?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Plantain is a staple food in many African and Asian countries yet it is underutilized and experiences huge post-harvest losses annually. This study attempts to increase plantain utilization through processing into more shelf stable products. Blends of plantain flours with or without the peels and Moringa oleifera leaf powder were produced and analysed for the nutritional composition, antioxidative potential, functional and pasting properties. The dough meals from the flour blends were analysed for acceptability. The results obtained showed that incorporation of plantain peel and moringa leaves into the flours led to increase in crude fibre, and also raised the soluble and insoluble dietary fibres content by more than 50%. Flour blends also had increased water absorption capacity, but a reduced oil absorption and foaming capacity. The breakdown and final viscosities of the blends decreased significantly with inclusion of peel and moringa. Antioxidative potential of the blends was observed to have highest in flours containing peel and pulp. Addition of moringa leaves led to significant increase (p < 0.05) in the antioxidant potential of the flours. No significant difference was observed in the acceptability of the dough meals (amala) produced from different blends.
Keywords:
Public Interest Statement
The staple foods in many developing countries are mainly starchy in nature lacking the biochemical diversity required for normal healthy living. The wherewithal to diversify is a major problem with many living on less than USD1/day. Plantain grows well in most of these countries and suffers huge post-harvest losses. There is therefore the need to utilize the plantain when mature but still green before it ripens and deterioration rate increases. Combination of plantain (with the peels) and moringa in the appropriate proportion promises to give a meal rich in dietary fibre with good antioxidant properties and a composition that is suitable for the day-to-day commonly consumed dough meals with excellent sensory acceptability. The outcome of this research could be used by food processors and the government to serve local communities and internally displaced people (IDPs) who can easily be taught the combination to make the meal by themselves.
Competing Interest
The authors declare no competing interests.
1. Introduction
Plantain and Banana are economic crops in many tropical countries of the world providing income and food security to millions in Africa, Asia and Latin America. In sub-Saharan Africa, Uganda is the largest producer followed by Rwanda, Ghana, Nigeria, and Cameroon (IITA, Citation2016).
The cultivation of plantain is attractive to farmers due to the low labour requirements for production compared with cassava, maize, rice, and yam (Marriott & Lancaster, Citation1983). Although the production rate is increasing annually, post-harvest losses account for 35–60% due to poor handling, transportation, inadequate access to market, storage facilities and inappropriate food processing technologies (Affognon, Mutungi, Sanginga, & Borgemeister, Citation2015).
Banana pseudo-stem which once constitute waste and environmental problem can now be greatly utilized for nutritious food (Elanthikkal, Gopalakrishnapanicker, Varghese, & Guthrie, Citation2010). The peels of plantain, also regarded as waste, are rich in minerals such as potassium, calcium, magnesium, phosphorus, copper, and iron except sodium while the pulp is laden with vitamin C, B6, minerals (Aziz et al., Citation2011). Plantain and plantain peels are potential source of dietary fibre and pectin (Happi Emaga, Robert, Ronkart, Wathelet, & Paquot, Citation2008). Various types of delicacy can be made from plantain for human consumption when processed by boiling, smoking, frying (chips), or processed to flour for making dough meal (Famakin, Fatoyinbo, Ijarotimi, Badejo, & Fagbemi, Citation2016). In almost all the processing operations, the peels are often removed and used for animal feeding and if not properly discarded can constitute environmental problem (Happi Emaga et al., Citation2008).
Moringa oleifera is an essential plant in meeting global food security and sustaining the livelihoods of many millions of people. It is a highly valued plant cultivated in many countries of the world. Individual parts of M. oleifera tree have been attributed with numbers of medicinal properties such as the root, gum, bark, leaf, flowers, seed and seed oil thus M. oleifera has been used in the treatment of several diseases (Anwar, Latif, Ashraf, & Gilani, Citation2007).
Several products have been developed from composite flours having two or more of plantain, wheat, cassava and soybean for bread and pastries production (Akubor, Citation2003; Ho, Abdul Aziz, & Azahari, Citation2013; Olaoye, Onilude, & Idowu, Citation2006). There has been no information on the development of composite flour with plantain pulp, peels and the leaves of M. oleifera for dough meal. The current study evaluates the nutritional composition, dietary fibre contents, antioxidant composition, functional and pasting properties of the composite flours and dough meal.
2. Materials and methods
2.1. Materials
2.1.1. Source of materials and production of plantain-moringa composite flour
Mature green plantain and moringa leaves were obtained from the Teaching and Research Farm of the Federal University of Technology, Akure Ondo State, Nigeria. Moringa leaves were processed into powder as previously described (Badejo, Damilare, & Ojuade, Citation2014). Freshly harvested unripe plantain was divided into two portions. After washing, the first portion was peeled and blanched in hot water at 80°C for 5 min to prevent browning while the second portion was left unpeeled. Both portions were then sliced uniformly and oven dried at 65°C for 6–8 h. The dried chips were then milled to produce instant plantain flour. The moringa powder was mixed with the different plantain flours in the ratio 1:19.
2.1.2. Determination of proximate composition
The plantain-moringa flour blends were analysed for protein (950.48), fat (983.23), total ash (940.26) and moisture (925.09) by Association of Official Analytical Chemists [AOAC] (Citation2000) methods. The carbohydrate was determined by difference.
2.1.3. Determination of soluble and insoluble fibre content
The modified Klason dietary fibre method derived from the standardized method using AOAC (Citation2000) was deployed in this study. In the modified method, 300 mg of the defatted fibres were impregnated with 10 ml of 72% sulphuric acid and incubated at 30°C for 2 h in a water bath. Demineralised water (100 ml) was added to the sample and autoclaved at 125°C for 1 h, followed by cooling and filtration. The insoluble fibre (residual left) was washed with demineralised water until neutral pH 7.0 was obtained and then dried in a hot air oven at 105°C until the weight became constant. The insoluble fibre content was calculated by subtracting the final weight from the original. The filtrate obtained was used to determine the soluble fibre content in sulphuric acid by the spectrophotometric method where, 5 ml of 3% sulphuric acid was added to 5 ml of the filtrate and the absorbance at 205 nm was measured with UV Spectrophotometer (JENWAY Inc., Staffordshire, UK).
2.2. Functional properties
2.2.1. Determination of water and oil absorption capacity
The water and oil absorption capacity (WAC, OAC) was determined as described by Omowaye-Taiwo, Fagbemi, Ogunbusola, and Badejo (Citation2015) adapted from Sathe, Deshpande, and Salunkhe (Citation1982). Briefly, 1.0 g of each sample was dispersed in 10 ml distilled water (ρ = 1 g/ml) or Executive Chef ® vegetable oil (ρ = 0.92 g/ml) for WAC or OAC respectively. The content was stirred for 5 min with a magnetic stirrer. The content was centrifuged at 2,500 × g for 30 min and the volume of the supernatant obtained was measured. The water absorbed by the flour was calculated as the difference between the initial water used and the volume of the supernatant obtained after centrifuging, ditto for the oil absorbed but the density of the oil was used to obtain the weight absorbed. The water/oil absorbed by the flour was expressed as percentage.
2.2.2. Determination of bulk density
The bulk density of plantain flour was determined by the method of Ige, Ogunsua, and Oke (Citation1984). Briefly, certain quantity of each sample was weighed into a pre-weighed measuring cylinder (W1) and the new weight of the sample and measuring cylinder (W2) as well as volume occupied in the measuring cylinder (V) was taken. The bulk density was then calculated as shown below:
The standard method described by Leach, McCowen, and Schoch (Citation1959) modified by Fagbemi, Adeoya, and Badejo (Citation2012) was used in the determination of the swelling capacity.
2.2.3. Determination of foaming capacity
The foaming capacity of the plantain flour was determined by the method of Coffmann and Garcia (Citation1977). One gram of the sample was weighed into a measuring cylinder containing 50 ml distilled water and the volume of the resultant solution was recorded (V1). The solution was then homogenized with a high speed mechanical stirrer inside the cylinder for 5 min. The new volume with the foam was measured (V2). The foaming capacity was expressed as the percentage volume increase as shown below:
2.3. Antioxidant capacity determination
2.3.1. Preparation of the ethanol extract
Ethanolic extracts of the flour blends were prepared according to the method described by Burdock, Carabin, and Crincoli (Citation2009). Briefly, 10 ml of ethanol was added to 1 g of the powdered samples and subjected to continuous shaking in a laboratory mixing rotator. After 24 h, the solution was filtered through Whatman filter paper No.1 to recover the ethanolic extract of the samples.
2.3.2. DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging assay
The free radical scavenging ability of the extract against DPPH (1, 1-diphenyl-2-picrylhydrazyl) was determined using the method of Gyamfi, Yonamine, and Aniya (Citation1999). An aliquot of 1 ml of the extract was mixed with 1 ml of 0.4 mM DPPH solution and the mixture was left in the dark for 30 min before measuring the absorbance at 517 nm on JENWAY UV-visible spectrophotometer (JENWAY Inc.). The DPPH inhibition ability was calculated in percentages.
2.3.3. FRAP (Ferric Reducing Antioxidant Power)
The reducing property of the extract was determined as described by Pulido, Bravo, and Saura-Calixto (Citation2000). An aliquot of 0.25 ml of the extract was mixed with 0.25 ml of 200 mM of sodium phosphate buffer pH 6.6 and 0.25 ml of 1% Potassium Ferrocyanate (KFC). The mixture was incubated at 50°C for 20 min followed by the addition of 0.25 ml of 10% Tricarboxylic acid (TCA). The mixture was centrifuged at 2,000 ×g for 10 min and 1 ml of the supernatant was mixed with equal volume of distilled water and 0.1% of iron (III) chloride (FeCl3) and the absorbance was measured at 700 nm using a JENWAY UV–visible spectrophotometer. FRAP values were obtained by comparing the absorption change in the test mixture with those obtained from increasing concentrations of Fe2+, and expressed as mmol of Fe2+ equivalents per gram of the sample.
2.3.4. ABTS (2, 2-azino-bis 3-ethylbenz-thiazoline-6-sulfonic acid)
The ABTS scavenging ability of the extract was determined according to the method described by Re et al. (Citation1999). The ABTS was generated by reacting 7 mM ABTS with K2S2O8 (2.45 mmol/L, final concentration) in the dark for 16 h and adjusting the absorbance at 734 nm to 0.70 ± 0.02 with ethanol. Appropriate dilution of the extract was then added to 2.0 ml of ABTS solution and the absorbance was read at 734 nm after 5 min. The results were expressed as mmol of Trolox per gram sample.
2.4. Pasting properties of the plantain-moringa blends
The pasting properties of the samples were determined using a Rapid Visco Analyser (RVA) (RVA model RVA 3D+, Newport Scientific Australia). The sample (3.5 g) was weighed into a vessel prior to transfer into the test canister with 25 ml of water. The paddle was placed into the canister and the blade was vigorously swirled through the sample several times to ensure an intimate mix. The slurry was heated at 50°C with 3 min holding time. The measurement cycle was initiated by depressing the motor tower of the instrument. The rate of heating and cooling was recorded, peak time, pasting temperature as well as peak, breakdown, final, and setback viscosities were read from the pasting profile with the aid of thermocline for windows software connected to a computer.
2.5. Sensory evaluation
The flour blends were made into dough according to the method described by Akissoe et al. (Citation2001). The dough meals were subjected to sensory evaluation using a 9-point Hedonic scale with 1 representing dislike extremely and 9 like extremely. The dough meals were evaluated on the basis of taste, texture, mouldability, aroma, colour, and overall acceptability.
2.6. Statistical analysis
All the analyses were performed in triplicates. Data obtained from this study were subjected to analysis of variance (ANOVA) using Statistical Package for Social Sciences (SPSS) version 18.0. The means were separated using Duncan New Multiple Range Test (DNMRT).
3. Results and discussion
3.1. Proximate composition of the flour blends
The moisture content of the various blends ranged from 8.26 ± 0.90 to 9.76 ± 0.12% (Table ). Moisture content of unripe plantain has been found to vary depending on the drying method used (Fagbemi, Citation1999; Pacheco-Delahaye, Maldonado, Pérez, & Schroeder, Citation2008). Apart from the flour from plantain pulp (P), there was no significant difference between the crude fibres of all the samples. The values obtained in this study ranged from 1.67 to 2.09%. These values were similar to 2.00% reported for blanched plantain (Fagbemi, Citation1999) but higher than 1.18% reported for plantain banana (Pelissari, Andrade-Mahecha, Sobral, & Menegalli, Citation2012). The crude fat contents ranged from 6.80 ± 0.13 to 9.38 ± 0.56%. The flours containing moringa (PM and PPM) had higher values of crude fat compared to those without moringa. The low fat content of the plantain flours will prevent the development of rancid flavour during storage. The total ash content of all samples ranged from 0.85 ± 0.16 to 3.85 ± 0.12%. The unpeeled plantain flour with moringa leaves powder (PPM) had the highest value of 3.85 ± 0.12%. The peel that was incorporated into the flour must have contributed greatly to the higher total ash content. The plantain (pulp) flour (P) had total ash content of 1.92 ± 0.11% which is similar to earlier report on unripe plantain flour (Fagbemi, Citation1999; Pacheco-Delahaye et al., Citation2008; Pelissari et al., Citation2012). The crude protein content of all samples ranged from 4.44 ± 0.02 to 7.11 ± 0.12%. The protein contents obtained are higher in value compared to 3.6% reported by Fagbemi (Citation1999). The carbohydrate content ranged from 70.92 ± 0.79 to 75.31 ± 0.73%. These values are similar to previous reports (Pacheco-Delahaye et al., Citation2008; Pelissari et al., Citation2012).
Table 1. Proximate composition of plantain-moringa flour blends
3.2. Functional properties of unripe plantain flour
The bulk density of the samples ranged between 0.53 ± 0.02 and 0.84 ± 0.03 g/ml (Table ). The values are comparable to previous reports on plantain flours (Fagbemi, Citation1999; Yadav, Yadav, & Dhull, Citation2012). Low bulk density flour can find application in baby foods where high nutrient intake is of more importance than the bulk. It also plays a role in determining the packaging requirement for the flour. There was no significant difference observed in the swelling capacity of all the plantain blends which ranged from 0.40 ± 0.03 to 1.05 ± 0.06%. However, the unpeeled plantain flour (PP) with swelling capacity of 0.40 ± 0.03% was significantly lower than the rest (Table ). The swelling power is related to the water holding index and it is an indication of the associative force within the flour granules. The water absorption capacity of all the samples ranged from 180.52 ± 7.33 to 205.69. Apart from flour from the plantain pulp (P) that had the lowest water absorption capacity of 180.52 ± 7.33, there was no significant difference observed amongst the other plantain flour samples. Closely related water absorption capacities have been reported earlier for ordinary plantain flours (Mepba, Eboh, & Nwaojigwa, Citation2007; Yadav et al., Citation2012). The presence of hydrophilic constituent like the soluble fibre could have resulted in the increase observed in water absorption capacity as the peel and moringa powders were incorporated into the blends. This high water absorption capacity is very important in making foods that require high viscosity such as dough and as good thickening agent which may increase yield. The reverse was the case for the oil absorption capacity. The plantain flour from the pulp had the highest value of 204.33 ± 5.39% which was highly significant when compared to other samples. This is an indication that there are more hydrophobic groups on the surface of the protein in the pulp compared to the hydrophilic groups. These values compared favourably well with that reported by Fagbemi (Citation1999) but are higher than 144% reported by Yadav et al. (Citation2012) on blanched and unblanched plantain flours respectively. The foaming capacity of the samples ranged from 1.96 ± 0.08 to 7.84 ± 0.20%. The peeled plantain flour (P) and the sample with moringa powder (PM) have higher foaming capacity compared to the other two. This may be due to higher protein content of the samples. This property will make them useful in the food industry where improved texture and consistency is required.
Table 2. Functional properties of plantain-moringa flour blends
3.3. Soluble and insoluble fiber of the plantain flour blends
Dietary fibre is a group of non-starch polysaccharides and lignin, which are resistant to digestion and absorption in the small intestine but ends up being fermented in the large intestine. Within the body they promote beneficial physiological effects such as reduction in blood cholesterol and modulation of blood glucose (Brennan, Citation2005). Dietary fiber can be classified as insoluble (lignin, cellulose, and hemicelluloses) or soluble fiber (pectin, beta-glucans, and galactomanan gums) (Happi Emaga et al., Citation2008). Table showed that the samples were significantly different in dietary fibre composition. The insoluble dietary fibre values ranged between 28.09 ± 0.12 and 41.23 ± 0.33% with plantain (pulp only) (P) having the lowest and the unpeeled plantain with moringa (PPM) having the highest value. It had been reported that plantain peels generally have higher insoluble fibres compared to banana peel (Happi Emaga et al., Citation2008). Aziz et al. (Citation2011) analysed pseudo-stem of banana processed into flour and found them to be very high in insoluble dietary fibre. The peel and the moringa leaves included in the PPM might have contributed to the high insoluble dietary fibre content recorded. The soluble fibre contents also ranged between 0.92 ± 0.08 and 3.74 ± 0.12% with plantain flour (P) having the lowest value and unpeeled plantain flour with moringa (PPM) having the highest value. It is clear from the result that addition of moringa to the plantain increased the dietary fibre contents. This will make the dough meal from this flour a very good food that will ease defecation by virtue of the high percentage of insoluble fibre and may serve as a meal for the diabetic.
Table 3. Insoluble, solube and total dietary fibre content of plantain-moringa flour blends
3.4. Antioxidant properties of the plantain flour blends
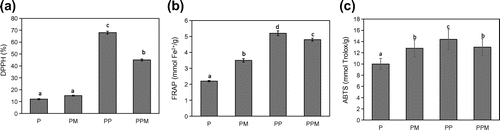
Antioxidants are considered important nutraceuticals (Gul, Singh, & Jabeen, Citation2016). They scavenge free radicals generated in the body due to metabolic processes. Plants and their by-products laden with antioxidants have been found to be of health benefit to human (Gul et al., Citation2016; Hou et al., Citation2003). The use of DPPH as an assay method is due to the good stability, simplicity and feasibility and the ability to form stable radicals (Aparadh, Naik, & Karadge, Citation2012). Figure (a) showed that unpeeled plantain flour (PP) had the highest DPPH values of 67.03%. The value was 6-fold higher than the activity recorded with plantain (pulp) flour (P), thus showing that the peel contributed greatly to the high activity. It has been shown that banana peels possess high antioxidant capacity (González-Montelongo, Gloria Lobo, & González, Citation2010). When pseudo-stems of banana were processed into flour, they also showed high antioxidant capacity (Aziz et al., Citation2011). There was no significant difference in the DPPH values of the plantain flour (pulp only) and the sample with moringa (PM). This indicates that incorporation of plantain peel into plantain flour significantly increased the radical scavenging ability of the flour. The reducing power of a compound is related to electron transfer ability of the compound which could lead to the neutralization of free radical (Zhu et al., Citation2001). Figure (b) showed that all the samples were able to reduce Fe3+ to Fe2+. The unpeeled plantain (peel and pulp) flour (PP) had a FRAP value of 5.23 mmol Fe2+/g which was not significantly different after the addition of moringa (PPM). However, addition of moringa to plantain flour (PM) showed a significant increase in the FRAP value over the ordinary plantain flour (P). Although both were significantly lower in value compared to the flours containing peel and pulp. The reducing power of a compound may serve as a significant indicator of its potential antioxidant activity (González-Montelongo et al., Citation2010) which is confirmed in this present study whereby the inclusion of peels in the plantain flour resulted in greater antioxidant activity. Figure (c) followed similar trend with the result from DPPH and FRAP showing the highest value recorded for unpeeled plantain (peel and pulp) flour having 14 mmol Trolox/g while the plantain flour (pulp only) had the lowest value of 10.05 mmol Trolox/g. There was no significant difference between the two samples when moringa was added.
3.5. The pasting properties of the plantain flour blends
The pasting properties of flour are of great importance in the food industry as it determines the extent of its use (Adebowale, Adeyemi, & Oshodi, Citation2005). The result for the pasting properties of the various plantain flour blends is shown in Table . The pasting temperatures of the samples ranged between 82.30 and 84.00°C. Since there was no significant difference in the temperatures observed for all the samples, it is an indication that gelatinization of the starch granules occur at similar temperature range for all the samples. The peak time is a measure of the cooking time and the values ranged between 4.87 and 5.27 min. Inclusion of moringa led to a significant reduction in the peak time for both plantain flours (peel and pulp, and pulp only). This shows that plantain flour containing moringa will cook faster perhaps due to lower starch proportion contained in moringa leaves.
Table 4. Pasting properties of plantain-moringa flour blends
Peak viscosity is the point at which maximum viscosity of a gelatinized starch is developed during heating of the dough in water. It provides an indication of the viscous load likely to encounter during mixing and its correlation to final product quality (Maziya-Dixon, Dixon, & Adebowale, Citation2004). The highest value of 367.4 RVU was recorded in the plantain flour (pulp only) and the lowest value of 257.8 RVU for the unpeeled plantain flour (peel and pulp) with moringa (PPM) blend (Table ). All the blends apart from the flour made from the pulp only, showed tendency to be more resistant to mechanical fragmentation. This is also evident from the values got for the breakdown viscosity. Breakdown viscosity is the index for the stability of swollen starch granule to rupture when held at high temperature and with continuous shearing. The values ranged between 65 and 104.7 RVU. Aryee et al. (2006), reported that high paste stability is an indication of very weak cross linkage within the starch granules. This means that such starches are not recommended for products where stability at very high temperature is required to prevent their breakdown. The low values in breakdown viscosity have the tendency for better resistance to heating and shear-thinning.
Setback is the stage where retrogradation or reordering of starch molecule occurs after cooling to 50°C. It is the tendency of starch gel to become firmer. Setback viscosity values ranged between 105.8 and 169.6 RVU with the lowest value of 105.8 RVU observed in the unpeeled plantain flour with moringa (PPM). Incorporation of moringa and/or peel into plantain flour will give a non-cohesive paste. This means that such starches have a low tendency toward retrogradation while the plantain flour (P) in the current study had the highest tendency toward retrogradation. However when banana starch and the flour were compared, the starch was reported to have higher tendency to retrograde due to the high value of setback viscosity (Pelissari et al., Citation2012). The final viscosity values ranged between 289.3 and 432.3 RVU. Incorporation of moringa led to a significant reduction in the final viscosity of the plantain flour. This trend might be due to interference in the aggregation of amylase molecules in the starch granules of the plantain flour. The trough is the period when the sample is subjected to constant temperature and mechanical shear stress accompanied by a breakdown in viscosity. The lower the value of trough, the more stable the starch gel. The trough values ranged between 177.1 and 262.8 RVU with the plantain flour blend with moringa having the lowest value (177.1 RVU) and plantain flour (P) observed to have the highest value (262.8 RVU). This indicates that plantain flour with moringa blend will constitute the most stable starch gel during cooking.
3.6. Sensory evaluation of the dough meal produced from various plantain flour blends
The sensory evaluation of the plantain dough (amala) from the various blends is presented in Table . The result showed no significant difference in the values for all the parameters tested in all the samples. This is contrary to recent report that showed significant differences in the sensory parameters when plantain was combined with soycake to make a dough meal (Famakin et al., Citation2016). Although incorporation of moringa into the plantain flour for dough meal production is novel, the acceptability in terms of the organoleptic properties (aroma, colour, taste, texture and mouldability) was not different from the dough meal made from the common plantain (pulp only) flour. This shows that acceptable dough meals can be produced from the entire samples tested.
Table 5. Sensory evaluation of dough meals made from plantain-moringa flour blends
4. Conclusion
The incorporation of plantain peels and moringa powder in the production of different plantain flour blends gave variety of products laden with dietary fibre and antioxidant potentials thus having nutraceutic potential. The pasting properties showed that these products can find application in the food industries. The high acceptability, from the sensory evaluation of the dough meals, is an indication that these combinations will lead greatly to diversification of plantain use and thus reduce postharvest losses while generating income to the local farmers.
Additional information
Funding
Notes on contributors
Adebanjo A. Badejo
Adebanjo A. Badejo is a senior lecturer at the Federal University of Technology, Akure, Nigeria. He holds a PhD from Hiroshima University, Japan. He works on plant food biotechnology and food product development. He has published several papers on newly developed food products that can serve as functional foods in collaboration with TNF.
Adeboye P. Osunlakin
Adeboye P. Oshunlakin and Ademola Famakinwa are graduate students in the Department of Food Science and Technology, Federal University of Technology, Akure, Nigeria. They are both working on Food Product Development under the supervision of Adebanjo A. Badejo and Tayo N. Fagbemi.
Atinuke O. Idowu
Atinuke O. Idowu is a senior lecturer in the Department of Food Science and Technology, Mountain Top University. She had her PhD in Food Technology and specializes in Food processing and product development using underutilized crops.
Tayo N. Fagbemi
Tayo N. Fagbemi is a professor in the Department of Food Science and Technology. He specializes in Food Chemistry and the development of functional foods and nutraceutical using under exploited local materials. He has published several papers on functional foods using plantain, soybean cake and rice bran.
References
- Adebowale, Y. A., Adeyemi, I. A., & Oshodi, A. A. (2005). Functional and physicochemical properties of flour of six Mucuna species. African Journal of Biotechnology, 4, 1461–1468.
- Affognon, H., Mutungi, C., Sanginga, P., & Borgemeister, C. (2015). Unpacking postharvest losses in Sub-Saharan Africa: A meta-analysis. World Development, 66, 49–68.10.1016/j.worlddev.2014.08.002
- Akissoe, N. H., Hounhouigan, J. D., Bricas, N., Vernier, P., Nago, M. C., & Olorunda, O. A. (2001). Physical, chemical and sensory evaluation of dried yam (Dioscorea rotundata) tubers, flour, and “amala” a flour-derived product. Tropical Science, 41, 151–156.
- Akubor, P. (2003). Functional properties and performance of cowpea/plantain/wheat flour blends in biscuits. Plant Foods for Human Nutrition, 58(3), 1–8.
- Anwar, F., Latif, S., Ashraf, M., & Gilani, A. H. (2007). Moringa oleifera: A food plant with multiple medicinal uses. Phytotherapy Research, 21, 17–25.10.1002/(ISSN)1099-1573
- Aparadh, V. T., Naik, V. V., & Karadge, B. A. (2012). Antioxidative properties (TPC, DPPH, FRAP, metal chelating ability, reducing power and TAC) within some cleome species. Annali Di Botanica, 2, 49–56.
- Aryee, F. N. A., Oduro, I., Ellis, W. O., & Afuakwa, J. J. (2006). The physico-chemical properties of flour samples from the roots of 31 varieties of cassava. Food Control, 17, 916–922.
- Association of Official Analytical Chemists. (2000). Official methods of analysis (17th ed.). Gaithersburg, MD: Author.
- Aziz, N. A. A., Ho, L. H., Azahari, B., Bhat, R., Cheng, L. H., & Ibrahim, M. N. M. (2011). Chemical and functional properties of the native banana (Musa acuminate x balbisiana Colla cv. Awak) pseudo-stem and pseudo-stem tender core flours. Food Chemistry, 128, 748–753.10.1016/j.foodchem.2011.03.100
- Badejo, A. A., Damilare, A., & Ojuade, T. (2014). Processing effects on the antioxidant activities of beverage blends developed from Cyperus esculentus, Hibiscus sabdariffa, and Moringa oleifera extracts. Preventive Nutrition and Food Science, 19, 227–233.10.3746/pnf.2014.19.3.227
- Brennan, C. (2005). Dietary fibre, glycaemic response, and diabetes. Molecular Nutrition and Food Research, 49, 560–570.10.1002/(ISSN)1613-4133
- Burdock, G. A., Carabin, I. G., & Crincoli, C. M. (2009). Safety assessment of kola nut extract as a food ingredient. Food and Chemical Toxicology, 47, 1725–1732.10.1016/j.fct.2009.04.019
- Coffmann, C. W., & Garcia, V. V. (1977). Functional properties and amino acid content of a protein isolate from mung bean flour. Journal of Food Technology, 12, 473–484.
- Elanthikkal, S., Gopalakrishnapanicker, U., Varghese, S., & Guthrie, J. T. (2010). Cellulose microfibres produced from banana plant wastes: Isolation and characterization. Carbohydrate Polymers, 80, 852–859.10.1016/j.carbpol.2009.12.043
- Fagbemi, T. N. (1999). Effect of blanching and ripening on functional properties of plantain flour. Plant Foods for Human Nutrition, 54, 261–269.10.1023/A:1008153404357
- Fagbemi, T. N., Adeoya, A. S., & Badejo, A. A. (2012). Effect of sulphiting on the physical and functional properties of acetylated cassava (Manihot esculenta) starch. Food, 6, 38–43.
- Famakin, O., Fatoyinbo, A., Ijarotimi, O. S., Badejo, A. A., & Fagbemi, T. N. (2016). Assessment of nutritional quality, glycaemic index, antidiabetic and sensory properties of plantain-based functional dough meals. Journal of Food Science and Technology, 53, 3865–3875.10.1007/s13197-016-2357-y
- González-Montelongo, R., Gloria Lobo, M., & González, M. (2010). Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chemistry, 119, 1030–1039.10.1016/j.foodchem.2009.08.012
- Gul, K., Singh, A. K., & Jabeen, R. (2016). Nutraceuticals and functional foods: The foods for the future world. Critical Reviews in Food Science and Nutrition, 56, 2617–2627.10.1080/10408398.2014.903384
- Gyamfi, M. A., Yonamine, M., & Aniya, Y. (1999). Free-radical scavenging action of medicinal herbs from Ghana. General Pharmacology: The Vascular System, 32, 661–667.10.1016/S0306-3623(98)00238-9
- Happi Emaga, T., Robert, C., Ronkart, S. N., Wathelet, B., & Paquot, M. (2008). Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresource Technology, 99, 4346–4354.10.1016/j.biortech.2007.08.030
- Ho, L. H., Abdul Aziz, N. A., & Azahari, B. (2013). Physico-chemical characteristics and sensory evaluation of wheat bread partially substituted with banana (Musa acuminata X balbisiana cv. Awak) pseudo-stem flour. Food Chemistry, 139, 532–539.10.1016/j.foodchem.2013.01.039
- Hou, W. C., Lin, R. D., Cheng, K. T., Hung, Y. T., Cho, C. H., Chen, C. H., ... Lee, M. H. (2003). Free radical scavenging activity of Taiwanese native plants. Phytomedicine, 10, 170–175.10.1078/094471103321659898
- Ige, M. M., Ogunsua, A. O., & Oke, O. L. (1984). Functional properties of the proteins of some Nigerian oilseeds: Conophor seeds and three varieties of melon seeds. Journal of Agricultural and Food Chemistry, 32, 822–825.10.1021/jf00124a031
- IITA. (2016). Banana and plantain. Retrieved March 10, 2016, from http://www.iita.org/banana-and-plantain
- Leach, H. W., McCowen, L. D., & Schoch, T. J. (1959). Structure of the starch granule. I. Swelling and solubility patterns of various starches. Cereal Chemistry, 36, 534–544.
- Marriott, J., & Lancaster, P. A. (1983). Bananas and plantains. In H. T. Chan (Ed.), Handbook of tropical foods (pp. 85–143). New York, NY: Marcel Dekker.
- Maziya-Dixon, B. A., Dixon, G. O., & Adebowale, A. A. (2004). Targeting different end uses of cassava: Genotypic variations for cyanogenic potentials and pasting properties. International Journal of Food Science and Technology, 42, 969–976.
- Mepba, H. D., Eboh, L., & Nwaojigwa, S. U. (2007). Chemical composition, functional and baking properties of wheat-plantain composite flours. African Journal of Food, Agriculture, Nutrition and Development, 7(1), 1–22.
- Olaoye, O. A., Onilude, A. A., & Idowu, O. A. (2006). Quality characteristics of bread produced from composite flours of wheat, plantain and soybeans. African Journal of Biotechnology, 5, 1102–1106.
- Omowaye-Taiwo, O. A., Fagbemi, T. N., Ogunbusola, E. M., & Badejo, A. A. (2015). Effect of germination and fermentation on the proximate composition and functional properties of full-fat and defatted cucumeropsis mannii seed flours. Journal of Food Science and Technology, 52, 5257–5263.10.1007/s13197-014-1569-2
- Pacheco-Delahaye, E., Maldonado, R., Pérez, E., & Schroeder, M. (2008). Production and characterization of unripe plantain (Musa paradisiaca L.) flours. Intercientia, 33, 290–296.
- Pelissari, F. M., Andrade-Mahecha, M. M., Sobral, P. J. A., & Menegalli, F. C. (2012). Isolation and characterization of the flour and starch of plantain bananas (Musa paradisiaca). Starch - Stärke, 64, 382–391.10.1002/star.201100133
- Pulido, R., Bravo, L., & Saura-Calixto, F. (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. Journal of Agricultural and Food Chemistry, 48, 3396–3402.10.1021/jf9913458
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26, 1231–1237.10.1016/S0891-5849(98)00315-3
- Sathe, S. K., Deshpande, S. S., & Salunkhe, D. K. (1982). Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrates. Journal of Food Science, 47, 491–497.10.1111/jfds.1982.47.issue-2
- Yadav, R. B., Yadav, B. S., & Dhull, N. (2012). Effect of incorporation of plantain and chickpea flours on the quality characteristics of biscuits. Journal of Food Science and Technology, 49, 207–213.10.1007/s13197-011-0271-x
- Zhu, N., Wang, M., Wei, G. J., Lin, J. K., Yang, C. S., & Ho, C. T. (2001). Identification of reaction products of (−)-epigallocatechin, (−)-epigallocatechin gallate and pyrogallol with 2,2-diphenyl-1-picrylhydrazyl radical. Food Chemistry, 73, 345–349.10.1016/S0308-8146(00)00308-3