utilization. The lipid stores as an energy source utilized instead of glucose as part of this utilization (Jarrett, Filsell, & Ballard, Citation1976). The secretion of glucagon and insulin can be influenced by blood glucose, amino acids, VFAs, nervous stimulation, adrenal medullary hormones and gastrointestinal hormones, additionally, by stress and other physiological disorders (Bassett, Citation1972). Thus, this paper reviews the role of insulin and other related hormones in energy metabolism. In addition, it’s concerned with role of insulin and other related hormones in maintaining constant levels of glucose in the blood.
2. Secretion, regulation and metabolic effects of insulin and glucagon
The pancreas contains two major tissue types and is a dual-function gland, endocrine and exocrine (Figure ). As exocrine gland, the acini secrets pancreatic juice including digestive enzymes to facilitate absorption and digestion of nutrient molecules in the duodenum. As an endocrine gland, the islets of Langerhans of pancreas secrete insulin and glucagon into the bloodstream directly involved in metabolism (Suckale & Solimena, Citation2008). The islets contain many of cells: alpha (α), beta (β) and delta (δ) cells; the α cells secrete glucagon hormone (increase glucose in blood), the β cells, constituting about 60% of all cells of the islets, secrete insulin (decrease glucose in blood- inhibits glucagon secretion) and δ cells secrete somatostatin (regulates/stops α and β cells; inhibits the secretion of both insulin and glucagon). The gamma (γ) cells; secrete pancreatic juice (Suckale & Solimena, Citation2008). In ruminants, glucagon secretion is more sensitive than is insulin secretion to inhibitory influences of somatostatin (Brockman, Citation1979). Mechanism of insulin and glucagon on carbohydrate metabolism occurs as glucose concentration is high, such as after eating, insulin secreted by β cells into the blood stream to promote glycolysis to lower glucose levels by increasing removal of glucose from blood stream to most body cells. As same taken, when glucose concentration drops, such as during exercise, glucagon released by α cells promotes gluconeogenesis to raise the blood glucose levels by increasing breakdown of glycogen and release of glucose by the liver (Figure ). Insulin activates β cells and inhibits α cells, while glucagon activates α cell. Glucagon promotes amino acids absorption from the blood by the liver, and converts them to glucose. Rising blood glucose levels inhibit glucagon via a negative feedback mechanism. Thus, insulin and glucagon make together to maintain homeostasis of glucose. In the starvation or fasted state, secretion of insulin is reduced and glucagon secretion is enhanced, that leading to stimulation of catabolic processes and mobilization of glucose and free fatty acids (FFA) (Frayn, Citation2009).
Figure 1. Histological structure of pancreas (Longnecker, Citation2014).
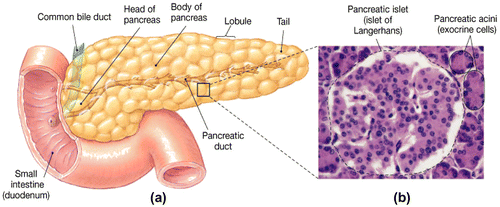
Figure 2. Blood sugar insulin cycle graphic (Winter, Citation2013).
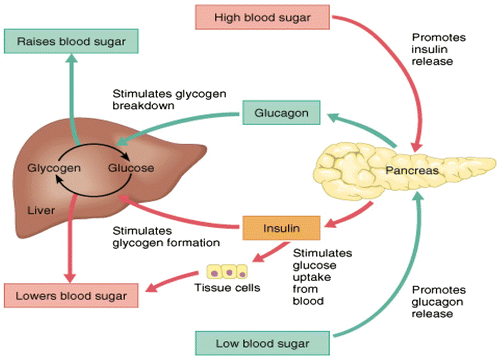
3. Insulin as hypoglycemic, storage and anabolic hormone
Insulin is; a polypeptide hormone, composed of two amino acid chains (A chain: 21 amino acids; B chain 30 amino acids). The chains are connected to each other by disulfide linkage; those chains contain 51 amino acids with a molecular weight of 6,000. It is secreted by the β cells of the pancreas when blood glucose levels rise. As insulin binds to insulin receptors of the target cell and signal transduction, it stimulates the cell to combine glucose transport proteins into its membrane, lead to fall blood glucose levels, hypoglycemic, or “low sugar”, which inhibits β cells to release further insulin through a negative feedback mechanism. It can be caused by low levels of insulin, or by reduced sensitivity of tissue cells to insulin. Insulin stops the use of fat by inhibiting the release of glucagon. Consequently, insulin is used medically to treat some diabetes mellitus forms. Insulin may be considered a storage hormone by promoting movement of glucose and participates in directing absorbed acetate and amino acids into peripheral tissues. These insulin effects serve to promote body gain by encouraging stimulating synthesis of carbohydrate, protein and fat (lipogenesis); in the long run is an anabolic hormone. Insulin is the primary hormonal regulator of metabolism in the resting animal (Brockman & Laarveld, Citation1986; Squires, Citation2011). In absence of insulin, glucose uptake decreases in the tissues and increases mobilization of lipids in adipocytes. In presence of insulin, glucose uptake increases in the tissues and decreases mobilization of lipids in adipocytes (Frayn, Citation2009).
Insulin inhibits gluconeogenesis and glycogenolysis, stimulates glycolysis and glycogenesis, stimulates uptake and incorporation of amino acids into protein, inhibits protein degradation, stimulates lipogenesis, and suppress lipolysis (Bassett, Citation1975). Immediately after a high-carbohydrate meal, the absorbed glucose into the blood stream causes rapid secretion of insulin. The insulin causes rapid uptake, storage, and use of blood glucose by almost all tissues of the body directly (e.g. brain, intestine), or indirectly by affecting the mobilization of fuels from adipose tissue (Dimitriadis, Mitrou, Lambadiari, Maratou, & Raptis, Citation2011). Insulin increases glucose usage by most other cells of the body -except brain- and effects on glucose transport and usage in the muscle cells. Glucose transportation into adipose cells provides glycerol for fat molecule. Thus, insulin promotes deposition of fat in these cells. Insulin promotes storage of metabolic fuels within cells. Insulin increases the movement of glucose into many peripheral tissues (West & Passey, Citation1967) including both muscle (Jarrett, Filsell, & Ballard, Citation1974) and fat (Khachadurian, Adrouni, & Yacoubian, Citation1966) (Tables and ; and Figures ).
Table 1. Metabolic effects of hormones (Marilou, Citation2004)
Table 2. Mechanism of action of key metabolic regulators (Shrayyef & Gerich, Citation2010)
Figure 3. Actions of insulin (Martin, Citation1983).
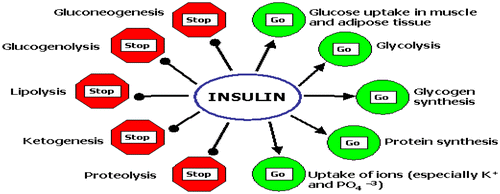
Figure 4. Utilization of glucose in metabolic specialization organs and cells (McKee & McKee, Citation2012b).
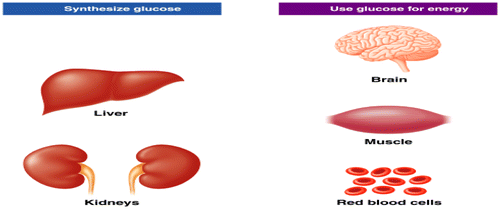
Figure 5. Role of insulin and glucagon in glucose regulation (Rajendran, Pharm, & Santhi, Citation1979).
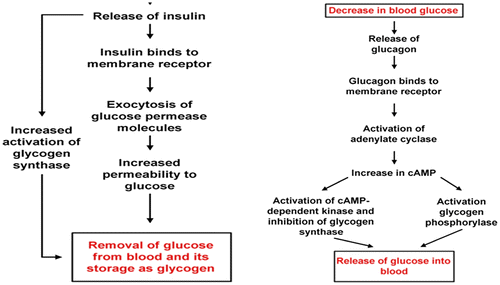
Figure 6. Role of insulin and glucagon in metabolic processes of carbohydrates (Dashty, Citation2013; McKee & McKee, Citation2012b).
4. Glucagon as hyperglycemic, catabolic or energy mobilizing hormone
Glucagon is a pancreatic polypeptide hormone composed of 29 amino acids with molecular weight of 3,500 and is secreted from the α-cells of pancreas. Glucagon acts in regulating metabolism and stimulating glucose output, which is achieved by acceleration of hepatic glucose output by glycogenolysis and gluconeogenesis. Glucagon stimulates the uptake of amino acids and the rate of protein catabolism by the liver (Bassett, Citation1975; Squires, Citation2011) to prevent insulin hypoglycemia (Bloom et al., Citation1975; Brockman, Citation1977). It decrease in plasma amino acid concentrations and may impair protein synthesis in muscle (Trenkle, Citation1981). Glucagon activates phosphofructokinase-2 (PFK-2), which reduces the level of fructose-2,-6-bisphosphate in the cell. For that, phosphofructokinase-1 (PFK-1) activity and flux through glycolysis are decreased. In liver, glucagon also inactivates pyruvate kinase (McKee & McKee, Citation2012a). Glucagon, as a counter regulatory hormone for insulin, stimulates glucose release by the liver where it binds to specific glucagon binding sites and maintains glucose homeostasis.
Glucagon stimulates the production of ketone bodies from amino acids (AA) and fatty acids (FAs). When blood glucose levels return to normal, secretion of glucagon stops through negative feedback mechanism. Glucagon is energy mobilizing, and increases hepatic glucose output and lipolysis. When amino acids are utilized in gluconeogenesis, glucagon enhances hepatic uptake of amino acids for non-hepatic tissues. In the long term, then, it is catabolic (Brockman, Citation1978). Glucagon is a potent hyperglycemic hormone. It stimulates both glycogenolysis and gluconeogenesis in sheep. Reduction of 15–20% glucagon concentration in sheep leads to reduction of glucose production (Brockman & Johnson, Citation1977). Glucagon enhanced gluconeogenesis is associated with increased activity of hepatic pyruvate carboxylase (Brockman & Manns, Citation1974), a key gluconeogenic enzyme, as well as augmented hepatic extraction of gluconeogenic substrates, such as alanine, pyruvate/lactate and glutamine, but not glycerol. Glucagon may enhance the conversion of propionate to glucose (Clark, Filsell, & Jarrett, Citation1976; Savan, Jeacock, & Shepherd, Citation1976).
5. The hormonal regulation of carbohydrate metabolism
Insulin, glucagon and GH (somatotropin) are the most important hormones that affect carbohydrate, protein, and lipid metabolism (Tables and ). The effects of insulin, glucagon, epinephrine, T3, T4 and growth hormones on carbohydrate, lipid and protein metabolism are shown in Figures , and .
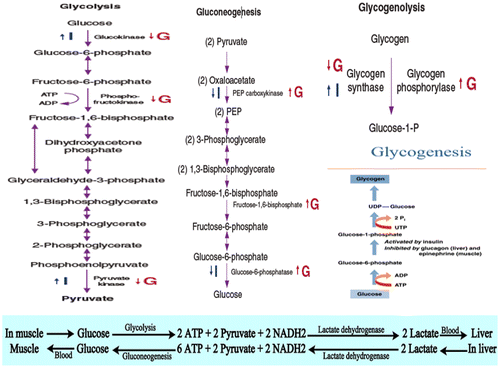
5.1. Glucose transport and glycolysis
Glycolysis is a cytoplasmic non-oxidative reaction for glucose degradation and is regulated by the glucagon and insulin hormones (Dashty, Citation2013). Glucose transport controls the rate of glucose utilization. In skeletal muscles and adipose tissues there are three glucose transporters: Glucose transporter 1, 3 and 4 (GLUT1, GLUT3 and GLUT4). Insulin increases uptake rate of glucose mainly by stimulating the translocation of the GLUT4 isoforms from intracellular pools to the surface cell membrane (Dimitriadis et al., Citation1988; Epstein, Shepherd, & Kahn, Citation1999) by increasing rate of glucose transport (Epstein et al., Citation1999) and the activities of hexokinase and 6-phosphofructokinase which increases the level of glucose-6-phosphate and fructose 2,6-bisphosphate in the cell, respectively (Dimitriadis et al., Citation1997, Citation1992; Mandarino et al., Citation1995). The stimulation of these two enzymes by insulin in muscle, which leads to enhanced glycolysis (Dimitriadis & Newsholme, Citation2004; Hawthorne, Citation1984), is of fundamental metabolic importance for the following reasons: (1) when glycogen store in muscle is replete, the glucose is converted to lactate, to maintain enhanced glucose utilization (Dimitriadis & Newsholme, Citation2004; Hawthorne, Citation1984; Shulman, Rossetti, Rothman, Blair, & Smith, Citation1987), and (2) lactate produced and released by muscle (and adipose tissue) is taken up by liver and converted to glycogen (known as the “indirect pathway” of glycogen synthesis) (Consoli, Nurjahan, Gerich, & Mandarino, Citation1992; Dimitriadis & Newsholme, Citation2004).
5.2. Glycogenesis
Glycogenesis is the process of glycogen synthesis (Dashty, Citation2013; Marilou, Citation2004). Increased ingestion of carbohydrate lead to increased glycogen storage in the liver and muscles. All excess carbohydrate cannot be stored as glycogen and is converted under the stimulus of insulin into fats and stored in the adipose tissue. The liver and muscle are essential tissues in intermediary metabolism of glycogen in ruminants. The liver glycogen depot plays a central role in intermediary metabolism, storing and mobilizing glycogen during the fed and fasted metabolic states, with these responses modulated during pregnancy, lactation, and exercise (Gardner, McGilchrist, & Pethick, Citation2014). Glycogenesis is stimulated by insulin and growth hormone in muscle, adipose and liver tissues, and inhibited by glucagon, epinephrine and T3 and T4 (Table ). Glycogenesis is the key-regulating enzyme for glycogen synthesis and it is activated by a direct effect of insulin and correlated to level of glucose 6-phosphate (Dimitriadis et al., Citation1997; Dimitriadis & Newsholme, Citation2004; Hawthorne, Citation1984; Mandarino, Citation1989; Rossetti & Giaccari, Citation1990).
5.3. Glycogenolysis
Glycogenolysis is the process of glycogen degradation or breakdown (Dashty, Citation2013). Glucagon stimulates glycogenolysis in the liver. Insulin decreases the rate of glycogen breakdown in muscle and liver. Both of these effects greatly enhance the availability of glucose to the other organs of the body. Increase in glucagon enhances the strength of the heart, increases blood flow in some tissue especially in the kidneys, enhances bile secretion, and inhibits gastric acid secretion. Increased blood glucose inhibits glucagon secretion. Conversely, increase in blood amino acids stimulates both insulin and glucagon secretion. Thus, in this instance the glucagon and insulin responses are not opposite (Dashty, Citation2013; Marilou, Citation2004).
5.4. Gluconeogenesis
Gluconeogenesis is a vital process for maintaining a supply of glucose for various metabolic needs in which non-carbohydrate molecules (pyruvate, lactate, glycerol, alanine and glutamine) are converted to glucose in the liver, kidneys, brain, testes and erythrocytes (Dashty, Citation2013). Insulin also inhibits gluconeogenesis. Mainly, glucagon increases gluconeogenesis in the liver by decreasing the quantities and activities of the liver enzyme required for gluconeogenesis. Amino acids released from muscles and other extra-hepatic tissues by an action of insulin are necessary precursors required for gluconeogenesis (McKee & McKee, Citation2012b). When muscle glycogen store is replete, this allows glucose to be converted to a glyconeogenic precursor, and makes as a “temporal buffer” for glucose. Thus conversion to lactate, circulation in the blood and then uptake and conversion to glycogen in liver could “smooth-out” the increase in the blood glucose level. This would help to maintain a constant blood glucose level after ingestion of carbohydrate. This is consistent with the idea that maintenance of the blood glucose level provides the condition for anabolism (Dimitriadis & Newsholme, Citation2004).
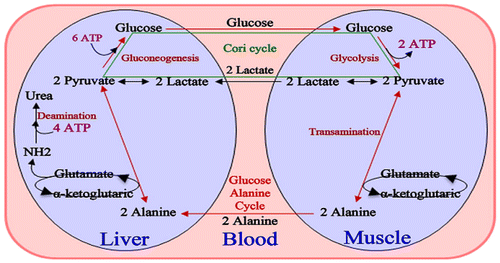
6. The Cori cycle
As shown in Figure , the Cori cycle, defined as conversion of glucose to lactate in muscle and adipose tissue and the conversion to glucose in the liver, represents a cyclic flow of carbon (Dimitriadis & Newsholme, Citation2004; Newsholme & Crabtree, Citation1975). By maintaining a continuous flux, the Cori cycle provides a dynamic “buffer” of lactate to remains concentration constant in both tissues and bloodstream (Dimitriadis & Newsholme, Citation2004; Newsholme & Dimitriadis, Citation1996). The benefit of this mechanism can be used by tissues whenever required for anabolic purposes. Lactate is converted to pyruvate, which is a precursor for acetyl-CoA. The latter has many important anabolic functions in the body. Thus, maintenance of a normal blood glucose level via the Cori cycle is essential for anabolic processes as increases Cori cycle flux during sepsis and hyperthyroidism conditions (Dimitriadis et al., Citation1988, Citation1997; Dimitriadis & Raptis, Citation2000; Leighton et al., Citation1989).
Figure 7. Cori cycle (Dashty, Citation2013).
7. The hormonal regulation of protein metabolism
The normal protein level in cells is maintained as a balance between synthesis and degradation. Contrary to that, in the muscle insulin increases the synthesis and decreases degradation of protein to favor anabolic process by increasing incorporation between amino acid into protein to maintain anabolic conditions in the body (Dimitriadis & Newsholme, Citation2004; Hawthorne, Citation1984; Liu & Barrett, Citation2002).
7.1. Protein synthesis
Protein synthesis is one of nature’s essential processes of transcription and translation of gene expression to convert amino acids to protein. An increases in proteins ingesting leads to increased insulin secretion to enhance amino acid uptake by cells, consequently converting them into protein. Then, it inhibits breakdown of proteins in the cells. Insulin, GH, cortisol, T3 and T4 and IGF-I play a key role in stimulating protein synthesis. The glucagon and epinephrine inhibit protein synthesis (Umpleby & Russell-Jones, Citation1996).
7.2. Proteolysis (protein degradation)
Proteolysis is the process of protein degradation to amino acids and peptides. Decreased insulin decreases protein synthesis and consequently increases proteins catabolism, and so large amount of amino acids are dumped into the plasma, and most of the excess of amino acids are used either as directly for energy supply or as precursor for gluconeogenesis. This degradation of amino acid resulting protein wasting as urea exertion in the urine, which is one of the most serious of all effects of severe diabetes mellitus. Insulin, GH, cortisol, T3 and T4 and IGF-I play a dominant role in inhibit proteolysis. The glucagon and epinephrine stimulate protein proteolysis (Umpleby & Russell-Jones, Citation1996).
8. The hormonal regulation of lipid metabolism
Lipid metabolism (lipogenesis and lipolysis) is influenced by Insulin and other related hormones (Table and Figure ). All aspects of lipid breakdown and use for providing energy are enhanced in the absence of insulin or minimum secretion, for instance diabetes mellitus when secretion of insulin is almost zero. Lipolysis of storage fat occurs during insulin deficiency and release of free fat acids, increase plasma cholesterol and phospholipids levels. Thus, excess usage of fats during insulin lack causes ketosis and acidosis (McKee & McKee, Citation2012c).
8.1. Lipogenesis
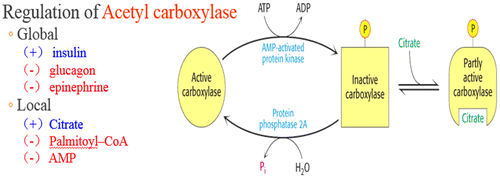
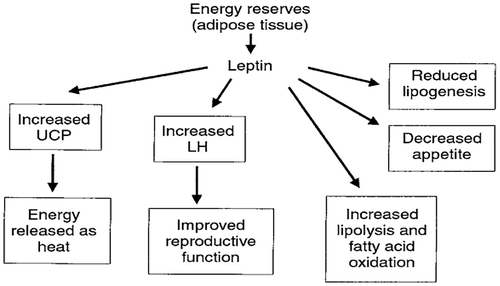
Lipogenesis is a process of fat synthesis from fatty acid and triacylglycerol. Insulin promotes lipogenesis, and increases the uptake of triglycerides from blood stream into adipose tissue and muscle. Leptin hormone and β-adrenergic agonists (β-AA) reduce lipogenesis (Figures and ).
8.2. Lipolysis
Lipolysis is a process of degradation fat to fatty acid and triacylglycerol. Insulin decreases the rate of lipolysis and leads to fat storage in adipose tissue and hence lowers the plasma fatty acid level and decreases the utilization of fat. It decreases the rate of fatty acid oxidation in muscle and liver (Figure ). Insulin inhibits the action of hormone-sensitive lipase and promotes glucose transport thought the cell membrane into the fat cells. Some of this glucose is then used to synthesize minute amounts of fatty acids, it also forms large quantity of α-glycerol phosphate. In diabetes or prolonged starvation its lead to insulin deficiency, results an increased lipolysis and increases FFAs, which finally increases formation of ketone bodies (ketonemia, ketonuria and ketosis) (Dimitriadis et al., Citation2011). Also, β-AA (Figure ) and leptin (Figure ) increases lipolysis and fatty acid oxidation.
Figure 8. Regulation of fatty acid synthesis (McKee & McKee, Citation2012c).
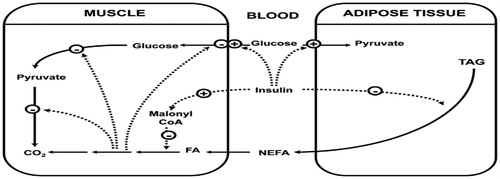
9. Randle cycle (The glucose/fatty acid cycle)
The major store of triacylglycerol in the body is present in adipose tissue and it is mobilized in the form of long chain fatty acids, which are carried to other tissues via the blood stream. Muscle oxidizes non-esterified fatty acids (NEFA) derived from adipose tissue and obtains energy. However, NEFA oxidation not only provides energy, but also regulatory mechanism by which some hormones can decrease the rates of glucose utilization and oxidation. In addition, glucose decreases the rate of NEFA oxidation, so that there is a reciprocal relationship between the oxidation of these two fuels; this control mechanism is known as the glucose/fatty acid cycle (Randle, Citation1998) (Figure ). This cycle enables a different way as insulin regulates the blood glucose concentration. Insulin has two effects in the glucose fatty acid cycle, both of these effects leading to the same result in a marked increase in the rate of glucose utilization in muscle: (1) a decrease in the plasma level of NEFA via the effect on adipose tissue lipolysis when an increased concentration of insulin, because insulin is a potent antilipolytic hormone; and (2) a specific decrease in the rate of NEFA oxidation via an increase in malonyl-CoA content within the muscle (Kelley & Mandarino, Citation2000; Ruderman, Saha, Vavvas, & Witters, Citation1999). These and other observations have led to the concept of “lipotoxicity” in the pathogenesis of hyperglycemia in type 2 diabetes (Kraegen, Cooney, Ye, Thompson, & Furler, Citation2000).
Figure 9. Overview of the randle cycle (the glucose/fatty acid cycle) (Dimitriadis et al., Citation2011).
10. Factors affecting insulin and glucagon secretion
The secretion of insulin and glucagon is explained in Table . These hormones are influenced by many things such as postprandial (after eating) periods, VFAs levels, nervous system and gastrointestinal hormones (Brockman, Citation1978).
Table 3. Factors and conditions increased and decreased insulin and glucagon secretion (Bassett, Citation1972, Citation1974; Evans, Buchanan-Smith, & Macleod, Citation1975; Ross & Kitts, Citation1973)
11. Diabetes mellitus
It is a syndrome of impaired carbohydrate, fat, and protein metabolism caused by either lack of insulin secretion or decreased sensitivity of the tissue to insulin. It has two forms: Type 1 (insulin dependent diabetes mellitus, IDDM) is caused by lack of insulin secretion. Type 2 (Non-insulin dependent diabetes mellitus, NIDDM, or also called insulin resistance) is caused by decrease sensitivity of target tissue to the metabolic effects of insulin lead to may suffer from a “relative” insulin deficiency. Over 40% of patients with Type 2 diabetes require insulin as part of their diabetes management plan (American Diabetes Association, Citation2014).
12. Somatostatin, growth hormone-inhibiting hormone (GHIH or SRIF)
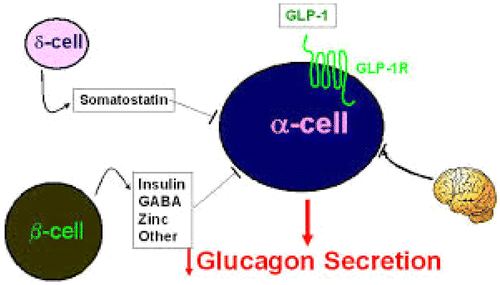
Somatostatin inhibits glucagon and insulin secretion. Definition, action, origin, metabolic effect and effect on blood glucose of somatostatin are shown in Tables , and and Figures and . Somatostatin secretion is increased by increased blood glucose, AA, FA and several of the gastrointestinal hormone concentrations released from the upper gastrointestinal tract in response to food uptake. This hormone has multiple inhibitory effects as: acts locally within the islets of langerhans themselves to depress the secretion of both insulin and glucagon (Hsu, Xiang, Rajan, Kunze, & Boyd, Citation1991; Strowski, Parmar, Blake, & Schaeffer, Citation2000).
Table 4. Summary of metabolic effects of somatotrophin (Squires, Citation2011)
Table 5. Hormones involved in regulation of blood glucose and their metabolic effect & actions (Rajendran et al., Citation1979)
Figure 10. Action and metabolic effect of somatostatin on insulin and glucagon (Rajendran et al., Citation1979).
13. Role of other hormones in the maintenance of blood glucose level
In addition to insulin, glucagon, growth hormone and several hormones including cortisol and catecholamines contribute to the regulation of glucose metabolism by the liver (Cotrozzi, Casini, Relli, & Buzzelli, Citation1996). Other hormones which increase the blood glucose level are: growth hormone, thyroxine, cortisol and adrenaline. These hormones are explained in Table and Figures –.
Figure 11. Regulation of blood glucose level by insulin and glucagon and other related hormones (Rajendran et al., Citation1979).
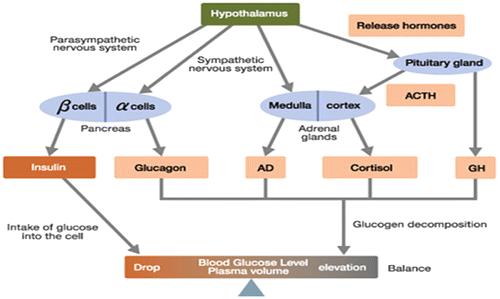
Figure 12. Role of cortisol in converted protein and fat to glucose blood glucose in stress (Rajendran et al., Citation1979).
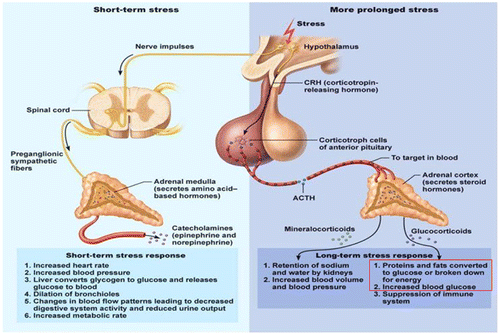
Figure 13. Metabolic disorder during stress (Marilou, Citation2004).
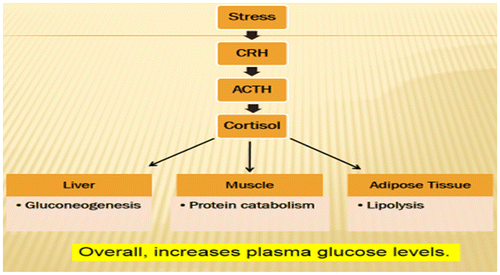
Figure 14. Glucose counter-regulatory hormones: effect on liver (Rajendran et al., Citation1979).
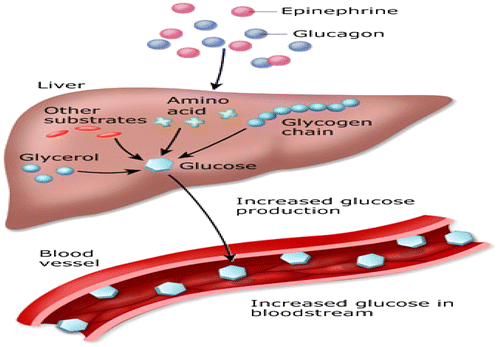
Figure 15. Metabolic effects of β-adrenergic agonists (Squires, Citation2011).
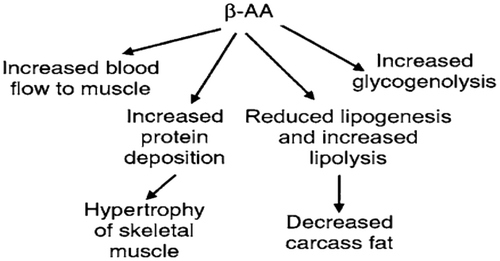
Figure 16. Regulation of growth hormone release (Squires, Citation2011).
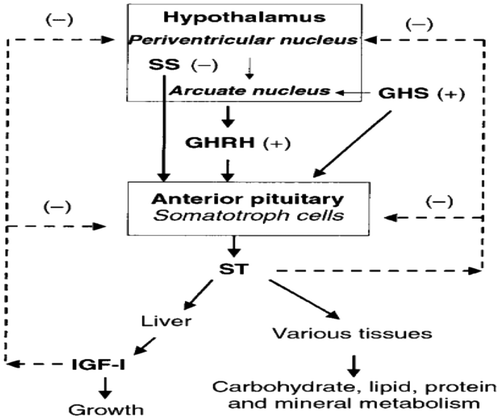
Figure 17. Effects of thyroid hormones on metabolism, growth and development. BMR, basal metabolic rate (Squires, Citation2011).
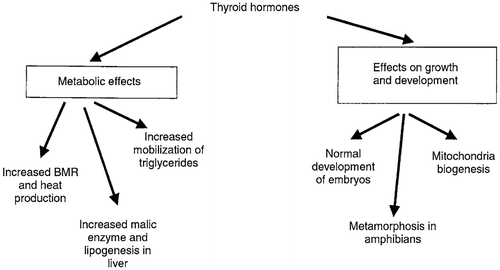
Figure 18. Metabolic effects of leptin (Squires, Citation2011).
14. Conclusion
Insulin and glucagon play important roles in regulating metabolism, including glycolysis, glycogenesis, glycogenolysis, gluconeogenesis, lipogenesis, lipolysis, and protein synthesis. Insulin appears to be primarily involved in regulation at peripheral sites, specifically muscle and adipose tissue.
Glucagon and other related hormones appears to direct its action primarily in the liver to increase glucose production. In ruminants, fermentation of carbohydrates to VFAs in the rumen limits the amount of glucose that reaches the small intestine. The resulting hypoglycemia initiates numerous hormonal changes. These hormonal changes are intended to stimulate glucose synthesis from protein and to spare glucose utilization by providing alternate energy sources from adipose tissue. The provision of alternate energy sources, such as FFA, can exceed the availability of gluconeogenic precursors, causes impaired oxidation of FFA and results in hyperketonemia, and hypoglycemia that can lead to development of lactation ketosis. Changes in the hormones, especially glucagon, control carbohydrate, protein, and lipid metabolism. β-cells are essentially “fuel sensors” that monitor and react to elevated nutrient load by releasing insulin. Thus, any failure in pancreatic β-cell function may lead to the development of type 2 diabetes mellitus. An increasing blood glucose, FFA and AA, gastrointestinal hormones and glucagon, growth hormone, cortisol, parasympathetic stimulation, insulin resistance and obesity are factors or conditions that increase insulin secretion. In contrary, hypoglycemia, fasting, somatostatin and α-adrenergic activity are factors decreasing insulin secretion.
Funding
I would like to thank King Saud University, Deanship of Scientific Research, College of Food & Agriculture Sciences, Agriculture Research Center to financial support of this scientific research.
Additional information
Notes on contributors
Mohammed M. Qaid
Dr Mohammed M. Qaid completed Bachelor of Science (BSc) in Veterinary Medicine and Surgery from Thamar University, and completed Masters of Sciences in Poultry (MSc) in Poultry Immunology in King Saud University, and presently PhD student.
Mutassim M. Abdelrahman
Prof Mutassim M. Abdelrahman working as Teacher and Scientist of Ruminant Nutrition at King Saud University, College of Food and Agricultural Sciences. He had his PhD degree in Ruminant Nutrition.
References
- Annison, E., & Armstrong, D. (1970). Volatile fatty acid metabolism and energy supply. Physiology of digestion and metabolism in the Ruminant. Newcastle Upon Tyne: Oriel Press Ltd.
- American Diabetes Association (2014). Diagnosis and classification of diabetes mellitus. Diabetes Care, 37, S81–S90.
- Ballard, F., Hanson, R., & Kronfeld, D. (1969). Gluconeogenesis and lipogenesis in tissue from ruminant and nonruminant animals. Federation Proceedings, 1969, 218–231.
- Bassett, J. (1972). Plasma glucagon concentrations in sheep: Their regulation and relation to concentrations of insulin and growth hormone. Australian Journal of Biological Sciences, 25, 1277–1288.10.1071/BI9721277
- Bassett, J. (1974). Early changes in plasma insulin and growth hormone levels after feeding in lambs and adult sheep. Australian Journal of Biological Sciences, 27, 157–166.10.1071/BI9740157
- Bassett, J. (1975). Dietary and gastro-intestinal control of hormones regulating carbohydrate metabolism in ruminants. Digestion and Metabolism in the Ruminant, 383–398.
- Bergman, E. (1973). Glucose metabolism in ruminants as related to hypoglycemia and ketosis. Cornell Veterinarian, 63, 341–382.
- Bergman, E., Katz, M., & Kaufman, C. (1970). Quantitative aspects of hepatic and portal glucose metabolism and turnover in sheep. American Journal of Physiology, 219, 785–793.
- Bergman, E., Starr, D. J., & Reulein, S. (1968). Glycerol metabolism and gluconeogenesis in the normal and hypoglycemic ketonic sheep. American Journal of Physiology Legacy Content, 215, 874–880.
- Bloom, S., Edwards, A., Hardy, R., Malinowska, K., & Silver, M. (1975). Endocrine responses to insulin hypoglycaemia in the young calf. The Journal of Physiology, 244, 783–803.10.1113/jphysiol.1975.sp010826
- Brockman, R. (1977). Glucose-glucagon relationships in adult sheep. Canadian Journal of Comparative Medicine, 41, 95.
- Brockman, R. (1978). Roles of glucagon and insulin in the regulation of metabolism in ruminants. A review. The Canadian Veterinary Journal, 19, 55.
- Brockman, R. P. (1979). Effect of somatostatin suppression of glucagon secretion on glucose production in sheep. Canadian Journal of Physiology and Pharmacology, 57, 848–852.10.1139/y79-129
- Brockman, R. P., & Johnson, M. R. (1977). Evidence of a role for glucagon in regulating glucose and β-hydroxybutyrate metabolism in sheep. Canadian Journal of Animal Science, 57, 177–180.10.4141/cjas77-021
- Brockman, R. P., & Laarveld, B. (1986). Hormonal regulation of metabolism in ruminants; a review. Livestock Production Science, 14, 313–334.10.1016/0301-6226(86)90012-6
- Brockman, R., & Manns, J. (1974). Effects of glucagon on activities of hepatic enzymes in sheep. The Cornell Veterinarian, 64, 217.
- Clark, M. G., Filsell, O. H., & Jarrett, I. G. (1976). Gluconeogenesis in isolated intact lamb liver cells. Effects of glucagon and butyrate. Biochemical Journal, 156, 671–680.10.1042/bj1560671
- Consoli, A., Nurjahan, N., Gerich, J. E., & Mandarino, L. J. (1992). Skeletal muscle is a major site of lactate uptake and release during hyperinsulinemia. Metabolism, 41, 176–179.10.1016/0026-0495(92)90148-4
- Cotrozzi, G., Casini, R. V., Relli, P., & Buzzelli, G. (1996). Role of the liver in the regulation of glucose metabolism in diabetes and chronic liver disease. Annali Italiani di Medicina Interna: organo ufficiale della Societa, Italiana di Medicina Interna, 12, 84–91.
- Dashty, M. (2013). A quick look at biochemistry: Carbohydrate metabolism. Clinical Biochemistry, 46, 1339–1352.10.1016/j.clinbiochem.2013.04.027
- Dimitriadis, G., & Newsholme, E. (2004). Integration of biochemical and physiologic effects of insulin on the control of blood glucose concentrations. In D. LeRoith, S. Taylor, & J. Olefsky (Eds.), Diabetes mellitus: A fundamental and clinical text (3rd ed., pp. 183–197). Philadelphia, PA: Lippincott Williams & Wilkins.
- Dimitriadis, G., & Raptis, S. (2000). Thyroid hormone excess and glucose intolerance. Experimental and clinical endocrinology & diabetes: Official journal. German Society of Endocrinology and German Diabetes Association, 109, S225–239.
- Dimitriadis, G., Leighton, B., Vlachonikolis, I., Parry-Billings, M., Challiss, R., West, D., & Newsholme, E. (1988). Effects of hyperthyroidism on the sensitivity of glycolysis and glycogen synthesis to insulin in the soleus muscle of the rat. Biochemical Journal, 253, 87–92.10.1042/bj2530087
- Dimitriadis, G., Parry-Billings, M., Bevan, S., Dunger, D., Piva, T., Krause, U., … Newsholme, E. (1992). Effects of insulin-like growth factor I on the rates of glucose transport and utilization in rat skeletal muscle in vitro. Biochemical Journal, 285, 269–274.10.1042/bj2850269
- Dimitriadis, G., Parry-Billings, M., Bevan, S., Leighton, B., Krause, U., Piva, T., … Newsholme, E. (1997). The effects of insulin on transport and metabolism of glucose in skeletal muscle from hyperthyroid and hypothyroid rats. European Journal of Clinical Investigation, 27, 475–483.10.1046/j.1365-2362.1997.1380688.x
- Dimitriadis, G., Mitrou, P., Lambadiari, V., Maratou, E., & Raptis, S. A. (2011). Insulin effects in muscle and adipose tissue. Diabetes Research and Clinical Practice, 93, S52–S59.10.1016/S0168-8227(11)70014-6
- Epstein, H., Shepherd, R., & Kahn, B. (1999). Glucose transporters and insulin action—Implications for insulin resistance and diabetes mellitus. New England Journal of Medicine, 341, 248–257.10.1056/NEJM199907223410406
- Evans, E., Buchanan-Smith, J., & Macleod, G. (1975). Postprandial patterns of plasma glucose, insulin and volatile fatty acids in ruminants fed low- and high-roughage diets. Journal of Animal Science, 41, 1474–1479.10.2527/jas1975.4151474x
- Frayn, K. N. (2009). Metabolic regulation: A human perspective (3rd ed.). Oxford: University of Oxford.
- Gardner, G., McGilchrist, P., & Pethick, D. (2014). Ruminant glycogen metabolism. Animal Production Science, 54, 1575–1583.10.1071/AN14434
- Hanson, R., & Ballard, F. (1967). The relative significance of acetate and glucose as precursors for lipid synthesis in liver and adipose tissue from ruminants. Biochemical Journal, 105, 529–536.10.1042/bj1050529
- Hawthorne, J. (1984). Biochemistry for the Medical Sciences. Journal of the Royal Society of Medicine, 77, 712.
- Hsu, W., Xiang, H., Rajan, A., Kunze, D., & Boyd, A. (1991). Somatostatin inhibits insulin secretion by a G-protein-mediated decrease in Ca2+ entry through voltage-dependent Ca2+ channels in the beta cell. Journal of Biological Chemistry, 266, 837–843.
- Jarrett, I., Filsell, O., & Ballard, F. (1974). Metabolic and endocrine interrelationships in normal and diabetic sheep. Hormone and metabolic research= Hormon-und Stoffwechselforschung= Hormones et métabolisme, 111–116.
- Jarrett, I., Filsell, O., & Ballard, F. (1976). Utilization of oxidizable substrates by the sheep hind limb: Effects of starvation and exercise. Metabolism, 25, 523–531.10.1016/0026-0495(76)90006-8
- Kelley, D. E., & Mandarino, L. J. (2000). Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes, 49, 677–683.10.2337/diabetes.49.5.677
- Khachadurian, A., Adrouni, B., & Yacoubian, H. (1966). Metabolism of adipose tissue in the fat tail of the sheep in vivo. Journal of Lipid Research, 7, 427–436.
- Kraegen, E., Cooney, G., Ye, J., Thompson, A., & Furler, S. (2000). The role of lipids in the pathogenesis of muscle insulin resistance and beta cell failure in type II diabetes and obesity. Experimental and clinical Endocrinology & Diabetes: Official journal. German Society of Endocrinology and German Diabetes Association, 109, S189–201.
- Leighton, B., Dimitriadis, G. D., Parry-Billings, M., Bond, J., de Vasconcelos, P. R., & Newsholme, E. A. (1989). Effects of insulin on glucose metabolism in skeletal muscle from septic and endotoxaemic rats. Clinical Science, 77, 61–67.10.1042/cs0770061
- Leng, R. (1970). Glucose synthesis in ruminants. Advances Vet Science, 14, 209–260.
- Liu, Z., & Barrett, E. J. (2002). Human protein metabolism: Its measurement and regulation. American Journal of Physiology-Endocrinology and Metabolism, 283, E1105–E1112.10.1152/ajpendo.00337.2002
- Longnecker, D. (2014). Anatomy and histology of the pancreas. The Pancreapedia: Exocrine Pancreas Knowledge Base. Retrieved from http://www.pancreapedia.org/reviews/anatomy-and-histology-of-pancreas
- Mandarino, L. J. (1989). Regulation of skeletal muscle pyruvate dehydrogenase and glycogen synthase in man. Diabetes/Metabolism Reviews, 5, 475–486.10.1002/dmr.v5:6
- Mandarino, L. J., Printz, R. L., Cusi, K. A., Kinchington, P., Odoherty, R. M., Osawa, H., … DeFronzo, R. A. (1995). Regulation of hexokinase II and glycogen synthase mRNA, protein, and activity in human muscle. American Journal of Physiology-Endocrinology and Metabolism, 269, E701–E708.
- Marilou, B. (2004). Hormone metabolism. Valenzuela: Department of Biochemistry & Nutrition. Fatima College of Medicine. Our Lady of Fatima University.
- Martin, D. W. (1983). Insulin and glucagon. Actions of insulin. Arper’s Review of Biochemistry (pp. 257–274). New York, NY: Lange Medical Publications.
- McKee, T., & McKee, J. R. (2012a). Biochemistry: The molecular basis of life. Carbohydrate metabolism (3rd ed.). Oxford: Oxford University Press.
- McKee, T., & McKee, J. R. (2012b). Biochemistry: The molecular basis of life. 8. Carbohydrate Metabolism. Oxford: Oxford University Press.
- McKee, T., & McKee, J. R. (2012c). Biochemistry: The molecular basis of life. 12. Lipid Metabolism. Oxford: Oxford University Press.
- Newsholme, E., & Crabtree, B. (1975). Substrate cycles in metabolic regulation and in heat generation. Biochemical Society Symposium, 1975, 61–109.
- Newsholme, E., & Dimitriadis, G. (1996). Some thoughts on the importance of insulin in the regulation of the blood glucose level. Experientia, 52, 421–425.10.1007/BF01919310
- Rajendran, S. S., Pharm, M., & Santhi, N. (1979). Hormonal regulation of blood glucose. (Diabetes mellitus). Sulur: RRVS College of Pharmaceutical Sciences.
- Randle, P. J. (1998). Regulatory interactions between lipids and carbohydrates: The glucose fatty acid cycle after 35 years. Diabetes/Metabolism Reviews, 14, 263–283.10.1002/(ISSN)1099-0895
- Ross, J., & Kitts, W. (1973). Relationship between postprandial plasma volatile fatty acids, glucose and insulin levels in sheep fed different feeds. The Journal of Nutrition, 103, 488–493.
- Rossetti, L., & Giaccari, A. (1990). Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. Journal of Clinical Investigation, 85, 1785.10.1172/JCI114636
- Ruderman, N. B., Saha, A. K., Vavvas, D., & Witters, L. A. (1999). Malonyl-CoA, fuel sensing, and insulin resistance. American Journal of Physiology-Endocrinology and Metabolism, 276, E1–E18.
- Savan, P., Jeacock, M., & Shepherd, D. (1976). Hepatic gluconeogenesis in foetal and suckling lambs. The Proceedings of the Nutrition Society, 35, 30A–30A.
- Shoemaker, W. C., Yanof, H. M., Turk, L. N., & Wilson, T. H. (1963). Glucose and fructose absorption in the unanesthetized dog. Gastroenterology, 44, 654–663.
- Shrayyef, M. Z., & Gerich, J. E. (2010). Normal glucose homeostasis. In L. Poretsky (Ed.), Principles of diabetes mellitus (pp. 19–35). Philadelphia, PA: Springer Science + Business Media.
- Shulman, G., Rossetti, L., Rothman, D., Blair, J., & Smith, D. (1987). Quantitative analysis of glycogen repletion by nuclear magnetic resonance spectroscopy in the conscious rat. Journal of Clinical Investigation, 80, 387.10.1172/JCI113084
- Squires, E. J. (2011). Chapter 3: Manipulation of growth and carcass composition. In Applied animal endocrinology (2nd ed., pp. 89–155). Guelph: Department of Animal and Poultry Science, University of Guelph.
- Strowski, M., Parmar, R., Blake, A., & Schaeffer, J. (2000). Somatostatin inhibits insulin and glucagon secretion via Two receptor subtypes: An in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice 1. Endocrinology, 141, 111–117.
- Suckale, J., & Solimena, M. (2008). Pancreas islets in metabolic signaling-focus on the beta-cell. Frontiers in Bioscience, 13, 7156–7171.10.2741/3218
- Trenkle, A. (1981). Endocrine regulation of energy metabolism in ruminants. Federation Proceedings, 1981, 2536–2541.
- Umpleby, A. M., & Russell-Jones, D. L. (1996). The hormonal control of protein metabolism. Baillière’s Clinical Endocrinology and Metabolism, 10, 551–570.10.1016/S0950-351X(96)80711-7
- West, C., & Passey, R. (1967). Effect of glucose load and of insulin on the metabolism of glucose and of palmitate in sheep. Biochemical Journal, 102, 58–64.10.1042/bj1020058
- Winter, G. (2013). Blood sugar insulin cycle graphic. Retrieved from http://www.allthingsgym.com/blood-sugar-insulin-cycle-graphic/
