Abstract
Shrimps are one of the most important commodities of the global fishery trade, are consumed by humans and also used in formulation of feed for animals to enhance nutrition.
Enumeration and identification of fungi and bacteria present on shrimp was checked. Gamma radiation doses of 0, 4, 8 and 10 kGy at a dose rate of 1.7 kGy/h from a Cobalt-60 source (SLL-515, Hungary) was evaluated. Mycological analysis was done by direct plating method on Oxytetracycline Glucose Yeast Extract and Dichloran Rose Bengal Chloramphenicol (DRBC) media. Bacteriological analysis was done by decimal reduction method on Plate Count Agar. Some toxigenic species of the genus Aspergillus (A. alutaceus, A. flavus, A. candidus and A. fumigatus), Fusarium (F. verticillioides) and Penicillium (P. expansum, P. citrinum and P. cyclopium) were isolated from the shrimp. Comparatively higher initial fungal and bacterial counts of 5.0–5.4 log 10 CFU/g and 4.3–4.7 log 10 CFU/g, respectively, were recorded for non-pretreated while lower counts of 1.7–2 and 1.6–2.1 log 10 CFU/g were recorded for pretreated smoked dry shrimp. Gamma radiation significantly (P < 0.05) reduced initial total fungal and bacterial populations by an average of 3 and 2.5 log cycles, respectively.
Keywords:
PUBLIC INTEREST STATEMENT
In Ghana, shrimp meat is revered owing to its wide usage in the food industry. It is consumed as food to complement a range of cereal-based foods to curb protein malnutrition, used to prepare animal feed and also used to prepare pepper sauce (“shito” local name) and some other delectable dishes. Studies on the microorganisms (with particular emphasis on fungi) associated with the shrimp as it moves through the food chain and their subsequent control with gamma irradiation is lacking in Ghana. The knowledge of some of these pathogens and their toxins in foods are of high significance in food safety and public health as many of these can cause mycotoxicosis and mycosis.
1. Introduction
There has been an increase in awareness about the nutritional value and health benefits of fish consumption in the last two decades. Shrimps are estimated to contain nearly 20% protein (wb - Body weight) with well-balanced amino acids and significantly high amounts of other nutrients including micronutrients such as calcium and selenium. According to research by Yanar and Celik (Citation2006) and Shriket et al., (Citation2007), lipids in shrimps are largely made up of polyunsaturated fatty acids which are essential for human health. In Ghana, they are fished on both commercial and artisanal scale along the coastal areas (Entsua-Mensah et al., Citation2002) whilst the post-harvest processing of shrimp is essentially assumed by women of these fishing communities.
Drying and smoking remains one of the best options of preprocessing this seafood since it is one of the oldest means of food preservation and is applicable to a wide range of food products including shrimps (Akonor, Ofori, Dziedzoave, & Kortei, Citation2016). The principle behind drying is primarily reduction of moisture to levels low enough to prevent microbial growth and also slow down enzymatic and other biological reactions that may contribute to food spoilage (Akonor et al., Citation2016; Kortei, Citation2015). Dried shrimps are popular and widely acceptable and they are used (in whole or powdered form) in soups and sauces as a major protein source and for their delectable flavor (Akonor et al., Citation2016) and can help address the protein malnutrition problem in Africa.
By and large, seafoods have been responsible for a significant percentage of foodborne diseases (Karunasagar et al., Citation2005; Wallace et al., Citation1999). Consumption of raw or undercooked seafood is the factor most commonly associated with infection (Butt et al., Citation2004). There is ample epidemiological evidence, particularly from Japan, that consumption of raw fish is indeed the cause of many outbreaks of foodborne diseases. Biotoxins and histamines make up a large proportion of these outbreaks (Huss, Citation1997). Though viruses are the most common cause of seafood-related infections, most of the hospitalisation and deaths are due to bacterial agents (Butt et al., Citation2004). Previous studies have also reported the presence of toxigenic moulds and mycotoxins such as aflatoxins and ochratoxins in smoked dry fish collected in retail markets in tropical conditions (Bukolo et al., Citation2008; Essien, Ekpo, & Brooks, Citation2005). As a consequence, food safety and quality aspect in handling of this seafood has become important, since fresh food is more prone to certain microbiological contamination (Sawhney, Citation2005).
Gamma irradiation as a physical treatment effectively eliminates spoilage and pathogenic microorganisms in foods (IAEA-TECDOC, Citation2005; Neimera and Fan, Citation2005) and has been utilized for the reduction and elimination of pathogens in foods (Farkas, Citation2001; ICGFI, Citation2002). The World Health Organization/IAEA Joint Committee on Food Irradiation in 1980 concluded that irradiation of any food commodity up to 1 Mrad (l0 kGy) causes no toxicological hazards and therefore needs no further toxicological testing of such foods (Varanyanond, Hirata, & Ishitani, Citation1990). Gamma irradiation also introduces no nutritional or microbiological problems (Selman, Citation1982). Irradiation treatment of foods may cause certain changes in flavour, odour and colour which could affect the product and cause problems with consumer acceptance (Egan et al., Citation1985).
In this paper, the microbiological quality (with particular reference to fungi) was studied before and after gamma irradiation, with the view to identifying potential mycotoxin-producing fungal species which may endanger consumer health.
2. Materials and methods
2.1. Collection of samples
Smoked shrimp (Penaeus notialis) were purchased from the local market. The samples were dominated mainly by P. notialis although there were other few varieties found in the population, which were identified as P. monodom and P. kerathucus. The samples were then carefully sorted to ensure a homogenous population of P. notialis. They were then wrapped in a brown paper and then placed in a black polyethylene bag (main mode of packaging by the local producers) and then transported to the Department of Plant and Environmental Biology laboratory of the University of Ghana. The shrimps were poured into dense polypropylene containers and kept at a temperature (6–8°C) appropriate for storage and further studies.
2.2. Determination of moisture content
According to AOAC (1995) methods described.
2.3. Enumeration of mycoflora
This was done according procedure outlined by Kortei et al. (Citation2015) with modifications. The media used were Oxytetracycline Glucose Yeast Extract (OGYE; Oxoid CM 0325) agar, Dichloran Rose Bengal Chloramphenicol (DRBC) agar and Plate Count Agar (PCA). These media were prepared according to manufacturer’s instructions. One gram of each test sample was added separately to 100 ml of 0.1% peptone in a 250 ml Erlenmeyer flask and allowed to settle for 5 min. Each flask was then shaken at 120 rev/min for 5–10 min on an Orbital Shaker (Gallenkamp, England). The decimal serial dilution technique was used, and samples were diluted up to 1:104. One milliliter of each aliquot was squirted into petri dishes using a sterile syringe (Digatrading, Belgica-Brussels, Belgium) and 20 ml of the different media was poured onto the aliquots in the petri dishes and swirled in clockwise and anticlockwise directions (four times) each and horizontal and vertical directions (two times) each. This was done in order to ensure equal distribution of the media and aliquot. Each sample was duplicated and plates containing OGYE and DRBC (for fungi) were incubated at 30°C for 5 days.
2.3.1. Characterization and identification of fungal isolates
Fungal isolates were examined under stereo binocular microscope (Leica 261, Germany) using the needle mounts technique. Their identification was performed according to macro and micro morphological characteristics. All the isolates were identified up to the species using keys and manuals (Barnet and Hunters, Citation1972; Samson et al., Citation1995).
2.4. Irradiation and dosimetry methods
Irradiation was done according to Varanyanond et al, (2000) with modifications. The samples were packaged in dense polypropylene bowls which was able to satisfy this condition (packaging material which is strong enough to resist piercing/ puncture by the antenna of the shrimp was considered) in accordance to the East African Standard (EAS, CD/K/512:2010: ICS 67.120.30) for dried shrimp packaging. The samples were treated with gamma irradiation (cobalt-60 irradiator) in a Category Four (4) Wet Storage Irradiator at the Radiation Technology Centre at the Ghana Atomic Energy Commission. Doses applied were 0 kGy (control), 4 kGy, 8 kGy and 10 kGy within the range used by for dried shrimp in East Asia. The dosimeter used was the Ethanolchlorobenzene (ECB) Dosimetry system, which comprise of the Ethanolchlorobenzene Dosimeter and the High Frequency Dosimeter Reader (HFDR). The dosimeter was calibrated against an International standard set by the International Atomic Energy Agency and read using the HFDR. To minimize variations in radiation dose absorption, the bags were turned at different angles halfway through the procedure. Samples were analysed in triplicates for each source. The dose rate used was 1.7 kGy / hour in air.
2.5. Enumeration of aerobic mesophilic bacteria
Procedure was done as outlined by Kortei et al. (Citation2014) with modifications. One gram of each sample was mixed with 9 ml peptone water and serial dilutions of each shrimp sample homogenate were made to 10−4 dilutions. Approximately 1 ml aliquot portions of the dilutions were spread onto duplicate sterile plates of PCA (Oxoid, England),
Cultures were incubated at 37°C for 24–48 h. After the incubation, the different culture plates were examined for microbial growth. Colonies were counted using the colony counter (Gallenkamp, England), counts were expressed as colony forming unit per gram of sample homogenate (CFU/g).
2.5.1. Bacterial identification and characterization
For the identification and characterization of the microorganisms in the samples, some morphological and biochemical examinations were done as described by Prescott, Harley, and Klein (Citation1996). These included Gram Staining, Catalase test, Peroxidase test, Triple Sugar Iron agar test, Sulphur Indole Motility test and the Simmons Citrate test.
2.6. Statistical analysis
The values obtained for total fungal and bacterial counts were transformed to logarithm conversions and subjected to analysis of variance using SPSS (Chicago, IL) version 9 for windows.
3. Results and discussion
3.1. Fungal population
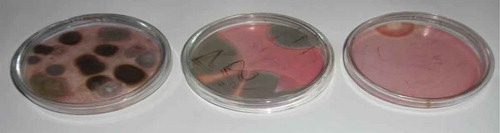
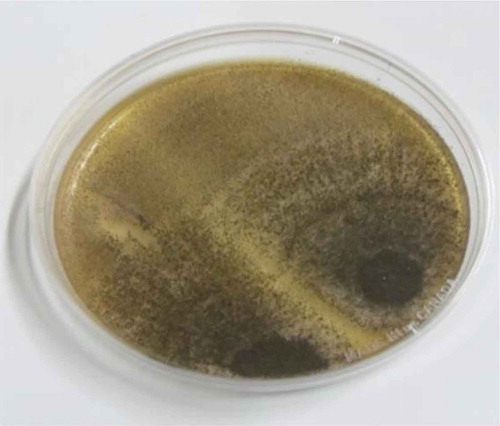
Table shows the list of 26 different fungi isolated from dried smoked shrimp from different water sources. Fourteen different fungi belonging to seven genera (Aspergillus, Alternaria, Cladosporium, Curvalaria, Fusarium, Penicillium, Rhodotorula) and yeast were isolated from the shrimps harvested from the sea. Seventeen different fungi belonging to six genera (Aspergillus, Cladosporium, Fusarium, Pencillium, Syncephalastrum, Mucor) contaminated the dry shrimp from the river water. On the other hand, the lagoon shrimp harboured 12 fungal species belonging to 5 genera (Aspergillus, Cladosporium, Fusarium, Penicillium, Rhodotorula). In all these instances, Aspergillus species predominated over other fungi isolated, followed by Penicillium and Cladosporium species. Some toxigenic species of the genus Aspergillus (A. alutaceus, A. flavus, A. candidus, A. fumigatus), Fusarium (F. verticillioides) and Penicillium (P. expansum, P. citrinum, P. cyclopium) were isolated from the shrimp (Table ). The shrimp are therefore susceptible to fungal infections and this cannot be discounted. Plates – show some of the fungi isolated.
Table 1. Total list of fungi isolated from shrimp in different water bodies on different media
The dynamics of a fungal community may be attributed generally to abiotic variables and nature of substrate (Venugopal, 2010). More than 92 fungal species belonging to over 15 genera have been isolated from cereals, grains and pulses, medicinal plants, spices and other food products (Addo, Citation2008; Allotey & Odamtten, Citation1996; Odamtten, Citation1986) in Ghana. In a recent study, Kortei, Odamtten, Obodai, and Wiafe-Kwagyan (Citation2017) isolated similar nine fungal species belonging to six genera which were associated with the sorghum grains. Among these fungi were Cladosporium macrocarpum, Trichoderma harzianum, Fusarium oxysporum, Rhodotorula spp., Penicillium spp., Aspergillus niger, Aspergillus fumigatus, Aspergillus ochraceous and Aspergillus flavus as they investigated the comparative occurrence of resident fungi on gamma irradiated and steam sterilized sorghum grains (Sorghum bicolor L.) for spawn production.
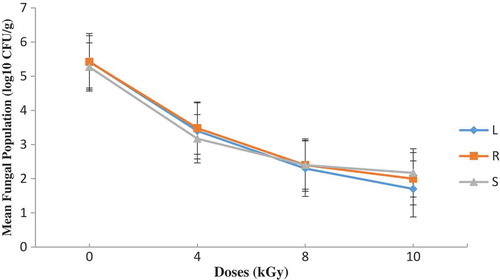
Figure 1. Total fungal population on DRBC before and after irradiation with the indicated doses (L—lagoon; R—river; S—sea)
In this study, Aspergillus predominated the fungal species, followed by Penicillium and Cladosporium species. Some toxigenic species of the genus Aspergillus and Penicillium were also isolated from the shrimp (Table ). Occurrence of other mycotoxins and aflatoxins at high levels in a particular food raises concerns about its safety. Aflatoxins, especially aflatoxin B, are recognized in both humans and animal as responsible for toxic signs and lesions, reduced growth, immune suppression and liver cancer (Odamtten, Citation1986; Osweiler, Citation2010; Turner et al.., Citation2003). The International Agency for Research on Cancer has classified AFB1 as a probable human carcinogen (IACR, Citation1993).
Figures – summarize results obtained. A dose of 8 kGy and 10 kGy reduced the initial fungal load by nearly 3 log cycles on both media. The differences observed with residual fungal load on the sample after treatments were not statistically significant (P ≥ 0.05). After 4 months of storage in dense polyethylene containers, the fungal load remained nearly the same (lowered by nearly 3 log cycles) (Figures and ) and the isolating media had no significant (p ≥ 0.05) influence on the mycoflora population. The mycoflora population in the shrimp coming from different water sources did not vary significantly (P > 0.05) after 4 months of storage (Figures –). Storage period did not therefore influence the mycological quality of the irradiated shrimp. The residual fungi after four months was predominated by Rhodotorula and unidentified yeast. The role of yeasts in the spoilage of foods is not well documented in Ghana and their growth on foods can cause major problems.
Figure 2. Total fungal population on DRBC four months after irradiation and storage in dense polyethylene containers (L—lagoon; R—river; S—sea).
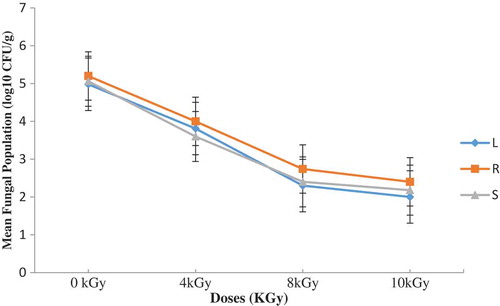
Figure 3. Total fungal population on OGYE before and after irradiation with the indicated doses (L—lagoon; R—river; S—sea)
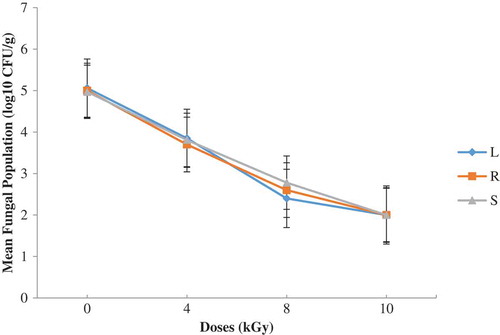
Figure 4. Total fungal population on OGYE four months after irradiation and storage in dense polyethylene containers (L—lagoon; R—river; S—sea)
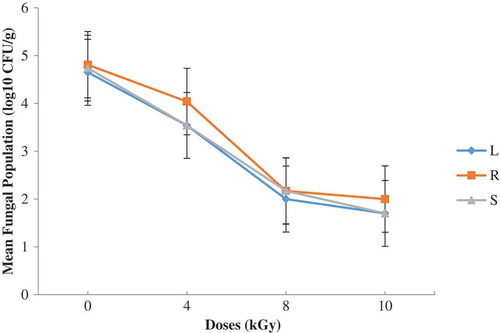
Figure 5. Total aerobic bacteria on PCA (before and after irradiation with the indicated doses (L—lagoon; R—river; S—sea)
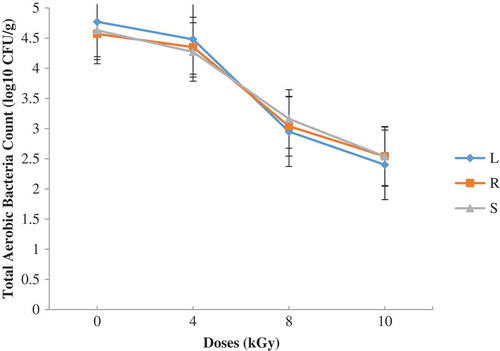
Figure 6. Total aerobic bacteria on PCA four months after irradiation and storage in dense polyethylene containers with the indicated doses (L—lagoon; R—river; S—sea)
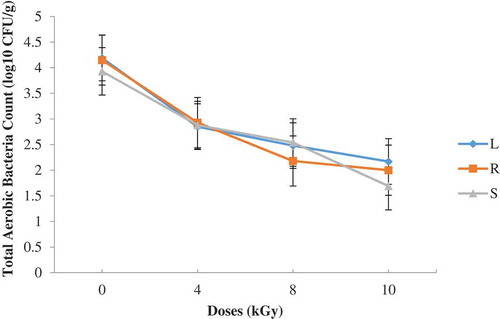
Kpoclou et al. (Citation2013) reported mould counts of 2.7 × 102 and 3.4 × 102 for entire smoked shrimp and smoke shrimp powder, respectively, collected in some local markets in Benin. Kortei et al. (Citation2014) also recorded a log cycle of range 1.2–1.4 of fungal counts in fresh and dried mushrooms stored in packaging materials by 1–5 kGy gamma radiation. Similarly, an average of 2.2 log cycles of fungal counts was achieved by Kortei et al. (Citation2017) after application of 1–5 kGy radiation doses on the mycoflora population of dried fruitbodies of Pleurotus ostreatus stored in either polythene or polypropylene. Presumably, the reduction of fungal counts could be attributed to the killing action of gamma radiation as it interacts with the DNA strand of the microorganism (Diehl et al., Citation1995). Fungal counts obtained after gamma irradiation were found to be below acceptable limits set by The International Commission for Microbiological Specification for Foods (ICMSF, Citation1998) which states that ready-to-eat foods with plate counts between 0 and 103 is acceptable, between 104 and ≤105 is tolerable and 106 CFU/g and above is unacceptable.
3.2. Total aerobic bacteria (TAB) count
Figures and show results obtained. A dose of 8 kGy reduced TAB count by 2.5 log cycles (Figure ). There were no statistical differences (P ≥ 0.05) between the samples so far as the bacteria flora after irradiation was concerned. Storage for four months after irradiation did not influence the TAB counts on the different shrimp samples (Figure ). There was a general decrease in microbial counts after gamma irradiation. Prior to storage, counts for 0 and 4 kGy statistically differed (P < 0.05) from 8 and 10 kGy. This observation could be attributed to the killing action of ionizing radiation which produces chemical and physical changes in substrate which act in concert to inactivate microorganisms. The energy of ionizing radiation affects directly the microbial DNA (genome), causing the damage on fungal or bacterial cells.
The vulnerability of microorganisms and their spores to gamma radiation has been well documented by many researchers (Adu- Gyamfi and Appiah, Citation2012; Diehl, Citation1995; Kortei, Citation2015; Kortei et al., Citation2015). The ability of an organism to withstand a physical stress (gamma radiation and/or steam) depends on how quickly it is able to repair its damaged DNA as a result of denaturing (IAEA-TECDOC, Citation2005).
Results obtained were below levels of acceptability suggested by ICMSF (1996) (106). Results were in agreement with published findings Kpoclou et al. (Citation2013) who recorded an Aerobic Mesophilic Bacteria (AMB) count up to 1.4 × 104 CFU/g and 3.2 × 104 CFU/g for whole smoke shrimp and smoke shrimp powder, respectively, as they studied the microbiological and physico-chemical quality of smoked shrimp in Benin. Similar results were reported by Essien et al. (Citation2005) and Bukola et al. (Citation2008) on smoked dry fish collected in retail markets in Nigeria. In Bangladesh, Anwar, Shankar, Mohammad, Md, and Mahmud (Citation2010) reported 8.13 × 104 CFU/g of total aerobic counts of bacteria after investigating the microbiological quality of processed black tiger shrimps. Recently, Kortei et al. (Citation2014) recorded an aerobic mesophilic count of range 1.5 × 104–8.6 × 107 CFU/g and achieved an average of 3.7 log cycles reduction with gamma radiation as they studied the microbiological qualities of gamma-irradiated fresh and dried mushrooms kept in polythene and polypropylene packs in Ghana.
The AMB count enumerated in these samples examined before gamma irradiation could be due to the growth of microorganism which resisted to the smoking treatment. It could also probably be due to the contamination during the post-processing handling. Indeed, hot smoking process is a pasteurization method and could not eliminate all the microorganisms of the raw shrimp. Plahar et al. (Citation1999) have reported that the initial microbial types and viable numbers decrease during traditional hot smoking (60–80°C for 2–5 h) but are not completely eliminated.
The shrimp were confirmed to be contaminated by Bacillus cereus and Staphylococcus aureus. B. cereus was isolated from the lagoon and sea shrimp, and S. aureus contaminated all the three types of shrimp from the different water sources. Abolagba and Igbinevbo (Citation2010) and Ola and Oladipo (Citation2004) have shown that these Bacillus spp. constitute the normal flora of fish.
Abolagba et al. (Citation2010) and Ola et al. (Citation2004) have shown that these Bacillus spp. constitute the normal flora of fish. These flora of the fish are not initially harmful, as they even help in preventing the invasion of the fish flesh by other microorganisms but they become pathogenic when there is an enabling environment (bad handling which can lead to bruises, poor hygiene and delayed processing and preservation of the fish after harvest) which promotes their growth. Smoking plays a bacteriostatic and antioxidant role in foods (CAC/RCP 68, Citation2009). Several reports, for example, Abolagba and Melle (Citation2008), Kumolu-Johnson et al. (Citation2009), Olokor et al . () and Sengor, Kalafatoglu, and Gun (Citation2004), have noted that apart from giving the product a desirable taste and odour, smoking provides a longer shelf-life through its antibacterial and oxidative effect.
4. Conclusion
From our studies, it can be deduced that the dried shrimps (Penaeus notialis) harboured 14 different fungi belonging to 7 genera (Aspergillus, Alternaria, Cladosporium, Curvalaria, Fusarium, Penicillium, Rhodotorula) and yeast were isolated from the shrimps harvested from the sea. Seventeen different fungi belonging to six genera (Aspergillus, Cladosporium, Fusarium, Pencillium, Syncephalastrum, Mucor) contaminated the dry shrimp from the river water. On the other hand, the lagoon shrimp harboured 12 fungal species belonging to 5 genera (Aspergillus, Cladosporium, Fusarium, Penicillium, Rhodotorula). Generally, a significant (P < .05) reduction in mycofloral population by >2 log cycles with the application of gamma irradiation was observed.
Application of gamma irradiation treatment is a vital tool for the control of fungal microorganisms in foods and other materials. These products are often consumed raw or with minimum processing. Data obtained showed the type of fungi and an estimate of microbial loads on the smoke dry shrimps as well as the level of reduction obtained when pretreated with gamma radiation (low to medium doses) and was capable of reducing these fungal and bacteria populations of smoke dry shrimps stored in polypropylene packs sufficiently to achieve the recommended levels of The International Commission for Microbiological Specification for Foods (ICMSF, 1996).
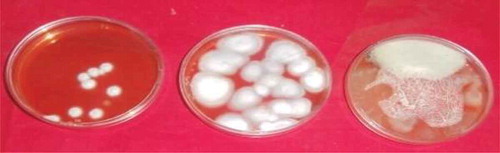
Plate 1. A photograph showing some of the fungi resident in the shrimp cultured on OGYE media at 30°C. Fungi isolated include Aspergillus candidus (left), Fusarium verticillioides (middle) yeast and Curvularia
Competing interests
The authors declares no competing interests.
Authors’ contributions
The principal author (Felicia Akuamoa) was the one who conducted the laboratory analyses. She also researched on the topic and came out with the relevant literature for the write-up. The second author (George Tawia Odamtten) supervised the study from start to finish and he was also responsible for all the necessary corrections in the write-up. The third author (Nii Korley Kortei) assisted in carrying out the experiment, assemblage of the write-up and making the needed corrections as well as submitting it to a suitable journal for publication. All authors read and approved the final manuscript.
Acknowledgements
Authors would also like to express their heart-felt gratitude to Mr G. Akwetey of Mycology Unit, Department of Plant and Environmental Biology, University of Ghana. Also to Messers S.N.Y. Annan, E.A Quarcoo and S.W.N.O Mills of Radiation Technology Centre, Ghana Atomic Energy Commission for carrying out irradiation of samples. Finally, we express our gratitude to our respective families for their undying support throughout the study.
Additional information
Funding
Notes on contributors
Felicia Akuamoah
Mrs Felicia Akuamoa is an MPhil (Radiation Processing) holder from the Graduate School of Nuclear and Allied Sciences, University of Ghana. She is a doctoral student at the Wageningen Agricultural University, Holland. She works as a research scientist at the Ghana Atomic Energy Commission. Her research areas include in-vitro antioxidant analysis and radiation processing of foods.
George Tawia Odamtten
Prof. George Tawia Odamtten holds a PhD in Agriculture & Food Safety from the Wageningen Agricultural University, Holland. He is a full professor of Mycology and Plant Pathology at University of Ghana. His research areas include fungal pathogens of crops, foodborne fungal contaminants, fungal biotechnology and mycotoxin analysis.
Nii Korley Kortei
Dr Nii Korley Kortei holds a PhD in Radiation Processing (Food Science option) from the University of Ghana. He is a lecturer of Food Science at the Department of Nutrition and Dietetics, University of Health and Allied Sciences, Ghana. His research areas are foodborne fungal contaminants, mycotoxins and myco chemicals in foods.
References
- Abolagba, O. J., & Igbinevbo, E. E. (2010). Microbial load of fresh and smoked fish marketed in benin metropolis, Nigeria. Research Journal of Fisheries and Hydrobiology, 5(2), 99–104. INSInet Publication.
- Abolagba, O.J, & Melle, O.O. (2008). Chemical composition and keeping qualities of a scaly fish tilapia (oreochromis niloticus) smoked with two energy sources. Afr. J. Gen. Agric. Klobex, 4(2), 113-117.
- Addo, A. A. (2008). Mycological profile and aflatoxigenic potential of resident Aspergillus species of six packaged Ghanaian dehydrated foods ( M. Phil. Thesis). Department of Botany, University of Ghana, Legon.
- Adu-Gyamfi, A., & Appiah, V. (2012). Enhancing the hygienic quality of some Ghanaian food products by gamma irradiation. Food and Nutrition Sciences, 3, 219–223. doi:10.4236/fns.2012.32032
- Akonor, P. T., Ofori, H., Dziedzoave, N. T., & Kortei, N. K. (2016). Drying characteristics and physical and nutritional properties of shrimp meat as affected by different traditional drying techniques. International Journal of Food Science, 2016, 1–5. Article ID 7879097. doi:10.1155/2016/7879097
- Allotey, J., & Odamtten, G.T. (1996). Hidden infestations from Kaneshie and Tema warehouses (Ghana) after 4 months storage under laboratory conditions in relation to resident mycoflora. In: Odamtten, G.T., & Clerk, G.C. (Eds.). Mitigation of Stackburn in woven polypropylene bag. Stack Maize Grains for Improved Food Security in Sub-Saharan Africa. University of Ghana. ISBN 999-7756-0-1: pp 80-85..
- Anwar, H., Shankar, C. M., Mohammad, S. R., Md, M. R., & Mahmud, H. (2010). Microbiological quality of processed frozen black tiger shrimps in fish processing plant. World Journal of Fish and Marine Sciences, 2(2), 124–128.
- A.O.A.C (2005). Determination of moisture, ash, protein and fat. Official methods of analysis of the Association of Analytical Chemists. 18th Edition. A.O.A.C, Washington DC.
- Barnett, H. L., & Hunter, B. B. (1972). Illustrated genera of fungi, imperfecti 3rd Edition, Burgess publishing Company, Minesota, U.S.A. p. 273.
- Bukola, C. A., Abiodun, A. O., & Ukpe, G. P. (2008). Mycofloral of smoke-dried fishes sold in Uyo, Eastern Nigeria. World Journal Agricultural Sciences, 4(3), 346–350.
- Butt, A. A., Aldridge, K. E., & Sanders, C. V. (2004). Infections related to the ingestion of seafood Part I: Viral and bacterial infections. The Lancet Infectious Diseases, 4, 201–212. doi:10.1016/S1473-3099(04)00969-7
- CAC/RCP 68. (2009). Code of practice for the reduction of contamination of food with polycyclic aromatic hydrocarbons (PAH) from smoking and direct drying processes.
- Diehl, J. F. (1995). Safety of irradiated foods (2nd ed.). New York: Marcel Dekker, Inc.
- Egan, A. F., & Wills, P. A. (1985). The preservation of meats using irradiation. CSIRO Food Researcher, Q.45, 49.
- Entsua-Mensah, M., De Graft-Johnson, K. A. A., Atikpo, M. O., & Abbey, L. D. (2002). The lobster, shrimp and prawn industry in Ghana—Species, ecology, fishing and landing sites, handling and export, Tech. Rep. CSIR-FRI/RE/E-MM/2002/012, Food Research Institute/AgSSIP. doi:10.1044/1059-0889(2002/er01)
- Essien, J. P., Ekpo, M. A., & Brooks, A. A. (2005). Mycotoxigenic and proteolytic potential of moulds associated with smoked shark fish (Chlamydoselachus anguincus). Journal Applications Sciences En-Viron Management, 9(3), 53–57.
- Farkas, J. (2001). Irradiation of minimally processed foods. In: Food Irradiation.- Principles and Applications (Ed. R. Molons). Wiley/Interscience. New York. pp. 273–290.
- Huss, H.H. (1997). Control of indigenous pathogenic bacteria in seafood. Food Control, 8(2), 91–98.
- IAEA-TECDOC-1530 (2005). Use of irradiation to ensure hygienic quality of fresh, pre-cut fruits and vegetables and other minimally processed food of plant origin. Proceedings of a final research coordination meeting organized by the joint FAO/IAEA programme of nuclear techniques in food and agriculture, Pakistan. pp 1–21.
- IARC (1993). Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC monographs on the evaluation of carcinogenic risks to humans. International Agency for Research on Cancer, Geneva. 56, 489–521.
- ICGFI. (2002). International consultative group on food irradiation. A global food safety tool. Vienna: Official Publication of IAEA.
- ICMSF. (1998). Microorganisms in foods.Volume 6, Microbial Ecology of Food Commodities. Blackie Academic and Professional, New York, pp. 521–577.
- Karunasagar, I., Karunasagar, I., & Umesha, R. K. (2005). Microbial diseases in shrimp aquaculture. In N. Ramaiah, Eds. Marine microbiology: monodon shrimp, 121–134. Fish & Shellfish Immunology, 13, 293–309.
- Kortei, N. K. (2015). Comparative effect of steam and gamma irradiation sterilization of sawdust compost on the yield, nutrient and shelf life of Pleurotus ostreatus (Jacq.ex. Fr) kummer stored in two different packaging materials (PhD thesis). Graduate school of Nuclear and Allied Sciences, University of Ghana.
- Kortei, N. K., Odamtten, G. T., Appiah, V., Obodai, M., Annan, T. A., Adu- Gyamfi, A., … Mills, S. W. O. (2014). Microbiological quality assessment of gamma irradiated fresh and dried mushrooms (Pleurotus ostreatus) and determination of D10 values of Bacillus cereus in storage packs. European Journal of Biotechnology and Biosciences, 2(1), 28–34.
- Kortei, N. K., Odamtten, G. T., Obodai, M., Appiah, V., Adu-Gyamfi, A., & Wiafe- Kwagyan, M. (2015). Comparative occurrence of resident fungi on gamma irradiated and steam sterilized sorghum grains (Sorghum bicolor L.) for spawn production in Ghana. British Biotechnology Journal, 7(1), 21–32. doi:10.9734/BBJ/2015/16747
- Kortei, N. K., Odamtten, G. T., Obodai, M., & Wiafe-Kwagyan, M. (2017). Mycofloral profile and the radiation sensitivity (D10 values) of solar dried and gamma irradiated Pleurotus ostreatus (Jacq. Ex. Fr.) Kummer fruitbodies stored in two different packaging materials. Food Science and Nutrition, 1–9. doi:10.1002/fsn3.545
- Kpoclou, E. Y., Anihouvi, V. B., Azokpota, P., Soumanou, M. M., Daube, G., Douny, C., … Hounhouigan, D. J. (2013). Microbiological and physico-chemical quality of smoked shrimp, an expanding food condiment in beninese local markets. Food and Public Health, 3(6), 277–283. doi:10.5923/j.fph.20130306.03
- Kumolu-Johnson, C. A., & Ndimele, P. E. (2001). Effect of salting, brining and sundrying on the shelf-life of Clarias gariepinus (LACEPEDE). Journal Researcher Reviews Sciences, 2, 21–25.
- Kumolu-Johnson, C.A, Aladetolun, N.F, & Ndimele, P.E. (2009). The Effects Of Smoking on The Nutritional Qualities and Shelf Life Of Clarias Gariepinus (Burchell 1822), 9(1), 73–76.
- Niemira, B.A., & Fan, X.(2005). Low-dose irradiation of fresh and fresh-cut produce: Safety, sensory and shelflife. In: Food Irradiation Research Technology. Ed. Sommers, C., & Fan, X., pp 169-181.Blackwell Publishing and the Institute of Food Technologists, Ames, Iowa, USA.
- Odamtten, G. T., (1986). Studies on the control of fungal contamination and aflatoxin contamination of treatment of heat and gamma irradiation. ( PhD Thesis), DUDOC Publication, Agricultural University, Wageninen, The Netherlands.
- Ola, J. B., & Oladipo, A. E. (2004). Storage of croaker in ice. African Journal of Biomedical Research, 7, 13–17.
- Olokor, J. O., Ihuahi, J. A., Omojono, F. S., Falayi, B. A., & Adelomo, E. O. (2007). Handbook of practical fisheries technology division, national institute of freshwater fisheries research (NIFFR) PMB 6006, (pp. 13–42). New-Bussa, Niger State, Nigeria: Remi-Thomas Press, pp 22-29.
- Osweiler, G. D. (2010). Clinical characteristics of specific mycotoxicoses in horses. In E. Gonçalez, J. D. Felício, & S. Aquino (Eds.), Mycotoxicoses in animals economically important (pp. 14–15). New York, NY: Nova Science Publishers.
- Plahar, A. W., Nerquaye-Tetteh, A. G., & Annan, T. N. (1999). Development of an integrated quality assurance system for the traditional Sardinella sp. and anchovy fish smoking industry in Ghana. Food Control, 10, 15–25. doi:10.1016/S0956-7135(98)00150-9
- Prescott, L., Harley, J., & Klein, D. (1996). Microbiology, William C. Brown (5th ed.). Wm. C. Brown Publishers, London. PubMed |Google Scholar
- Samson, A. R., Hoekstra, E. S., & Frisvad, J. C. (1995). Introduction of food-borne fungi (4th ed.). Netherlands: Pensen and Loogen. p12–20.
- Sawhney, A. (2005). Quality measures in food trade: the indian experience. The World Economy, 28(3), 329–348.
- Selman, J. D. (1982). How safe is irradiated food? (Food Manufa. 29. L2) Shewan J.M. 1971. The microbiology of fish and fishery products- A progress report. Journal of Applied Bacter, 34, 299.
- Sengor, G. F., Kalafatoglu, H., & Gun, H. (2004). The determination of microbial flora, water activity and chemical analysis in smoked mussels (Mytilus galloprivinciali s, L.) Turk. Journal Vitnery, Animals Sciences, 28, 793–797.
- Sriket, P., Benjakul, S., Visessanguan, W., & Kijroongrojana, K. (2007). Comparative studies on chemical composition and thermal properties of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) meats. Food Chemistry, 103(4), 1199–1207. doi:10.1016/j.foodchem.2006.10.039
- Turner, P. C., Moore, S. E., Hall, A. J., Prentice, A. M., & Wild, C. P. (2003). Modification of immune function through exposure to dietary aflatoxin in Gambian Children. Environmental Health Perspective, 111, 217–220. doi:10.1289/ehp.5753
- Varanyanod, W., Hirata, T., & Ishtani, T. (2000). Shelf life extension of dried shrimps by irradiation and packaging techniques. Thai Journal of Agricultural Science, 33(3–4), 179–188.
- Venugopal, P. (2016). Effects of climate, wood quality and fungal diversity on coarse wood decomposition of Scots pine. Dissertation Forestales 226. University of East Finland. Available at http/dx.doi.org/10.14214/df.226.
- Wallace, B. J., Guzewich, J. J., Cambridge, M., Altekruse, S., & Morse, D. L. (1999). Seafood-associated disease outbreaks in New York, 1980–1994. American Journal of Preventive Medicine, 17(1), 48–54.
- Yanar, Y., & Celik, M. (2006). Seasonal amino acid profiles and mineral contents of green tiger shrimp (Penaeus semisulcatus De Haan, 1844) and speckled shrimp (Metapenaeus monoceros Fabricus, 1789) from the Eastern Mediterranean. Food Chemistry, 94(1), 33–36. doi:10.1016/j.foodchem.2004.09.049