Abstract
Effects of various hormonally treated substrates on mycelial development, pinning and biomass of Pleurotus ostreatus were investigated. A 3 × 4 experimental layout was used i.e. three substrates: Urochloa panicoides, Zea mays and Datura stramonium and four hormonal treatments: cytokinins; auxins; gibberellins and control. Treatments were replicated three times and arranged in a Completely Randomize Block Design. The results indicated significant differences on mycelial, pinning, fruit caps and biomass (p ≤ 0.05). Mycelial development in respective substrates was as follows: U. panicoides (100%), Z. mays (84.78%) and D. stramonium (36.31%) and pinning rate was 65.48, 32.99, and 12.23% respectively. Hormones also had significant effect on cap size and style length (p ≤ 0.05). Cap sizes were: auxins: 13.42 cm, cytokinins: 9.9 cm and gibberellins: 7.13 cm and style lengths were: 6.93, 8.83 and 11.03 cm respectively. Mushroom biomasses from different substrates were: U. panicoides (7,609.56 g), Z. mays (7,296.42 g) and D. stramonium (4,368.15 g) indicating significant influence of substrate on mushroom biomass.
Public interest statement
Outcome of the study is development of a low cost food production technology that enables farmers in Arid and Semi-arid regions to convent a wide range of plant materials such as different crop residues and weeds into protein and essential mineral rich oyster mushroom. Production of this food source in-between cropping seasons helps to bridge the hunger gap period by providing the nutritional requirements of the local communities. This oyster mushroom enterprise also offers opportunities of generating income for their household needs. Furthermore, findings of this study also enable resource poor farmers to make informed decision on the choice of the best mushroom growth media that enables to improve mushroom productivity. The study similarly identifies plant growth hormones that can be used to improve the protein content of oyster mushrooms.
Competing Interests
The authors declare no competing interest.
1. Introduction
For more than a century, mushrooms have been harvested from the wild and some were cultivated outdoors i.e. without controlling the environmental conditions (Gupta, Citation1986). Over the past five decades growers who sought to increase production, initiated growing mushrooms indoors. They designed and constructed structures that enabled them to control growth conditions. Building of such structures is primarily meant to provide suitable microclimate throughout the year and therefore enabling continuous production of mushrooms (Sarker & Chowdhury, Citation2013). There are numerous species of mushrooms that produce well when grown indoors, among these species is the Pleurotus ostreatus commonly known as oyster mushroom. Oyster mushroom is one of the most important macro fungi that produce high levels of quality protein from various agro-wastes or forest wastes (Banik & Nandi, Citation2004).
Oyster mushrooms are mainly cultivated in the temperate and subtropical regions of the world (Quimio, Chang, & Royse, Citation1990). The initiative of using crop residues to grow oyster mushrooms stemmed from the realization that the incorporation of non-conventional crop production system into the existing agricultural systems can help to improve the socio-economic wellbeing of small scale farmers (Mukhopadhyay, Chatterjee, Chatterjee, & Guha, Citation2004). This mushroom species is highly tolerant to variations in temperature, humidity, light levels and carbon dioxide levels, and therefore an ideal crop for poor resourced smallholder farmers who often reside in unstable environmental conditions (Atikpo et al., Citation2008). Furthermore, oyster mushrooms can be produced on a small piece of land and have shorter production cycles and also require low production inputs (Gupta, Citation1986; Maniruzzaman, Citation2004).
In addition, these mushrooms can be produced after the main field crop has been harvested and therefore is an ideal food gap-bridging enterprise. It is also a potentially good income generating enterprise for the landless poor. Diversification of agriculture to high value crops and transformation of smallholder agriculture from subsistence to commercial business enterprise offer good promising options for revitalization of agriculture and wealth creation among rural poor.
Communal and smallholder farmers in semi-arid regions of South Africa generally experience low crop yields due to various biotic and abiotic factors. The biotic factors include the use of low yielding crop varieties some of which may not be climatically suitable to the area, and the abiotic factors include among other factors low rainfall averaging 450 to 550 mm per annum and, the relatively nutrient deficient soils. These soils are not able to sustain crop growth for many seasons without the application of commercial fertilizers (Shah, Ashraf, & IshtiaqCh, Citation2004). With this low land productivity, it is therefore advisable that alternative non-land based food production technologies be developed and introduced for adoption by the communal farmers in these dry regions. The success of commercialization of oyster mushroom lies in the development of technology that is easily adoptable by smallholder farmers particularly looking at utilization of locally available resources such as plant crop stover and other farm biomass. The objective of this study was therefore to investigate the effects of various substrates such as Urochloa panicoides, Datura stramonium and Zea mays inoculated with plant growth hormones: auxins, cytokinins and gibberellins on the development and yield of oyster mushrooms (P. ostreatus) in the semi-arid regions. In addition, to contribute into development of a low cost technology that is readily and easily adoptable by smallholder farmers in order to address food security at household level. The productivity of mushrooms is influenced by various factors such as growth media and other biochemical factors such as plant growth hormones (Khandakar, Citation2004). The type of substrate use to produce mushrooms and the trace elements contained by the substrates determines the development and the total biomass of the mushroom (Khandakar, Citation2004). It is therefore, the need to investigate effect of substrates and hormones on the production of oyster mushrooms.
2. Materials and methods
2.1. Study site
The study was carried out during 2015 winter cropping season at Molelwane farm, North-West University farm, North-West, South Africa located at 6.44 km away from the University Mafikeng Campus. Geographical location: 25′47′54″ south, 25′32′52″ north. In this study, plant capsule structures covered with a fabric cloth was used to create a growth environment/chamber for the oyster mushroom. Analysis of respective substrates for trace elements was carried out at NWU Animal Health laboratory and pH was analyzed at NWU Crop Science laboratory.
2.2. Descriptions of the experiment
2.2.1. Preparation of growth media
The individual substrates, that is, U. panicoides, Z. mays and D. stramonium were milled, and solar dried. The dry substrates were weighed before wetting i.e. U. panicoides - 1 kg, Z. mays - 1.23 kg and D. stramonium - 1.1 kg. All substrates were sterilized using an autoclave at a temperature of 121°C for 30 min at 100 kpa. After the sterilization of respective substrates, substrates were removed and allowed to cool in sterilized containers for sixteen hours. The following application rates of hormonal treatment were used to treat the respective substrates: Auxins (PoMaxa): 31.6%, Cytokinins (MaxCel): 31.6% and Gibberellins: 11.1% plus control—no hormone Determination of these rates was based previous similar work by various other workers (Khandakar, Citation2004; Sarker & Chowdhury, Citation2013). Distilled water was used to wet the hormonal treated substrates before inoculation with P. ostreatus spawn.
2.2.2. Spawn preparation
In order to minimise contamination, spawn preparation was carried in a laminar flow. A total bulk spawn of 11.77 kg was used to inoculate all the 36 experimental units at variable ratios for different substrates. These differences were because of the weight/volume differences between substrates. The inoculation ratios were therefore as follows: 1:3 for U. panicoides; 1:4 for Z. mays and 1:4 for D. stramonium.
2.2.3. pH analyses for the substrates
The pH analysis of all three individual substrates was carried out using the 1:25 probe and meter procedure with water. The pH was tested before the inoculation with oyster mushrooms and after harvest. The pH was not adjusted because the objective of study was to investigate influence of the inherent properties of the different substrates i.e. nutrient content, texture and pH. The following materials were used pH-Meter, reference, buffer pH 7.0, buffer solution pH 4.00, refillable electrode, distilled water, 150 ml glass beaker, magnetic stirrer, stir bar and a 100 ml graduated cylinder.
2.2.4. Trace elements analysis of substrates
As substrate trace element composition is an influential factor in the oyster mushroom growth and biomass production, it was therefore important that trace element composition of different test substrates to be analysed. The trace elements from the respective substrates were analyzed using the dry ashing macro and trace elements procedure provided by the Agri-Laboratory Association of Southern Africa guidelines. The substrates were dried at room temperature (26°C) for 72 h and milled thereafter; one gram (1 g) of substrates was placed in crucibles and further dried for 24 h. The crucibles with dry weight were placed into an ash machine at 600°C for a period of 8 h. The 4:1 ICP plant extract procedure was used, 8 ml Nitric acid (HNO3) and 2 ml Hydrochloric acid (HCl) were the chemicals used as reagents and incubated into a Microwave Reaction System Model 3000 for 45 min. Samples digested for 45 min, cooled and transferred into respective volumetric flask (100 ml) which were eventually topped-up with distilled water and left standing for 24 h. The samples were slowly transferred to McCartney bottles without disturbing the sediment. The ICP Mass Spectrometer (Perkin-Elmer, Citation1982, NexION 300Q) was used to analyze the trace elements of different substrates. The trace elements that were analyzed were as follows: Fe, Zn, Mg, Mn, K, Cl, Se, Cd, Pb, Cu, and Cr and these quantified in mg/100 g.
2.2.5. Experimental layout
The experiment was laid out in a 3 × 4 factorial treatment combination i.e. three substrates by four hormonal treatments. The treatment combinations were replicated three times making a total of 36 experimental units. The three growth substrates: Urochloa panicoides (Liverseed grass S1), Zea mays (Maize straw S2) and Datura stramonium (Thorn apple S3) and the hormone treatments were: 6-Benzynadine–a Cytokinins (H1); 2–4D- Auxin (H2); Gibberellic acid–a Gibberellin (H3) and Control (H0). The experimental units were arranged in Complete Randomized Block Design (CRBD). The twelve treatment combinations were: S1H0, S1H1, S1H2, S1H3, S2H0, S2H1, S2H2, S2H3, S3H0, S3H1, S3H2 and S3H3. The substrates from different treatment combinations were placed in each of the 70 cm diameter mushroom domes and each inoculated with 0.327 kg of spawn. The inoculated growth media was kept humid by using the scorpion irrigation system (Figure ). A water delivery of 1.5 L per hour was supplied in a form of drip by the water outlet of the scorpion irrigation system. The irrigation/watering schedule of the mushrooms was not fixed due to the dependence on temperature variations. Temperatures less than 30°C, the irrigation took place every second day for a period of two hours and temperatures above 30°C the irrigation intervals and scheduling were subjected to change.
2.2.6. Mycelia development, pinning and total biomass
Mushroom growth on different substrates and hormones was assessed by assessing mycelial development, fruiting body initiation and total biomass production. The data on mycelial development was collected at an interval of two days. The surface cover of mycelial development was measured inside each of the 70 cm diameter mushroom domes and measured in percentages dome cover. Data on pinning was also recorded at an interval of two days. The pinning heads inside the mushroom dome were measured for surface cover in percentages. The fruit bodies were weighed immediately after harvest using electronic balance and recording the total biomass in grams (g) at each harvest.
2.2.7. Fruit cabs and style length
At each harvest, the fruit cap diameters and style lengths of mushrooms harvested from each respective treatment combinations were measured using a tape measure.
2.2.8. Conversion efficiency
The means of spawn, substrates and total biomass of mushrooms for the three replicates for each treatment was calculated in order to determine the conversion efficiency ratio.
2.2.9. Temperature and humidity
The climatic condition i.e. temperature (degrees Celsius) and relative humidity (percentage) in the domes were recorded at an interval of two days from the first day of inoculation until harvest day using a multi-meter.
2.2.10. Statistical analysis
All collected and recorded data on mycelial development, pinning/fruiting and total biomass production for the different treatments combinations were subjected to Analysis of Variance (ANOVA) and all interpretations were based on mean comparison and standard deviation for the different treatments combinations. A Turkey’s HSD test was used to determine the significances of inter and intra treatments variations on growth characteristics and trace elements composition of substrates for the mean separation and standard deviation. The SAS 9.4 software package Version 2.0 (SAS, Citation2011) SAS Institute, Inc., 100 SAS Campus Drive, Cary, NC 27513–2414, USA, 2001–2011 was used to process all data.
3. Results and discussion
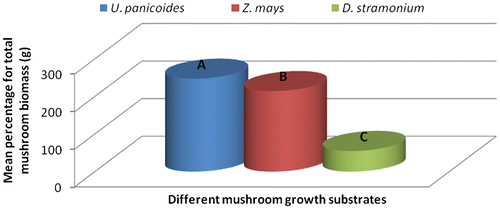
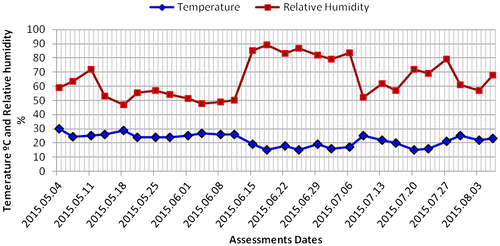
At the fourteenth assessment i.e. after 28 days from inoculation, mycelial colonization percentage was greater on mushrooms grown under the U. panicoides substrate followed by mushroom grown in Z. mays and D. stramonium as indicated in Figures and . The results on mycelial development indicated that U. panicoides experimental unit had the highest percentage of surface cover recording 100% followed by Z. mays with 84.78% and D. stramonium with 36.31% (Figure ). Trace elements such as Zn, Mn, and Cu are known to be anti-fungal and therefore are suppressive to fungal growth (Parashare, Pal, & Bhandari, Citation2013; Royse, Citation1995; Shah et al., Citation2004). The high percentage of mycelial development on U. panicoides and Z. mays could have been probably due to the low levels of anti-fungal trace elements such as Mn and Cu (Table ). Although, there was not much variation of pH levels between U. panicoides and Z. mays, however, mycelial development and fruiting rate was however noted to be higher in U. panicoides substrate. Z. mays had pH range of 6.83–7.93 which made it to be more alkaline than that of U. panicoides (Table ). The data analysis results further indicated that there were high levels of mycelial development, pinning and total biomass on U. panicoides straw and Z. mays substrates. This could have probably been because of the low levels of anti-fungal phytochemicals such as phenols, flavoids and alkanoids. These have previously been reported to be low in these two substrates (Dewanto, Wu, & Liu, Citation2002; MacLachlan et al., Citation2013). The delay on mycelial colonization in all three substrates could have been caused by effect of high temperatures which resulted in low humidity (Figure ). There is a relationship between temperature and relative humidity which greatly influences the mycelial development in oyster mushrooms. During the mycelial development phase, temperatures ranged from 20 to 30°C while relative humidity ranged from 45 to 60% (Figure ). However, relative humidity became more variable during other development phases of the mushroom reaching up to 90%. The mycelial development of oyster mushroom was reported to be optimum at temperatures ranging from 18 to 28°C and relative humidity range of 70–90% (Islam, Islam, Al Munsur, & Ali, Citation2007; Zervakis, Philippoussis, Ioannidou, & Diamantopoulou, Citation2001).
Figure 1a. Effect of different mushroom growth substrates on mycelial development rate assessed at 2-day interval for over a period of 28 days (%).
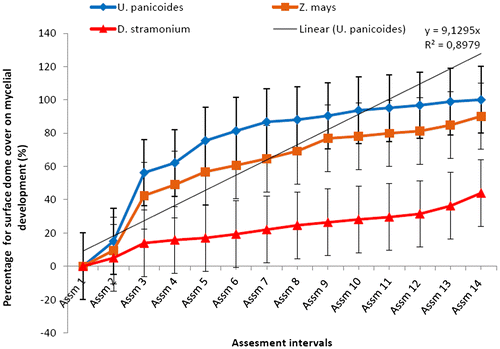
Figure 1b. Effect of different mushroom growth substrates on mycelial development over a period of 28 days (%).
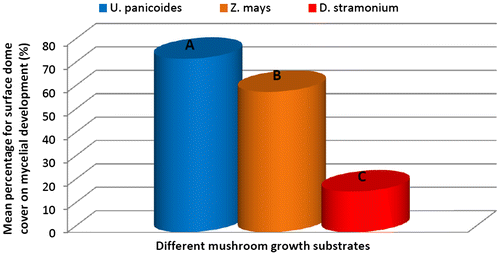
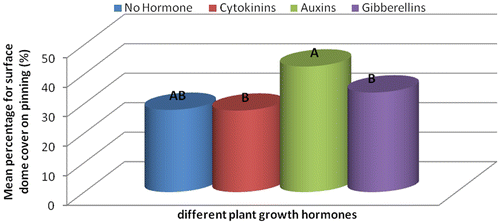
Figure 1c. Effect of different plant growth hormones on mycelial development over a period of 28 days (%).
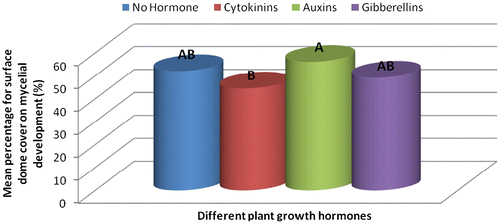
Figure 1d. Effect different treatment combinations (substrates X hormones interaction) on mycelial development over a period of 28 days (%).
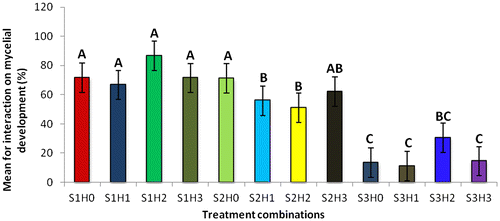
Figure 2a. Effect of different mushroom growth substrates on pinning rate over a period 64 days (%).
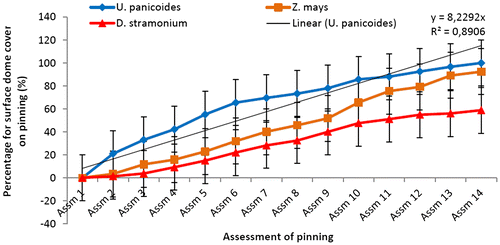
Figure 2b. Effect of different mushroom growth substrates on pinning rate over a period of 64 days (%).
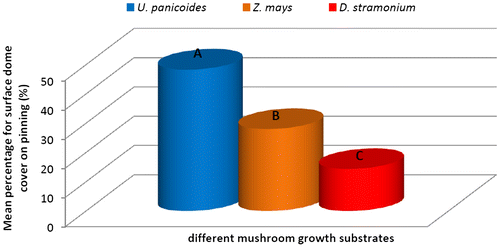
Figure 2d. Effect of different combinations (substrates X hormones interactions) on pinning over a period of 64 days.
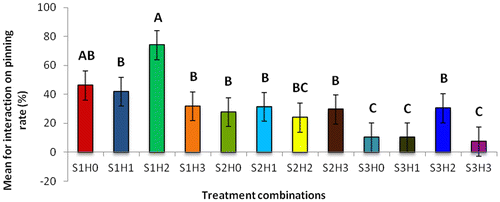
Figure 3a. Total mushroom biomass from different mushroom growth substrates for the production period (g).
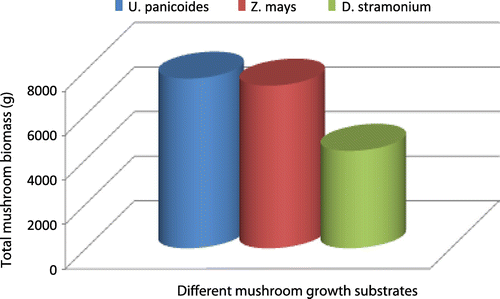
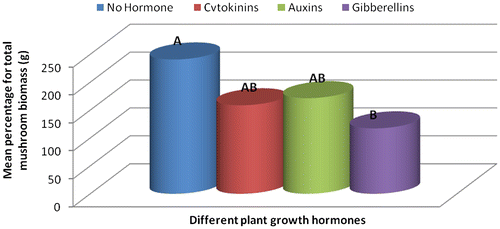
Figure 3d. Effect of substrates X hormones interactions on total biomass.
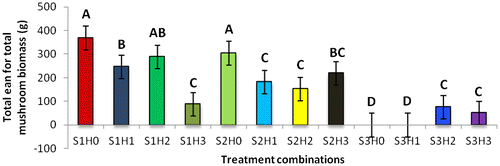
Table 1. pH analysis on substrates
Figure 4. Mean temperature (Degrees Celsius) and Relative humidity (%) recorded from 04 May 2015 to 03 August 2015 inside the mushroom domes during the experiment.
Furthermore, the poor mycelial development on substrate D. stramonium was probably due to the high pH and high levels of Mg, Ca and Cl (Tables and ). As indicated in Table , the pH of U. panicoides was 6.66 and according to Chitamba, Dube, Chiota, and Handiseni (Citation2012) fungus grow well in a pH ranging from 4.5 to 7 but their optimum pH is 6.5. The high pH level of 8.23 in D. stramonium which is highly alkaline could have had suppressive effect on mycelial growth. This could have subsequently caused delay in pinning and fruiting resulting in poor biomass production (4,368.15 g). Fanadzo, Zireva, Dube, and Mashingaidze (Citation2010) indicated that fungal growth was less prolific under high alkaline conditions. Buah, van der Puije, Bediako, Abole, and Showemimo (Citation2010) and Jonathan, Nwokolo, and Ekpo (Citation2013) both reported that pH of the substrate was one of the key factors that affect mycelial growth and subsequently the sporophore yields. It is also possible that the poor mycelial development which led to poor pinning on D. stramonium might have been because of the effect of high content of phytochemicals such as phenols, flavoids and alkanoids that is found in D. stramonium (Soni, Siddiqui, Dwivedi, & Soni, Citation2012). It has been reported that there is a negative correlation between the flavonoids and phenols content and the mushroom yield as well (Sreenivasa, Vinay, & Mohan, Citation2012). It was reported that on mushroom the higher the flavonoids, phenols and alkanoids content in the substrates the lower the yield of mushrooms (Chang, Citation1999).
Table 2. Analysis of trace elements on substrates composition (mg/100 g)
The data analysis results on pinning rates indicated significant difference between all three substrates (P ≤ 0.05). U. panicoides was first to reach 100% of pinning on day 64 (Figure ). The results indicated that among the three substrates, P. ostreatus pinning rate was comparatively more condensed and vigorous in U. panicoides growth media than any of the other two growth media (Figures and ). It was noted that the first pin heads (primordia) started appearing in U. panicoides within about 7–10 days after inoculation. The early mushroom pinning rates on U. panicoides was probably as a result of influence of low levels of trace elements such as Zn and Cu (Table ). High levels of these trace elements could have had an anti-fungal effect and therefore could have affected growth of the oyster mushroom. The pH levels of U. panicoides and Z. mays were 6.66 and 6.83 and were probably more appropriate for mushroom growth than that of Datura stramonium which was 8.23. (Table ).
The data analysis results of trace elements composition on the respective substrates (Table ), indicated that D. stramonium had the highest trace elements composition followed by Z. mays and U. panicoides. The high levels of Zn, Fe, Ca, and Mn in D. stramonium could have caused poor mushroom development. Several researchers reported that these trace elements had anti-fungal effect (Shah et al., Citation2004; Parashare et al., Citation2013). The low levels of N and K in D. stramonium (Table ) probably indicated that N and K were important elements for mushroom development and therefore when deficient in a substrate, mushroom yield would be compromised. Among these trace elements there was evidently remarkable high level of N, Cl, Mg, Ca and K in U. panicoides than the rest of the other substrates. High levels of trace elements K, Mg, and Ca on substrates are known to enhance fruiting cap diameter, style thickness and length which contribute to increase in weight, yield, protein, fibre and ash of the mushrooms (Hoa, Wang, & Wang, Citation2015). In addition, high levels of N had a close correlation with total colonization period, mushroom weight together with proteins content of mushroom (Hoa et al., Citation2015).
Substrates that were treated with auxins produced mushrooms with large fruit caps with an average diameter of 13.42 cm followed by those treated with cytokinins–9.9 cm, gibberellins–7.13 cm and the fruit caps size from substrates that had no hormonal treatment was 7.03 cm in diameter (Table ). Gibberellins treated substrates produced longer mushroom styles with a length of 11.03 cm, cytokinins–8.83 cm, no hormone–7.3 cm and auxins–6.93 cm. Despite that the mushrooms from substrates with no hormone had smaller fruit caps and shorter styles; they had the highest total biomass compared to all the hormonally treated substrates (Figure ). The large diameter of fruit caps (Table ) of mushrooms grown in auxin treated substrates was probably because of the enhanced cell elongation which is known to occur in plants treated with auxins (Grieneisen, Xu, Marée, Hogeweg, & Scheres, Citation2007). Mushrooms harvested from U. panicoides exhibited positive interaction with gibberellins in the influence of styles length recording the longest styles length (Table ). In plants, auxins are known to stimulate shoot elongation, seed germination, fruit and flower maturation (Khandakar, Citation2004).
Table 3. Fruit caps development (Diameter cm) and styles length (cm) over a period of 16 weeksTable Footnote*
The treatment combination S1H2 (U. panicoides + Auxins) was observed to be the best treatment combination for mycelial development and pinning rate (Figures and ). Similar findings were reported by Islam et al. (Citation2007) who observed the best colony production in oyster mushroom. This was probably due to the low content of anti-fungal trace elements found in substrate U. panicoides (Table ) and auxin which is known to enhanced mitotic cell division (Khandakar, Citation2004). As oyster mushrooms are grown on plant residues as substrates it is therefore logical to assume that plant growth hormones had an effect on the their growth (Cheng, Qin, Dai, & Zhao, Citation2007). Hormone auxins in plants are known to be responsible for cell elongation and control the differentiation of meristem into vascular tissue and promote leaf development and arrangement (Khandakar, Citation2004). Despite that S1H2 had the high percentage on mycelial development and pinning rate, S1H0 (U. panicoides + No hormone) had the highest total biomass (Figure ). Generally, it was observed that mushrooms produced in substrates that were not treated with hormones had the highest total biomass compared to the hormonal treated substrates (Figure ). The low total biomass of mushroom from hormonally treated substrates could have been because of increased mitotic cell division that probably created nutrient competition between the many individual pinnings and fruiting bodies. Competition in-between pinning populations could have caused a reduction in biomass of fruiting bodies. Carabelli et al. (Citation2007) highlighted that plant growth hormones affect many aspects of plant growth and development, including cell division, shoot initiation and growth, leaf senescence, apical dominance, sink/source relationships, nutrient uptake, phyllotaxis, and vascular, gametophyte, and embryonic development, as well as the response to biotic and abiotic factors. Similar influence could have been exerted on the development of these mushrooms.
Table shows that treatment combination S1H0 had a highest conversion efficiency ratio of 96.3% followed by S2H0–74.1% and S1H2–71.1%. Although the results indicated that treatment combination S1H0 (U. panicoides + No hormone) had highest conversion ratio it is essential that nutritional value i.e. trace element and protein content of these mushrooms should be analyzed.
Table 4. Substrate-mushroom conversion efficiency ratio
Funding
This work was supported by NWU Food Security and Safety Niche Area.
Additional information
Notes on contributors
Khosi Ramachela
This research was undertaken as part of Mr Sydwell M. Sihlangu’s MSc research programme under the supervision of K. Ramachela at North West University. Mr Sihlangu has developed a low cost oyster mushroom production technology and has interest in promoting the use of any readily available plant material as substrate for an appropriate food security intervention for smallholder farmers. Mr Sihlangu’s research findings have potential to address nutritional needs of the rural communities and his interest is to disseminate this technology to the rural communities.
Khosi Ramachela’s research interest is in mycology with particular focus in mycorrhizal fungi and oyster mushroom research. In mycorrhizal fungi, Ramachela’s research looks at use of mycorrhizal fungi in the management of soilborne diseases in both forestry and horticultural crops. His interest in oyster mushroom research is the development of various Food Security innovative approaches that can be employed to convert agricultural biomass into food sources.
References
- Atikpo, M., Onokpise, O., Abazinge, M., Louime, C., Dzomeku, M., Boateng, L., & Awumbilla, B. (2008). Sustainable mushroom production in Africa: A Case study in Ghana. African Journal Of Biotechnology, 7, 249–253.
- Banik, S., & Nandi, R. (2004). Effect of supplementation of rice straw with biogas residual slurry manure on the yield, protein and mineral contents of oyster mushroom. Industrial Crops and Products, 20, 311–319.10.1016/j.indcrop.2003.11.003
- Buah, J. N., van der Puije, G. C., Bediako, E. A., Abole, E. A., & Showemimo, F. (2010). The growth and yield performance of oyster mushroom (Pleurotus ostreatus) on different substrates. Biotechnology, 9, 338–342.
- Carabelli, M., Possenti, M., Sessa, G., Ciolfi, A., Sassi, M., Morelli, G., & Ruberti, I. (2007). Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes & Development, 21, 1863–1868.10.1101/gad.432607
- Chang, S. T. (1999). World production of cultivated edible and medicinal mushrooms in 1997 with emphasis on lentinus edodes (Berk.) sing, in China. International Journal of Medicinal Mushrooms, 1, 291–300.10.1615/IntJMedMushr.v1.i4
- Cheng, Y., Qin, G., Dai, X., & Zhao, Y. (2007). NPY1, a BTB-NPH3-like protein, plays a critical role in auxin-regulated organogenesis in Arabidopsis. Proceedings of the National Academy of Sciences, 104, 18825–18829.10.1073/pnas.0708506104
- Chitamba, J., Dube, F., Chiota, W. M., & Handiseni, M. (2012). Evaluation of substrate productivity and market quality of oyster mushroom (Pleurotus ostreatus) grown on different substrates. International Journal of Agricultural Research, 7, 100–106.10.3923/ijar.2012.100.106
- Dewanto, V., Wu, X., & Liu, R. H. (2002). Processed sweet corn has higher antioxidant activity. Journal of Agricultural and Food Chemistry, 50, 4959–4964.10.1021/jf0255937
- Fanadzo, M., Zireva, D. T., Dube, E., & Mashingaidze, A. B. (2010). Evaluation of various substrate and supplements for biological efficiency of Pleurotussajor-caju and Pleurotus ostreatus. African Journal of Biotechnology, 9, 2756–2761.
- Grieneisen, V. A., Xu, J., Marée, A. F., Hogeweg, P., & Scheres, B. (2007). Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature, 449, 1008–1013.10.1038/nature06215
- Gupta, R. S. (1986). Mushroom cultivation. Indian Horticulture, 31, 11.
- Hoa, H., Wang, C., & Wang, C. (2015). The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology, 43, 423–434.10.5941/MYCO.2015.43.4.423
- Islam, M. S., Islam, M. O., Al Munsur, M. A. Z., & Ali, M. S. (2007). Study on mycelial growth in different mushroom species and spawn production of Oyster mushroom in different substrates. Bangladesh Journal of Agricultural Science, 34, 19–22.
- Jonathan, S. G., Nwokolo, V. M., & Ekpo E. N. (2013). Yield performance of Pleurotus pulmonarius (Fries.) quelet, cultivated on different agro-forest wastes in Nigeria. World Rural Observations, 5, 22–30. ISSN: 1944- 6543.
- Khandakar, J. (2004). Effect of media composition and growth regulators on mycelial growth and spawn production of three mushroom species (MS Thesis). Department of Biotechnology, BAU, Mymensingh.
- MacLachlan, D. J., Blaney, B. J., Cook, L. G., Klim, E., Scholl, R., Sexton, M., … Watts, R. (2013). A review of potential contaminants in Australian livestock feeds and proposed guidance levels for feed Department of Agriculture and Food Western Australia. Animal Production Science, 53, 181–208.
- Maniruzzaman M. (2004). Influence of media composition and growth regulators on mycelial growth and spawn production of three mushroom species (MS Thesis). Department of Biotechnology, BAU, Mymensingh.
- Mukhopadhyay R., Chatterjee S., Chatterjee B. P., Guha A. K (2004). Enhancement of biomass production of edible mushroom Pleurotussajor-caju grown in whey by plant growth hormones. Process Biochemistry, 40, 1241–1244.
- Parashare V., Pal S., Bhandari A. (2013). Antimicrobial and nutritional studies on Agaricus bisporus and Pleurotus ostreatus. Acta Biological Indica 2013, 2, 310–315.
- Perkin-Elmer Corp. (1983). Analytical methods for atomic absorption spectrophometry. Norwalk, CT: Author.
- Quimio, T. H., Chang, S. T., & Royse, D. J. (1990). Technical guidelines for mushroom growing in the tropics. Food and Agriculture Organization of the United Nations, FAO Plant Production and Protection Paper 106, Rome.
- Royse, D. J. (1995). Specialty mushrooms: Cultivation on synthetic substrate in the USA and Japan. Inter disciplines Science Reviews, 20, 1–10.
- Sarker, R. R., & Chowdhury, A. K. M. S. H. (2013). Effect of different doses of GA3 application at primordia initiation stage on the growth and yield of Oyster mushroom. Journal of the Bangladesh Agricultural University, 11, 5–10.
- SAS. (2011). SAS user’s guide (10th edition). Cary, NC: Author.
- Shah, Z. A., Ashraf, M., & IshtiaqCh, M. (2004). Comparative study on cultivation of oyster mushroom (P. ostreatus) on different substrates (wheat straw, leaves, saw dust). In Pakistan Journal of Nutrition., 3, 158–160.
- Soni, P., Siddiqui, A. A., Dwivedi, J., & Soni, V. (2012). Pharmacological properties of Datura stramonium L. as a potential medicinal tree: An overview. Asian Pacific Journal of Tropical Biomedicine, 2, 1002–1008.10.1016/S2221-1691(13)60014-3
- Sreenivasa, S. Vinay, K., & Mohan, N. R. (2012, September). Phytochemicals analysis, antibacterial and antioxidant activity of leaf extract of datura stramonium. International Journal of Science Research, 1, 83–86.
- Zervakis, G., Philippoussis, A., Ioannidou, S., & Diamantopoulou, P. (2001). Mycelium growth kinetics and optimal temperature conditions for the cultivation of edible mushroom species on lignocellulosic substrates. Folia Microbiologica, 46, 231–234.10.1007/BF02818539