Abstract
Anthocyanins are known as the central players in kingdom Planta, elucidating their major role in various abiotic and biotic stresses including physiological events such as plant growth, development and defense responses. Plants synthesize a wide range of chemically diverse secondary metabolites that are distinctively important industrial biochemical compounds. The elucidation of regulatory mechanisms by flavonoids could facilitate the generation of new transgenic cotton varieties along the rational to increase fiber characteristics and yield at the verge of auxin transport. The anthocyanin gene expression and its structural genes encoding enzymes have already been explored in many ornamental flowers. But its particular role in crop plant stress regulatory mechanism is yet to be explored. This brief review summarizes the role of flavonoids against various abiotic factors including drought, light/ultraviolet (UV) stress, water scarcity, temperature variations and so on in the background of cotton crop. These factors adversely affect the growth and productivity of the crops. Moreover, information of the flavonoids metabolic interactions in relation to cotton fiber characteristics still needs to be unrevealed.
PUBLIC INTEREST STATEMENT
Plants are the major producers of food chain in ecosystem. A number of primary and secondary metabolites are produced by plants during their routine metabolism. Secondary metabolites are usually organic compounds not primarily involved in plant development but specifically work under specified conditions, for example, environmental stress conditions and adaptations. Phytohormones; phenolic compounds, that is, anthocyanins/flavonoids; and some other pigment-related compounds are categorized as secondary metabolites. Flavonoids are natural biosensors that boost up the plant defense mechanism during environmental stresses. The outstanding role of flavonoids in cotton fiber development has not been discussed so far. This article particularly throws light on flavonoid roles in the background of cotton. The exact mechanism of flavonoid genes in enhancing fiber characteristics is still unknown, but we have gathered various research studies in this regard to verify the role of flavonoid in the development of high-length fiber.
Competing Interest
The authors declare no competing interests.
1. Flavonoid as an important secondary metabolite
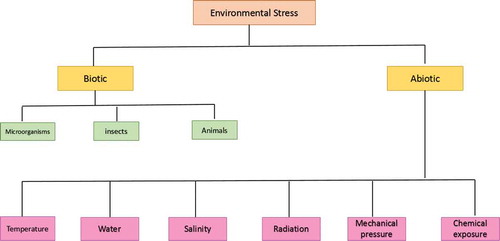
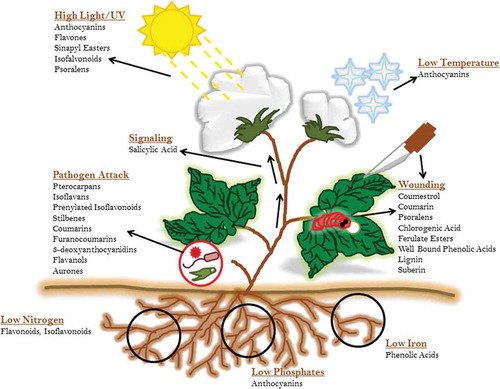
Plants get exposed to a number of biotic and abiotic environmental stresses (Figure ) and produce reactive oxygen species (ROS) in response to these stimulations. These excited ROS molecules cause the oxidation of different cellular biomolecules such as nucleic acids, proteins, lipids and eventually destruct the cellular existence. However, plants respond to ROS accumulation through antioxidative defense system and produce significant secondary metabolites for their survival (You & Chan, Citation2015). Secondary metabolites are those organic compounds which are not primarily involved in the plant development but accomplish specific functions under specified situations, for example, stress condition. Phytohormones, phenolic compounds, anthocyanins and some other pigment molecules are categorized as secondary metabolites, and a long-term impairment occurs in plants if these compounds are imbalanced. Phenolic molecules such as flavonoids (also known as natural biosensors) have been found to be accumulated during environmental stresses and secure the plant cells through inhibition of destructive ROS. Similarly, lignin precursor is also an important metabolite to protect abiotic stress conditions as shown in Figure (Bach et al., Citation2015). Phenylpropanoids comprise a diverse range of compounds grouped into different classes based on their biological functions (Lin et al., Citation2016). To adapt physiological changes, plants sense ambient light conditions and modulate their developmental processes by utilizing multiple photoreceptors such as phytochrome, cryptochrome, phototropin and photoprotectors (Ouzounis, Rosenqvist, & Ottosen, Citation2015). Anthocyanins, flavonols and related phenolics are considered among the flavonoids (Nix, Paull, & Colgrave, Citation2017).
2. Flavonoid as canvas element in cotton
The flavonoid compounds beautifully play their role to paint the nature with colorful flowers, fruits and seeds and more importantly protect the plant against different environmental stresses. Cotton flower color is also regulated by flavonoid pathway.Transcriptome analysis during the course of fiber development showed that pigmentation in naturally colored cotton fiber is due to flavonoid biosynthesis (Hua et al., Citation2007). Mainly, five structural genes, that is, GhCHI (chalcone isomerase), GhF3H (flavonoid 3 hydroxylase), GhDFR (dihydroflavonol-4-reductase), GhANS (anthocyanidin synthase) and GhANR (anthocyanidin reductase) found to play their role in cotton fiber pigmentation. As an instance, the reddening of cotton leaves due to biochemical changes such as increased peroxidase activity and proline content and loss of chlorophyll content indicates the accumulation of anthocyanin (Velikova, Tsonev, Edreva, Gürel, & Hakerlerler, Citation2002). This physiological change in the leaf color serves as an indicator of the major biochemical disturbances of the plant such as salt, temperature, mineral deficiency and eradication (Dixon & Paiva, Citation1995) demanding a careful nurturing of the stressed condition. It has been reported recently that humic acid (HA) application on Gossypium barbadense enhanced the stress defense response by increasing anthocyanin concentration along with a significant positive role on plant growth and fiber quality (Rady, El-Mageed, Abdurrahman, & Mahdi, Citation2016). One of the photoprotective flavonoids, anthocyanin, is responsible for orange to blue color development in various flowers, leaves, fruits, seeds and other tissues along with stress tolerance in cotton (Park et al., Citation2015).
3. Flavonoid as a stress-relieving tool
Cotton is regarded as the backbone of worldwide textile industry and is predominantly important agricultural commodity around the globe. Country-wise major market shares of the cotton textile exporting countries ranging from China US$55 billion, Hong Kong US$38 billion, Korea US$35 billion, Taiwan US$16 billion and Indonesia and Pakistan about US$14 billion (http://fp.brecorder.com). The flavonoid-related phenylpropanoids play an important role in the growth and development of cotton plant. Anthocyanin performs proficient activities against stresses, such as drought, herbivory and pathogen attack (Nakabayashi & Saito, Citation2015).
3.1. Response of anthocyanin against ultraviolet stress
The ultraviolet (UV)-absorbing properties of flavonoids have long been evident from the literature, especially quercetin 3-O and luteolin 7-O-glycosides in the flavonoid metabolic pathway perform notable antioxidant roles against solar UV-B radiation contributing to ROS detoxification in plant cells (Agati et al., Citation2013; Rozema et al., Citation2002). The response of plant flavonoids to sunlight is very influential. They respond to sunlight in the first instance through funneling the green light to plant cell for food synthesis and second by scavenging the ROS, thus reducing the photoinhibition losses caused by intense sunlight (Gould, Citation2004). Almost all species of reactive oxygen and nitrogen are scavenged by purified anthocyanin with efficiency up to four times greater than α-tocopherol and ascorbate.
Moreover, it is determined that elevated incidence of anthocyanins in the stress environment is the last defense line against photoinhibition and ROS when all other protective mechanisms have been exhausted (Gould, Citation2004). The highest concentration of anthocyanins is found in young leaves, to guard against the photobleaching of chlorophyll. Hoch, Singsaas, & McCown (Citation2003) postulated another distinguishing feature of anthocyanins in the protection of foliar nutrient resorption in the period of senescence by protecting photosynthetic tissues from excessive light. Furthermore, a comparison of anthocyanin-deficient mutants and wild type of deciduous woody species revealed that the wild-type plants retained higher photochemical efficiencies than mutants. Hence, they were more capable to recover easily from the abiotic stress environment than could the mutants (Hoch et al., Citation2003). Anthocyanins are predominantly synthesized in cytosol and mainly stored in vacuoles of epidermal cells in cotton leaves. Riar, Wells, Edmisten, Jordan, & Bacheler (Citation2013) also reported changes in pigmentation patterns in a cotton leaf after the abnormal exposure to sunlight. The developed red pigments on the abaxial side of cotton leaves were anthocyanins, which provide protection to the photosynthetic tissues.
3.2. Anthocyanin shields against temperature variation
Flavonoid composition and concentration are sensitive to temperature variations (Arcas, Botía, Ortuño, & Del Rio, Citation2000). Mori and his coworkers have demonstrated that anthocyanin biosynthetic genes are not only strongly downregulated but also degraded by high temperatures, leading to inhibition of messenger RNA (mRNA) transcription as proved through microarray analysis. Cotton plants are more responsive to high temperature. After the onset of 4–5 days of a heat wave, cotton plants shed their small bolls in mid to late bloom. Without adequate moisture, high temperatures during the day had an inverse effect; it decreases the crop yield by shedding of cotton bolls. Damaging effect is more severe on cotton during bloom period. Moreover, high temperatures often cut short the boll-setting period, followed by decreasing late set bolls that resulted in having lower micronaire values. The boll period has been found to be least accelerated by hot weather than other developmental stages, such as seedling growth. Plants modulate to water deficiency by accumulating anthocyanins and other phenolic compounds (Roby, Harbertson, Adams, & Matthews, Citation2004); however, the metabolic inducers of these effects are still ambiguous (Kennedy, Matthews, & Waterhouse, Citation2002).
Grimplet et al. (Citation2007) reported that water deprivation stimulated an upregulation of mRNA involved in several pathways of secondary metabolism. Up to 80% increase in anthocyanin accumulation correlates with mRNA accumulation of UFGT, CHS and F3H, considered as the major anthocyanin biosynthetic genes involved in the flavonoid pathway. Such mechanisms are mainly limited to pulp and skin tissues, while seeds remain scarcely involved. Stress conditions trigger the whole flavonoid biosynthetic pathway, including expression, transport and accumulation of proteins involved (Petrussa et al., Citation2013).
Drought stress has been documented to initiate oxidative stress due to inhibition of photosynthesis (Smirnoff, Citation1993) resulting from the production and accumulation of toxic oxygen species such as peroxide radicals, hydroxyl radicals and hydrogen peroxide (Foyer, Lopez‐Delgado, Dat, & Scott, Citation1997). Anthocyanins also play a direct role in regulating osmotic pressure and preventing water loss while maintaining the turgor pressure. Here exists evidence that anthocyanins function as a solute to slow down leaf osmotic pressure potential, thereby contributing maintenance of osmotic adjustment and turgor pressure in the presence of drought stress during senescence (Chalker‐Scott, Citation1999).
3.3. Osmotic stress
Cotton fiber characteristics have been adversely affected by the water-deficit stress (Hearn, Citation1994). Timing in water-deficit stress is also a crucial factor. Previous studies showed that this stress in early flowering season of cotton had no effect on fiber quality, but when the stress occurred shortly after flowering, it drastically decreased the fiber length. In actual, the cotton fiber elongation process is primarily dependent on turgor pressure (Dhindsa, Beasley, & Ting, Citation1976). Moreover, deficiency in plant water supply, photosynthetic rate and carbohydrate supply negatively affect the fiber growth. The similar idea is also supported by Cosgrove (Citation1993) who explained that increased growing plant cell volume is highly dependent on water uptake by the vacuole. So, the lint yield is a function of fiber qualities, number of fibers/seed and number of seeds/unit area (Lewis, Challinor, & Lasenby, Citation2000). A strong correlation exists among plant water content, accumulation of dry matter in the developing fiber and in the developing seed which showed that quick water uptake supports seed growth.
3.4. Role of flavonoids against biotic stresses
Flavonoid and its related secondary metabolites have distinguishing features to protect plants against pathogens attack. These secondary metabolites are more likely to be the principal mediators of plant defense against insects in accordance with the phytochemical coevolution theory. The C-glycosyl flavones is effective against Helicoverpa zea, as well as isoflavones and isoflavanones besides bacterial pathogen Pseudomonas syringae pv. glycinea (Yong & Man-Tian, Citation2005). Similarly, the upregulation of certain flavonoid structural genes that enhanced the production of isoflavone and isoflavonoid compounds in phenyl-propanoid pathway was found to be a natural effective remedy against causal agents of powdery mildew, for example, Erysiphe pisi in Medicago truncatula (Foster‐Hartnett et al., Citation2007). Phytoalexins such as 2,7-dihydroxycadalene and lacinilene C7-methyl ether usually accumulate at infection sites in the hypersensitive-resistant response of cotton foliage to Puccinia malvacearum, Xanthomonas campestris as well as toward the bacterial pathogen (Stipanovic, Wakelyn, & Bell, Citation1975c). As a result, sometimes, the adaxial epidermal cells covering the infection sites turn red. These red cells have the ability of higher absorption at the photoactivating wavelengths of in comparison to colorless epidermal cells. The role of these epidermal pigments is in protection of living cells that surround infection sites from the toxic effects of phytoalexins. Experiments with UV-absorbing substances obtained from epidermal strips from inoculated and mock-inoculated cotton cotyledons indicated that primary increase in capacity to absorb the photoactivating wavelengths was due to a yellow and red anthocyanin flavonol, which were mainly known as quercetin-3-O-b-glucoside and cyanidin-3-O-glucoside, respectively (Edwards et al., Citation2008).
Anthocyanins have gained attention as indicators against pest and pathogen resistance. Previous studies showed that cyanidin-3-glucoside compounds in cotton leaves are primarily involved in developing resistance against tobacco budworm and Heliothis virescens. Previous studies reported leucoanthocyanins effective for bacterial blight in infected resistant cotton leaves. In the study conducted under controlled environmental conditions, the similar relationship between Xanthomonas campestris pv. malvacearum and anthocyanins production was shown. The epidermal adaxial surface of the leaves was the main tissue involved in anthocyanin production, thus revealing anthocyanins’ protective role in damage by light-activated phytoalexins and infection ROS (Kangatharalingam, Pierce, Bayles, & Essenberg, Citation2002).
Another study conducted by Fan et al. (Citation2015) showed that increased anthocyanin content by over expression of LC (leaf color) transgene showed a significant resistance in cotton leaves when infested with third-instar cotton bollworm larvae (Helicoverpa armigera). Flavonols appear in the front-line defense mechanism and triggered the insect behavior. Pink bollworm is more sensitive to flavonols, while the cotton bollworm is less sensitive as compared to the tobacco budworm. The insecticidal activity of well-known flavonols or flavonoid pathway, such as kaempferol, quercetin, isoquercitrin and rutin in cotton crop were also tested in previous studies Appreciable concentrations of free quercetin and kaempferol have been identified as glucosides in cotton plant tissues. Rutin and isoquercitrin at concentrations above 0.2% inhibit larval growth, particularly pupation in cotton bollworm, tobacco budworm and pink bollworm (Chan, Waiss, Binder, & Elliger, Citation1978; Nix et al., Citation2017). The concentrations of 0.05–0.1% of rutin added to 0.1% gossypol greatly increased toxicity to bollworms, indicating a synergistic interaction between flavonoids and terpenoids in natural resistance to insects (Chan et al., Citation1978). Before designing a strategy on genetic manipulation for insect control using flavonoid compounds, there is a critical need for having more toxicity data on major glycosides that would prove lethal dose against insects in cotton crop (Bell, Citation1986). Different species of genus Gossypium have different quantities of glycosides such as rhamnoglucosides are more abundant in G. hirsutum but in trace amounts in G. barbadense whereas kaempferol-3-glucoside and quercetin-7-glycosides widely present in G. barbadense in comparison to G. hirsutum. Other numerous differences among different species have been reviewed by Parks, Ezell, Williams, & Dreyer (Citation1975).
4. Flavonoid role in enhanced fiber quality
Although the exact mechanism of anthocyanins in fiber development is yet not clear and need to be updated, the literature disclosed some relation between these phenolic compounds and phytohormones in establishing high fiber quality.
Phytohormones application can greatly modify the expression level of genes involved in biosynthesis of anthocyanin. Abscisic acid (ABA) somehow found to upregulate the transcription of genes which take part in anthocyanin pathway, that is, Chs, Chi, Dfr and Ufgt (Jeong, Goto-Yamamoto, Kobayashi, & Esaka, Citation2004). Similarly, application of another phytohormone, 2-chloroethylphosphonic acid (2-CEPA) stimulated the long-term expression of genes such as Chs, F3h, Ans and Ufgt but not Dfr of anthocyanin pathway. In another report, post-harvest application of this particular plant hormone improved the stability of anthocyanins (Bellincontro, Fardelli, Santis, Botondi, & Mencarelli, Citation2006). Other well-known phytohormones such as jasmonic acid and salicylic acid activated as a result of biotic as well as abiotic stresses that enhanced the various genes of flavonoid pathway, that is, phenylalanine ammonia-lyase, Chs and Ufgt. Therefore, plant hormones stimulate the anthocyanin production, and they together play significant role in producing high-quality fiber.
Flavonoid functions as endocrine effector to determine PIN gene expression along with protein localization and acts as an indirect modulation of auxin transport (Santelia et al., Citation2008). These PIN genes, that is, GhPIN1a_Dt, GhPIN6_At and GhPIN8_At, jointly play a critical role in fiber growth and enhanced fiber elongation by regulating the auxin transport which eventually causes the loosening of fiber cell wall in allotetraploid cotton, G. hirsutum (Zhang, Citation2017).
The differential expression of flavonoid pathway genes through transcriptomic analysis at various stages of fiber development is evident for the involvement of these genes in fiber elongation. Scientists also reported regulation of flavonol genes during fiber development in cotton. G. barbadense and G. hirsutum varieties were compared from fiber initiation stage to maturation through a transcriptome analysis (Al-Ghazi, Bourot, Arioli, Dennis, & Llewellyn, Citation2009). Expression of isoflavonol genes greatly differed among both transcriptomes as upregulated expression in G. barbadense and downregulated in G. hirsutum was observed which further confirmed their positive role in the development of extra-long fiber. Padmalatha et al. (Citation2012) also performed transcriptome study of G. hirsutum during different fiber developmental stages and reported downregulation of flavonoid genes during fiber initiation and upregulation before the onset of elongation phenomenon. Another meta-analysis study on functional classification of differentially expressed genes in extra-long fiber as compared to shorter fiber that showed positive regulation of flavonoid biosynthetic process. This work was carried out by gene expression omnibus (GEO) portal available at National Center for Biotechnology Information. Various types of flavonoid genes actively expressed during early stages of fiber formation. A well-known flavonoid naringenin (NAR) is found to reduce the fiber development by silencing flavanone-3-hydroxylase (F3H) gene. Although overexpression of the F3H gene also did not directly result in increased fiber development, but its silencing tendency significantly disturbs the fiber initiation. Hence, the imperfections in cell volume due to osmotic stress could be resulted in increased flavonoid production and eventually modify the auxin balance in plant cells as well as fiber development.
Critical analysis of published data on physiological roles of flavonoids shows that it has greater potency to resist environmental stress. A new dimension in research presented in this review is to generate new cotton lines by selecting appropriate genes from flavonoid pathway, and its over expression in them will make them able to produce high-quality fiber and insecticide-resistant varieties.
5. Flavonoids role in plant fertility
Recent work established a correlation between flavonoids and pollen fertility which was previously unknown. For the first time, this point was unrevealed in anthocyanin mutants of maize. Furthermore, these mutants failed to produce a functional pollen tube, leading to male sterility. Fertility could be restoring by the addition of the flavonol kaempferol at pollination. Quercetin (flavonols) particularly have essential roles in plant fertility by enhancing pollen germination. However, Arabidopsis plants completely devoid lacking all chalcone synthase activities showed no pollen tube growth aberrations, elucidating that pollen fertility is not universally dependent on flavonols.
In cotton, MYB genes are renowned for fiber development and elongation. Limited knowledge about their role in anther or pollen development in cotton is available. Among MYB genes, GhMYB24 was identified as novel gene in cotton which was predominantly expressed in pollen. Moreover, this gene-encoded transcriptional activator which further interacts with GhJAZ1/2 proteins of JA signaling pathway significantly induced male sterility. However, particular role and behavior of flavonoids with respect to plant fertility is a chapter which is still unexplored. Side by side, both positive and negative reports on flavonoid behavior in plant fertility are given. But exact mechanism in cotton is unknown, and its role could be attractive for genetic engineers because it could be a side bridge to conventional breeders as well as manual hard field practices could be avoided.
6. Conclusion
Flavonoids are essential plant secondary metabolites with a variety of functions in plant physiology. Phytohormones and flavonoid pigments are correlated and together contribute in number of ways such as in cotton plant physiology, fiber development, environmental stress tolerance as well as in plant fertility. To date, very few experimental reports are available on potency of flavonoid genes in successfully modifying fiber traits. New research area is to develop local cotton varieties equipped with flavonoid transgenes. As fiber is an end product of cotton, fiber with superior characteristics will prove a remarkable raw material for textile industry.
Authors’ contribution
A.A. and A.Y. contributed equally in writing this article, R.N. made the picture, A.G., A.Q.R., A.A.S. and T.H. reviewed this article and refined the final document.
Additional information
Funding
Notes on contributors

Ammara Ahad
Ammara Ahad and Amina Yaqoob are PhD research scholars from Centre of Excellence in Molecular Biology (CEMB), University of the Punjab, Lahore. They are working in Plant Biotechnology laboratory under the supervisions of Prof. Dr Tayyab Husnain (Director CEMB) and Prof. Dr Ahmad Ali Shahid, respectively. Their areas of research focus on plant molecular biology, genetic transformation, bioinformatics and transgenic crop analysis. They have been working in cotton transformation for more than a decade and, therefore, published a number of research/review articles in renowned journals.
References
- Agati, G., Brunetti, C., Di M, F., Ferrini, F., Pollastri, S., & Tattini, M. (2013). Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiology and Biochemistry, 72, 35–45. doi:10.1016/j.plaphy.2013.03.014
- Al-Ghazi, Y., Bourot, S., Arioli, T., Dennis, E. S., & Llewellyn, D. J. (2009). Transcript profiling during fiber development identifies pathways in secondary metabolism and cell wall structure that may contribute to cotton fiber quality. Plant and Cell Physiology, 50, 1364–1381. doi:10.1093/pcp/pcp084
- Arcas, M. C., Botía, J. M., Ortuño, A. M., & Del Rio, J. A. (2000). UV irradiation alters the levels of flavonoids involved in the defence mechanism of citrus aurantium fruits against Penicillium digitatum. European Journal of Plant Pathology, 106, 617–622. doi:10.1023/A:1008704102446
- Bach, A., Kopczynska, A., Dziurka, K., & Dziurka, M. (2015). The endogenous phenolic compounds during bulb formation in lachenalia sp. in vitro cultures under different lighting conditions. BioTechnologia Journal of Biotechnology Computational Biology and Bionanotechnology, 96 (pp. 41-53).
- Bell, A. A. (1986). Physiology of secondary products (pp. 597–621). Memphis, TN: Cotton Physiology The Cotton Foundation.
- Bellincontro, A., Fardelli, A., Santis, D. D., Botondi, R., & Mencarelli, F. (2006). Postharvest ethylene and 1‐MCP treatments both affect phenols, anthocyanins, and aromatic quality of aleatico grapes and wine. Australian Journal of Grape and Wine Research, 12, 141–149. doi:10.1111/j.1755-0238.2006.tb00054.x
- Chalker‐Scott, L. (1999). Environmental significance of anthocyanins in plant stress responses. Photochemistry and Photobiology, 70, 1–9. doi:10.1111/php.1999.70.issue-1
- Chan, B., Waiss, A., Binder, R., & Elliger, C. (1978). Inhibition of lepidopterous larval growth by cotton constituents. Entomologia Experimentalis Et Applicata, 24, 294–300. doi:10.1111/eea.1978.24.issue-3
- Cosgrove, D. J. (1993). Water uptake by growing cells: An assessment of the controlling roles of wall relaxation, solute uptake, and hydraulic conductance. International Journal of Plant Sciences, 154, 10–21.
- Dhindsa, R., Beasley, C., & Ting, I. (1976). Effects of abscisic acid on in vitro growth of cotton fiber. Planta, 130, 197–201. doi:10.1007/BF00384420
- Dixon, R. A., & Paiva, N. L. (1995). Stress-induced phenylpropanoid metabolism. The Plant Cell, 7, 1085. doi:10.1105/tpc.7.7.1085
- Edwards, W. R., Hall, J. A., Rowlan, A. R., Schneider-Barfield, T., Sun, T. J., Patil, M. A., … Essenberg, M. (2008). Light filtering by epidermal flavonoids during the resistant response of cotton to xanthomonas protects leaf tissue from light-dependent phytoalexin toxicity. Phytochemistry, 69, 2320–2328. doi:10.1016/j.phytochem.2008.05.021
- Fan, M.-J., Wang, I.-C., Hsiao, Y.-T., Lin, H.-Y., Tang, N.-Y., Hung, T.-C., … Chung, J.-G. (2015). Anthocyanins from black rice (Oryza sativa L.) demonstrate antimetastatic properties by reducing MMPs and NF-κB expressions in human oral cancer CAL 27 cells. Nutrition and Cancer, 67, 327–338. doi:10.1080/01635581.2015.990576
- Foster‐Hartnett, D., Danesh, D., Penuela, S., Sharopova, N., Endre, G., Vandenbosch, K. A., … Samac, D. A. (2007). Molecular and cytological responses of medicago truncatula to erysiphe pisi. Molecular Plant Pathology, 8, 307–319. doi:10.1111/j.1364-3703.2007.00395.x
- Foyer, C. H., Lopez‐Delgado, H., Dat, J. F., & Scott, I. M. (1997). Hydrogen peroxide‐and glutathione‐associated mechanisms of acclimatory stress tolerance and signalling. Physiologia Plantarum, 100, 241–254. doi:10.1111/j.1399-3054.1997.tb04780.x
- Gould, K. S. (2004). Nature’s Swiss army knife: The diverse protective roles of anthocyanins in leaves. BioMed Research International, 2004, 314–320.
- Grimplet, J., Deluc, L. G., Tillett, R. L., Wheatley, M. D., Schlauch, K. A., Cramer, G. R., & Cushman, J. C. (2007). Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics, 8, 187. doi:10.1186/1471-2164-8-109
- Hearn, A. (1994). OZCOT: A simulation model for cotton crop management. Agricultural Systems, 44, 257–299. doi:10.1016/0308-521X(94)90223-3
- Hoch, W. A., Singsaas, E. L., & McCown, B. H. (2003). Resorption protection. Anthocyanins facilitate nutrient recovery in autumn by shielding leaves from potentially damaging light levels. Plant Physiology, 133, 1296–1305. doi:10.1104/pp.103.027631
- Hua, S., Wang, X., Yuan, S., Shao, M., Zhao, X., Zhu, S., & Jiang, L. (2007). Characterization of pigmentation and cellulose synthesis in colored cotton fibers. Crop Science, 47, 1540–1546. doi:10.2135/cropsci2006.12.0835
- Jeong, S. T., Goto-Yamamoto, N., Kobayashi, S., & Esaka, M. (2004). Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Science, 167, 247–252. doi:10.1016/j.plantsci.2004.03.021
- Kangatharalingam, N., Pierce, M. L., Bayles, M. B., & Essenberg, M. (2002). Epidermal anthocyanin production as an indicator of bacterial blight resistance in cotton. Physiological and Molecular Plant Pathology, 61, 189–195. doi:10.1006/pmpp.2002.0434
- Kennedy, J. A., Matthews, M. A., & Waterhouse, A. L. (2002). Effect of maturity and vine water status on grape skin and wine flavonoids. American Journal of Enology and Viticulture, 53, 268–274.
- Lewis, A., Challinor, A., & Lasenby, A. (2000). Efficient computation of cosmic microwave background anisotropies in closed Friedmann-Robertson-Walker models. The Astrophysical Journal, 538, 473. doi:10.1086/309179
- Lin, D. R., Xiao, M. S., Zhao, J. J., Li, Z. H., Xing, B. S., Li, X. D., … Chen, S. Y. (2016). An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules (Basel, Switzerland), 21, 10. doi:10.3390/molecules21101374
- Nakabayashi, R., & Saito, K. (2015). Integrated metabolomics for abiotic stress responses in plants. Current Opinion in Plant Biology, 24, 10–16. doi:10.1016/j.pbi.2015.01.003
- Nix, A., Paull, C., & Colgrave, M. (2017). Flavonoid profile of the cotton plant. Gossypium Hirsutum: A Review - Plants, 6(4), 43.
- Ouzounis, T., Rosenqvist, E., & Ottosen, C.-O. (2015). Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience, 50, 1128–1135.
- Padmalatha, K. V., Dhandapani, G., Kanakachari, M., Kumar, S., Dass, A., Patil, D. P., … Rawat, B. (2012). Genome-wide transcriptomic analysis of cotton under drought stress reveal significant down-regulation of genes and pathways involved in fibre elongation and up-regulation of defense responsive genes. Plant Molecular Biology, 78, 223–246. doi:10.1007/s11103-011-9857-y
- Park, C. H., Chae, S. C., Park, S.-Y., Kim, J. K., Kim, Y. J., Chung, S. O., … Park, S. U. (2015). Anthocyanin and carotenoid contents in different cultivars of chrysanthemum (Dendranthema grandiflorum Ramat.) flower. Molecules., 20, 11090–11102. doi:10.3390/molecules200611090
- Parks, C., Ezell, W., Williams, D., & Dreyer, D. (1975). The application of flavonoid distribution to taxonomic problems in the genus Gossypium. Bulletin of the Torrey Botanical Club, 102, 350–361. doi:10.2307/2484761
- Petrussa, E., Braidot, E., Zancani, M., Peresson, C., Bertolini, A., Patui, S., & Vianello, A. (2013). Plant flavonoids—Biosynthesis, transport and involvement in stress responses. International Journal of Molecular Sciences, 14, 14950–14973. doi:10.3390/ijms140714950
- Rady, M., El-Mageed, T. A., Abdurrahman, H., & Mahdi, A. (2016). Humic acid application improves field performance of cotton (Gossypium barbadense L.) under saline conditions. Journal Animal Plant Sciences, 26, 485–493.
- Riar, R., Wells, R., Edmisten, K., Jordan, D., & Bacheler, J. (2013). Changes in cotton leaf pigmentation after abnormal exposure to sunlight. Journal of Agricultural Research and Development, 2(1), 007–013.
- Roby, G., Harbertson, J. F., Adams, D. A., & Matthews, M. A. (2004). Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Australian Journal of Grape and Wine Research, 10, 100–107. doi:10.1111/j.1755-0238.2004.tb00012.x
- Rozema, J., Björn, L. O., Bornman, J., Gaberščik, A., D-P, H., Trošt, T., … Sinha, R. (2002). The role of UV-B radiation in aquatic and terrestrial ecosystems—An experimental and functional analysis of the evolution of UV-absorbing compounds. Journal of Photochemistry and Photobiology B: Biology, 66, 2–12. doi:10.1016/S1011-1344(01)00269-X
- Santelia, D., Henrichs, S., Vincenzetti, V., Sauer, M., Bigler, L., Klein, M., … Geisler, M. (2008). Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. Journal of Biological Chemistry, 283, 31218–31226. doi:10.1074/jbc.M710122200
- Smirnoff, N. (1993). The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist, 125, 27–58. doi:10.1111/nph.1993.125.issue-1
- Stipanovic, R. D., Wakelyn, P. J., & Bell, A. A. (1975c). Lacinilene C, a revised structure, and lacinilene C7-methyl ether from Gossypium bracts. Phytochemistry, 14, 1041–1043. doi:10.1016/0031-9422(75)85183-1
- Velikova, V., Tsonev, T., Edreva, A., Gürel, A., & Hakerlerler, H. (2002). Effects of reddening of cotton (Gossypium hirsutum L.) leaves on functional activity of photosynthetic apparatus. Photosynthetica., 40, 449–452. doi:10.1023/A:1022695513060
- Yong, Z., & Man-Tian, M. (2005). Genistein stimulates hematopoiesis and increases survival in irradiated mice. Journal of Radiation Research, 46, 425–433.
- You, J., & Chan, Z. (2015). ROS regulation during abiotic stress responses in crop plants. Frontiers in Plant Science, 6, 1092. doi:10.3389/fpls.2015.01092
- Zhang, Y. E. (2017). Non-smad signaling pathways of the TGF-β family. Cold Spring Harbor Perspectives in Biology, 9, a022129. doi:10.1101/cshperspect.a022129