Abstract
The lack of a standardized protocol makes it difficult to compare studies on the efficacy of commercial rodenticides. To contribute to the knowledge of pest control technology, we compared the efficacy of 17 commercial baits with different active ingredients and types of formulation from various commercial suppliers, using a standardized efficacy protocol under laboratory conditions. All rats died in all experimental groups. First deaths occurred 3 days after the beginning of the trial; average survival varied between 4.17 ± 0.12 days (difethialone wax blocks) and 5.96 ± 0.35 days (difenacoum pellets). Results showed no consistent pattern of time to death according to active ingredient and type of formulation. For bromadiolone-based baits, the grains induced significantly shorter time to death than wax blocks and the individuals fed on wax blocks varied the consumption rate according to the commercial supplier, while those consuming grains showed more homogeneous values. Our results show that although mortality was 100% efficacy differed among baits. These differences could be explained by the combination of the formulation type and commercial supplier rather than by the active ingredient itself.
PUBLIC INTEREST STATEMENT
Poisoned baits are the most commonly used methods for controlling rats in large cities. Second-generation anticoagulant rodenticides based on different active ingredients are available for both professional and home use in a wide variety formulation of different commercial suppliers. Although efficacy of each product has been evaluated for its commercial approval, there are no studies comparing efficacy among products using a standardized protocol. The commercial products may vary in their active principle, formulation (block, grain or pellet) among suppliers. Understanding contribution of these factors in the differences in bait efficacy will improve the product choice. This work provides useful knowledge for decision-making in rodent control actions.
Competing Interests
The authors declare no competing interests.
1. Introduction
Commensal rodents are considered a serious pest in urban environments. Norway rats (Rattus norvegicus), roof rats (Rattus rattus) and house mice (Mus musculus) are responsible for a number of health and economic problems as a result of their interaction with humans (Cueto, Cavia, Bellomo, Padula, & Suárez, Citation2008; Hancke, Navone, & Suárez, Citation2011; Himsworth, Parsons, Claire, & Patrick, Citation2013). High population density, exposed garbage and abandoned buildings have been found to be strong promoters of rat infestations (Easterbrook, Shields, Klein, & Glass, Citation2005; Traweger, Travnitzky, Moser, Walzer, & Bernatzky, Citation2006). Moreover, rat infestations tend to be more prevalent in low socioeconomic neighborhoods (Cavia, Cueto, & Suárez, Citation2009; Cavia, Muschetto, Cueto, & Suárez, Citation2015; Fernández, Cavia, Cueto, & Suárez, Citation2007; Vadell, Cavia, & Suárez, Citation2010; Walsh, Citation2014). Improvements introduced to sanitation and infrastructure can greatly reduce rat-related problems, but in most cases control measures require the use of chemicals, specifically rodenticides.
Rodenticides are classified as non-anticoagulant acute poisons and anticoagulants. Anticoagulants are the preferred method for controlling rodent populations worldwide because of their higher efficacy and safety (Bajomi & Kis-Varga, Citation1990; Murphy, Citation2002). They interfere with the mechanisms of blood coagulation, thus leading to internal bleeding and, ultimately, death (Hadler & Buckle, Citation1992; Pelz et al., Citation2005).
Rodent resistance to warfarin and other first-generation anticoagulants (FGARs) encouraged the development of more toxic second-generation anticoagulants (SGARs) (Buckle, et al.,Citation1994; Jackson & Ashton, Citation1992; Jackson & Kaukeinen, Citation1972). These have a delayed action because the active ingredient is used at very low concentrations and rodents cannot associate the onset of illness due to poisoning with any particular bait, thus preventing bait shyness.
The chemicals used in rodenticide formulations are of varied nature and origin, having different modes of action and toxicity (Witmer, Eisemann, & Howald, Citation2007). Bromadiolone, brodifacoum and difenacoum are the most frequently used active ingredients in the manufacture of SGRAs, many of which are available in one or more formulations such as wax blocks, pellets or grains (Witmer, Horak, Moulton, & Baldwin, Citation2013). The formulations of rodenticide baits may also contain additives that enhance product quality, preserve the chemical and physical properties before the expiration date or facilitate application (Prakash et al., Citation2003).
Many studies have been conducted comparing the efficacy of products already available on the commercial market (Greaves, Shepherd, & Quy, Citation1982; Prakash et al., Citation2003). However, the lack of a standardized protocol makes it very difficult to compare the results across studies, limiting the scope of the results (Prescott & Johnson, Citation1996). In this article, we compare the efficacy of 17 commercial rodenticide baits with different active ingredients and types of formulation obtained from various commercial suppliers. We used a standardized efficacy protocol under indoor, controlled conditions with the expectation of contributing to the knowledge of pest control technology (Schneider & Hitch, Citation1982).
2. Materials and methods
The Rodents Urban Ecology Research Laboratory of the University of Buenos Aires, Argentina conducts efficacy tests using rodenticide products obtained from commercial manufacturers and suppliers. A database is maintained incorporating all rodenticide efficacy data generated since 2012, and the data presented below have been obtained by analyzing that database.
We tested the efficacy of the SGARs bromodialone (0.005%), brodifacoum (0.0025%), difenacoum (0.005%) and difethialone (0.005%), using one or more of the following formulation types: wax blocks, pellets and whole grains. They were provided by eight different commercial suppliers, being registered for use against commensal rodents in Argentina. Three commercial suppliers provide locally manufactured baits and the remaining five provide imported ones. Further information about commercial baits characteristics in Additional file 1.
Laboratory tests were conducted from July 2012 to November 2015 in the bioterium at the University of Buenos Aires, Argentina. Experiments on animals were approved by the Ethics Committee of Bioterium at the University of Buenos Aires, Argentina and were in adherence to the guidelines of the Sociedad Argentina para el Estudio de los Mamíferos (Giannoni, Mera Sierra, Brengio, & Jiménez Baigorria, Citation2003) and NationalAct 14346 for the protection of animals.
Adult male and female Wistar strain of brown rats (Rattus norvegicus) were individually placed in plastic cages (60x50x45 cm) and allowed to acclimate to standard laboratory conditions (21–24° C, 12 h light/dark cycle, 45–70% relative humidity) for 1 week prior to each trial. In this period, the animals were fed rat pellets and had ad libitum access to water.
We randomly assigned 24 adults (12 males + 12 females) to each treatment group (combination of active ingredients, formulation types and commercial suppliers). Initial mean body weights of males were 265.6 g (sd = 18.8g) and females 255 g. (sd = 15.3g.). The mortality rate without treatment was determined using 12 adults of both sexes (6 males + 6 females) ascontrol group for each commercial supplier; they were fed rat pellets and kept under the same laboratory conditions as mentioned above for 1 week.
All caged animals were starved for 24 h before being offered a cup with 60 g of poison bait (wax block, pellet or whole grain) and water ad libitum. Poison bait consumption was recorded individually and the cup was replenished at the end of each 24-h period. Rats were monitored daily for health condition (alive or dead) and their individual weight was recorded until time to death.
2.1. Data analysis
Differences in survival were compared between experimental and control animals using the Log Rank statistic (K = 2) obtained from the Kaplan-Meier algorithm (Infostat).
A general linear mixed model (GLMM) (Zuur, Ieno, Walker, Saveliev, & Smith, Citation2009) was used to analyze the effect of formulation type (wax block, grain and pellet) and active ingredient (bromadiolone, brodifacoum, difenacoum and difethialone) on time to death of rats (response variable). A single variable was introduced in the model representing all the possible combinations because the active ingredients were not available in all types of formulations. To assess the effect of sex, weight of rats and origin of baits (locally manufactured or imported ones) the inclusion of these variables into the model was tested. The commercial suppliers were added to the model as random factor to account for the variability among rats fed on products from different suppliers. Homogeneity of variance was examined by inspection of the residuals after model fitting (plots of standardized residuals vs. fitted values). Heterogeneity of residual variance was modeled using a power variance function (varPower function) (Zuur et al., Citation2009). Pairwise comparisons between mean treatments were performed by LSD Test.
A second analysis was conducted with rats fed on bromadiolone since this is the most common active ingredient used in Argentina (ANMAT). GLMM (Zuur et al., Citation2009) was performed to analyze the effect of formulation type (wax block or grain) on time to death of rats. We included the commercial suppliers as random factor and heterogeneous within-group variances were modeled using a constant variance function (varIdent function) (Zuur et al., Citation2009). To assess the effect of sex of rats and origin of baits the inclusion of both variables into the model was tested. Daily consumption (g bait consumed/g body weight) was incorporated to control potential effect of consumption on time to death. Daily consumption rate was calculated for the first 2 days to avoid changes in the consumption rate due to intoxication.
3. Results
There was no mortality of control rats but all rats tested with anticoagulant formulations (n = 17) died (Log Rank Test; p < 0.01 in all cases). The analysis of survival showed that the first deaths occurred 72 h after the beginning of the experiment for all four active ingredients (Table ). However, the experimental populations treated with different active ingredients showed variability in the time to reach 100% mortality. Rats treated with difethialone or brodifacoum required 7 days to reach 100% mortality, while those treated with difenacoum or bromadiolone took 3 and 4 days longer, respectively (Table ).
Table 1. Time to death of rats fed with different commercial rodenticide baits (combination of active ingredient and type of formulation)
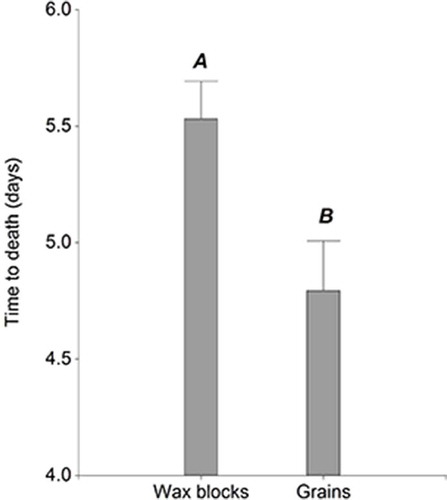
Most of the experimental rats were fed on bromadiolone (n = 216 vs n = 48 with other active ingredients; Table ) because most of Argentine baits are based on this active ingredient, being wax blocks and grain baits the most common formulations (ANMAT). The average time to death of experimental animals treated with different formulates (combination of the active ingredient and type of formulation) varied between a minimum of 4.17 ± 0.12 days for difethialone wax block baits and a maximum of 5.96 ± 0.35 days for difenacoum pellet baits (Table ). However, results did not indicate a consistent pattern of time to death according to the active ingredient and type of formulation. For example, rats fed on bromadiolone grains showed a significantly shorter time to death (4.69 ± 0.14 d) than those fed on wax blocks (5.53 ± 0.14 d) (Table ).No significant effects were registered in time to death, related to sex or weight of rats, or related to the origin of baits either (pvalue> 0.05 for the three variables).
The analysis of bromadiolone-based baits revealed that the individuals fed on wax blocks varied the total consumption according to the commercial supplier, while those fed on grain baits showed more homogeneous values (Figure ).
Figure 1. Total bait consumption (average ± standard error) of rats from different commercial suppliers treated with bromadiolone wax blocks and grains. Each commercial supplier was identified by a capital letter.
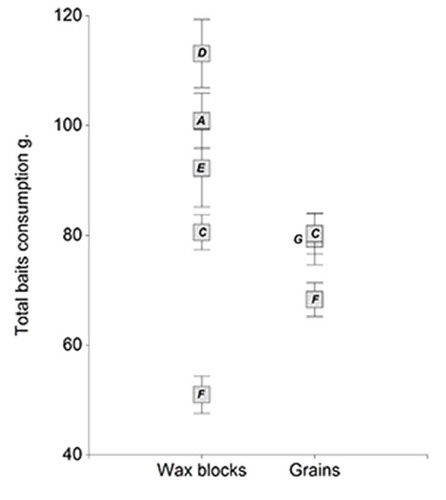
The time to death of animals fed on grain baits was significantly shorter than those of animals fed on wax blocks (Estimate = −0.74 (SE = 0.24); d.f. = 183; t = -3.13; p-value = 0.002) (Figure ). Rats fed on bromadiolone did not show significant differences in time to death, related to sex of individuals or origin of baits (pvalue > 0.05 for both variables). On the other hand, the variability in time to death of rats within the same trial varied among commercial suppliers. Standard deviation ranged between 0.87 days for the most homogeneous supplier (Label E) and 2.03 days for the most heterogeneous one (Label A). Therefore, AIC of GLMM showed a significant decrease when a VarIdent function was used to model different variances for each supplier.
4. Discussion
The experimental rats showed a wide range of time to death depending on the four active ingredients analyzed. However, the earliest deaths occurred 3 days after exposure to the poison baits in all assays. The delay corresponds to the time between the inhibition of the epoxide reductase and the degradation of the clotting factors that were already present in the blood; when the supply of these factors has been exhausted, the clotting mechanism fails and hemorrhaging begins. Therefore, SGARs prevent rodents from associating illness with poison bait consumption due to the time interval between the ingestion of a potentially lethal dose and the onset of symptoms (Hadler & Buckle, Citation1992).
There was not a clear pattern of time to death which allows explaining the differences among active ingredients and different formulation types. For example, the time to death between pellets and wax blocks showed substantial differences for difenacoum, but similar values for bromadiolone. However, these results should be treated with caution because most of the formulations were provided by the same commercial supplier (Table ). Bait efficacy (measured as time to death) could be explained not only by the active ingredient and formulation type but also by differences in the product quality among commercial suppliers.
Besides the active ingredient, the SGARs also include base material comprising solvents, diluents, stabilizers, additives, co-adjuvants and inert substances to achieve the desired attributes, preserve the chemical and physical properties before the expiration date or facilitate application. The efficacy of a rodenticide bait may be dependent on the quality of the bait base rather than on the active ingredient, as the latter may reduce the bait’s palatability (Rady, Hegab, Abdelnabby, & El-Bakhshwangi, Citation2012). Variations in the quality of the bait base components could play a key role in the differences observed in the rate of consumption of bromadiolone wax blocks among commercial suppliers. In Argentina, 17 out of 36 rodenticides registered for sale in the National Administration of Drugs, Food and Medical Technology (ANMAT) contain bromadiolone as active ingredient. We could compare the efficacy of bromadiolone-based baits among different types of formulation due to the variety of products in the market. Average time to death of rats was shorter in individuals fed on grain baits than on wax blocks and its dispersion varied among commercial suppliers. Moreover, rats fed on wax block showed more consumption variability among commercial supplier. These results might suggest that quality of the base components in the wax block baits from each commercial supplier may affect both the consumption rate and the efficacy of the product. However, these were not observed in Bromadiolone treated grains, probably because grain baits could have a lower number of inert substances than wax blocks, which would minimize differences in product quality among commercial suppliers. The identity and relative proportion of the base components of the wax block baits play a key role in product quality, thereby being treated as confidential information by the commercial suppliers.
Most commonly used anticoagulant rodenticides take several days to kill, causing distress, disability and/or pain during this time (Meerburg, Brom, & Kijlstra, Citation2008). Our results reveal that the success of a rodent-control program relying on the use of wax blocks is affected by the choice of high-quality products. Then, the animals treated with these products presumably have a reduced symptomatic period as the time to death is short. According to Mason and Littin (Citation2003), the methods that produce a quick death with minimal suffering are preferred from an animal welfare point of view. Furthermore, poisoned rats with clinical manifestations of intoxication such as external bleeding and bloody diarrhea (Littin et al., Citation2000) for longer periods of time represent hazards to public health. The contact with the blood of rodents strongly increases the probability of transmission of a number of zoonotic pathogens responsible for significant human morbidity and mortality (Cueto et al., Citation2008; Easterbrook et al., Citation2007; Hancke et al., Citation2011; Himsworth et al., Citation2013).
We agree with some rodent management practitioners suggesting a more ecologically based management for rodent damage (Singleton, Leirs, Hinds, & Zhang, Citation1999). Nonetheless, rodenticides remain as very important tools for the integrated pest management and the sustained contribution of technological development is thus necessary to improve the efficacy and safer use of the product.
5. Conclusions
Our results did not show a clear pattern of time to death which allows explaining the differences among active ingredients and different formulation types. Bait efficacy could be explained by the combination among active ingredient, formulation type and the differences in product quality between commercial suppliers. The efficacy of bromadiolone-based baits was higher in individuals fed on grain baits than on wax blocks. Rats fed on wax block showed more consumption variability among commercial suppliers. These results might suggest that quality of the base components in the wax block baits from each commercial supplier may affect both the consumption rate and the efficacy of the product.
Supplemental Material
Download Zip (10.2 KB)Acknowledgements
Authors are thankful to Bioterium at the University of Buenos Aires, Argentina, especially Dra. G. Lammel and Martin Viale for their assistance in laboratory experiments and Karina Hodara kindly revised the English version of the manuscript.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
Notes on contributors
Olga V. Suárez
Olga V. Suárez holds a PhD in Biological Science from the University of Buenos Aires (UBA). She is also an investigator for the National Council of Science and Technology of Argentina (CONICET). For 20 years, she has been the technically responsible for the Rodent Control Program of the city Buenos Aires (Argentina). Dra. Suárez also directs the Urban Rodent Ecology Laboratory of the Faculty of Exact and Natural Sciences at the University of Buenos Aires. Her research focuses on studies of rodent ecology in urban ecosystem, development of strategic programs for its control and prevention and zoonotic diseases associated with urban rodents
References
- Administración Nacional de Medicamentos, Alimentos y Tecnología Médica (ANMAT). Argentina: Ministerio de Salud. Presidencia de la Nación. Retrieved July 26, 2017 http://www.anmat.gov.ar/listados/Listado_Insecticidas.asp
- Bajomi, D., & Kis-Varga, A. (1990). A new modern anticoagulant rodenticide, Lanirat-B. Parasitology Hung, 23, 129–145.
- Buckle, A. P., Prescott, C. V., & Ward, K. J. (1994, March). Resistance to the first and second generation anticoagulant rodenticides: A new perspective. In W. S. Halverson & A. C. Crabb (Eds.). Proceedings of the 16th vertebrate pest conference (Vol. 16, pp. 138). Vertebrate pest conference. California, USA: University of California.
- Cavia, R., Cueto, G., & Suárez, O. V. (2009). Introduced and native rodents in an urban land scape. Landscape and Urban Planning, 90, 11–19. doi:10.1016/j.landurbplan.2008.10.017
- Cavia, R., Muschetto, E., Cueto, G. R., & Suárez, O. V. (2015). Commensal rodents in the city of Buenos Aires: A temporal, spatial, and environmental analysis at the whole city level. Ecohealth, 12, 468–479. doi:10.1007/s10393-015-1013-8.
- Cueto, G. R., Cavia, R., Bellomo, C., Padula, P., & Suárez, O. V. (2008). Prevalence of infection with Hantavirus in wild Rattus norvegicus and R. Rattus populations of Buenos Aires City, Argentina. Tropical Medicine and International Health, 13, 46–51. doi:10.1111/j.1365-3156.2007.01968.x
- Easterbrook, J. D., Kaplan, J. B., Vanasco, N. V., Reeves, W. R., Purcell, R. H., Kosoy, M. Y., … Klein, S. L. (2007). A survey of zoonotic pathogens carried by Norway rats in Baltimore, Maryland, USA. Epidemiology Infection, 135, 1192–1199. doi:10.1017/S0950268806007746.
- Easterbrook, J. D., Shields, T., Klein, S. L., & Glass, G. E. (2005). Norway rat population in Baltimore, Maryland, 2004. Vector Borne and Zoonotic Diseases, 5, 296–299. doi:10.1089/vbz.2005.5.227
- Fernández, M. S., Cavia, R., Cueto, G. R., & Suárez, O. V. (2007). Implementation and evaluation of an integrated program for rodent control in a shantytown of Buenos Aires City, Argentina. Ecohealth, 4, 271–277. doi:10.1007/s10393-007-0122-4
- Giannoni, S. M., Mera Sierra, R., Brengio, S., & Jiménez Baigorria, L. 2003. Guía para el uso de animales en investigaciones de campo y en cautiverio. Comisión de Ética de la Sociedad Argentina para el Estudio de los Mamíferos. Retrieved 18 July 2017 from http://www.sarem.org.ar/legislacion/
- Greaves, J. H., Shepherd, D. S., & Quy, R. (1982). Field trials of second-generation anticoagulants against difenacoum-resistant Norway rat populations. Journal Hygiene, 89, 295–301. doi:10.1017/S0022172400070820
- Hadler, M. R., & Buckle, A. P. (1992, March). Forty-five years of anticoagulant rodenticides past, present and future trend. In J. E. Borrecco & R. E. Marsh (Eds.). Proceedings of the15th Vertebrate Pest Conf (pp. 149). Vertebrate pest conference. California, USA: University of California.
- Hancke, D., Navone, G. T., & Suárez, O. V. (2011). Endoparasite community of Rattus norvegicus captured in a shantytown of Buenos Aires City, Argentina. Helminthologia, 48, 167–173. doi:10.2478/s11687-011-0025-3.
- Himsworth, C., Parsons, K. L., Claire, J., & Patrick, D. M. (2013). Rats, cities, people, and pathogens: A systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne and Zoonotic Diseases, 13, 349–359. doi:10.1089/vbz.2012.0964
- Jackson, W. B., & Ashton, A. D. (1992, March). A review of available anticoagulants and their use in the United States. In J. E. Borrecco & R. E. Marsh (Eds.). Proceedings of the15th vertebrate pest conf (pp. 156). Vertebrate pest conference. California, USA: University of California.
- Jackson, W. B., & Kaukeinen, D. E. (1972). Resistance of wild Norway rats in North Carolina to warfarin rodenticide. Science, 176, 1343–1344. doi:10.1126/science.176.4038.967
- Littin, K. E., O’Connor, C. E., & Eason, C. T. (2000). Comparative effects of brodifacoum on rats and possums. New Zealand Plant Protection, 53, 310–315.
- Mason, G., & Littin, K. E. (2003). The humaneness of rodent pest control. Animal Welfare, 12, 1–37.
- Meerburg, B. G., Brom, F. W., & Kijlstra, A. (2008). Perspective the ethics of rodent control. Pest Management Science, 64:, 1205–1211. doi:10.1002/ps.1623
- Murphy, M. J. (2002). Rodenticides. Veterinary Clinics of North America: Small Animal Practice, 32, 469–484. doi:10.1016/S0195-5616(01)00003-1
- Pelz, H. J., Rost, S., Hünerberg, M., Fregin, A., Heiberg, A., Baert, K., … Müller, C. R. (2005). The genetic basis of resistance to anticoagulants in rodents. Genetics, 170, 1839–1847. doi:10.1534/genetics.104.040360
- Prakash, S., Kumar, S., Veer, V., Gopalan, N., Purnanand, Pandey, K. S., & Rao, K. M. (2003). Laboratory evaluation of four rodenticides admixed in a cereal-based bait against commensal rat, Rattus rattus (L.) (Rodentia: Muridae: Murinae). Journal of Stored Products Research, 39, 141–147. doi:10.1016/S0022-474X(01)00026-1
- Prescott, C. V., & Johnson, R. A. (1996). The laboratory evaluation of rodenticides. In A. P. Buckle & R. H. Smith (Eds.), Rodent pests and their control (pp. 155–170). Wallingford: CABI.
- Rady, G. H. H., Hegab, A. M. I., Abdelnabby, H. M. E., & El-Bakhshwangi, M. I. A. (2012). Increasing efficacy of zinc phosphide rodenticide baits using the antispasmodic agent, otilonium bromide. Journal of the Egyptian Society of Toxicology, 45, 1–7.
- Schneider, B. A., & Hitch, R. K. (1982). Pesticide assessment guidelines: Subdivision G, product performance [MICROFICHE]. US Environmental Protection Agency, Office of Pesticide and Toxic Substances, Washington, D.C. USA.
- Singleton, G. R., Leirs, H., Hinds, L. A., & Zhang, Z. (1999). Ecologically-based management of rodent pests re-evaluating our approach to an old problem. In G. R. Singleton, L. A. Hinds, H. Leirs, & Z. Zhang (Eds.), Ecologically-based management of rodent pests (pp. 17–29). Canberra: Australian Centre for International Agricultural Research (ACIAR).
- Traweger, D., Travnitzky, R., Moser, C., Walzer, C., & Bernatzky, G. (2006). Habitat preferences and distribution of the brown rat (Rattus norvegicus Berk.) in the city of Salzburg (Austria): Implications for an urban rat management. Journal of Pest Sciences, 79, 113–125. doi:10.1007/s10340-006-0123-z
- Vadell, M. V., Cavia, R., & Suárez, O. V. (2010). Abundance, age structure and reproductive patterns of Rattus norvegicus and Mus musculus in two areas of the city of Buenos Aires (Argentina). International Journal of Pest Management, 56, 326–337. doi:10.1080/09670874.2010.499479
- Walsh, M. G. (2014). Rat sightings in New York City are associated with neighborhood socio demographics, housing characteristics, and proximity to open public space. Peer Journal, 2014(2), e533. doi:10.7717/peerj.533
- Witmer, G., Eisemann, J. D., & Howald, G. (2007). The use of rodenticides for conservation efforts. USDA National Wildlife Research Center-Staff Publications. Retrieved June 6, 2017 from http://digitalcommons.unl.edu/icwdm_usdanwrc/780
- Witmer, G., Horak, K., Moulton, R., & Baldwin, R. 2013. New rodenticides: An update on recent research trials. In: Wildlife damage management conferences proceedings Retrieved 2017, June 6 from http://digitalcommons.unl.edu/icwdm_wdmconfproc/164
- Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A., & Smith, G. M. (2009). Mixed effects models and extensions in ecology with R. New York: Springer.