Abstract
Plant growth regulators are synthetic chemical substances that are directly applied to crops to alter some structural processes. It is expected that these alterations modify hormonal balance and growth leading to increased yield, enhanced crop tolerance against abiotic stress and improved physiological trait of crops. Paclobutrazol is a member of the triazole family of plant growth regulators and has been found to protect several crops from various environmental stresses, including drought, chilling and heat radiation. Paclobutrazol impedes gibberellin biosynthesis through blocking ent-kaurene synthesis in the metabolic pathway of gibberellin production, resulting in reduced amounts of active gibberellins and consequent reduction in stem elongation. In this article, the current knowledge and possible applications of paclobutrazol, which can be used to improve the growth and yield of crops, have been reviewed and discussed. The role of paclobutrazol to mitigate the harmful effects of drought stresses in crops is also examined. Moreover, various biochemical and physiological processes leading to improved crop production under the effect of paclobutrazol are discoursed in detail.
PUBLIC INTEREST STATEMENT
Paclobutrazol is a synthetic chemical product that arrests vegetative growth. Also paclobutrazol has a role in improving drought tolerance of crops by enhancing physiological response (increased proline content and antioxidant enzyme activity). However, until now its mode of action has remained largely unknown. Due to lack of in-depth understanding about paclobutrazol’s mode of operation under drought stress limits its application in crop plants. Moreover, paclobutrazol application has found to increased grain of many crops. The increased grain yield accredited partly from decreased investment in above ground parts, due to a relatively stouter canopy of paclobutrazol treated plants, as well as enhanced grain filling in the treated plants due to the improved rooting system, which possibly increased the nutrients and water uptake. However, the mechanism of paclobutrazol effect on chlorophyll content, stress tolerance and soil residue effect remain unclear and needs further instigation.
Competing Interests
The author declares no competing interests.
1. Introduction
Plant growth regulators are widely used in contemporary agriculture to promote plant growth, yield and grain quality. Both beneficial and adverse effects of plant growth regulators on growth and development as well as plant metabolism have been documented (Ashraf, Akram, Al Qurainy, & Foolad, Citation2011).
The term growth retardants is used for all chemicals that retard cell division and cell elongation in shoot tissues and regulate plant height physiologically without formative effects (PGRSA, Citation2007). Paclobutrazol is a member of the triazole family of plant growth regulators and has been found to protect several crops from various environmental stresses, including drought, chilling, heat and UV radiation (Orabi, Salman, & Shalaby, Citation2010).
Paclobutrazol (PBZ) is a triazole derivative that inhibits sterol and gibberellin biosynthesis (Khan, Wagatsuma, Akhter, & Tawaraya, Citation2009). This compound can markedly affect plant growth and development by altering the photosynthetic rate and modifying the phytohormone levels (Kim, Wilson, Case, & Binder, Citation2012). Paclobutrazol inhibits the activity of ent-kaurene oxidase, which is an enzyme in the GA biosynthetic pathway that catalyzes the oxidation of ent-kaurene to ent-kaurenoic acid (Kondhare, Hedden, Kettlewell, Farrell, & Monaghan, Citation2014). PBZ application has reduced plant height, improved stem diameter and leaf number, altered root architecture (Pal et al., 2016) directly contributed to yield increase, and indirectly reduced the event of lodging (Syahputra, Sinniah, Ismail, & Swamy, Citation2016). It was also reported that application of paclobutrazol effectively reduced vegetative growth of rice plants and increased chlorophyll content. Rice seedlings treated with paclobutrazol allocated less photosynthates for vegetative growth; allocated more photosynthates for seed development compared to control plants or those plants treated with gibberellin (Dewi, Agustina, & Nurmalika, Citation2016). In corn (Zea mays L.) under drought stress, application of 50 ppm paclobutrazol increased yield and average weight of 1,000 seeds (Bayatand Sepehri, Citation2012).
Moreover, the possible hypotheses on drought tolerance regulation by PBZ have been proposed, which state that it maintains the endogenous cytokinin levels and stabilizes leaf water potential and causing increased leaf and epidermal thickness (Sankar, Karthishwaran, & Somasundaram, Citation2013). Alternatively, regulation of free proline and glycine betaine as major osmoprotectants (Hajihashemi & Ehsanpour, Citation2014) and promotion of enzymatic and non-enzymatic antioxidant activities, reduce the toxicity derived from drought stress (Hajihashemi & Ehsanpour, Citation2014; Jungklang, Saengnil, & Uthaibutra, Citation2016).In a view of this, the objective of this article is to review the effect of paclobutrazol on morphological, biochemical, yield and stress responses of crop.
2. Paclobutrazol induced responses in plants
2.1. Morphological response
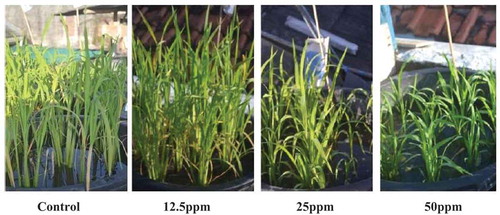
Paclobutrazol is used in high input crop management to shorten the stem, thereby reducing the risk of lodging. There are several reports describing the various effects of paclobutrazol on plant morphology of crops. For example, Sumit et al. (Citation2012) reported PBZ application significantly decreased plant height of Camelina sativa when compared to control and induced dwarfing effect and with highest concentration of PBZ in which maximum reduction (47.5% decrease) in plant height with respect to control was obtained. Similarly, paclobutrazol concentrations of 200 mg/L to 600 mg/L decreased gibberellin content in the leaves compared to that of control when applied to rice plant during preanthesis (Syahputra, Sinniah, Syed Rastan, & Ismail, Citation2013). Paclobutrazol application reduced plant height and the greater concentration of paclobutrazol caused severe dwarfism as indicated in Figure . Reduction in plant height is considered as the most imperative morphological outcome of paclobutrazol application. According to Tesfahun and Menzir (Citation2018), plant height reduction strongly associated with reduced elongation of the internodes, rather than lowering the number of internodes and they found uppermost internodes to be shortened under paclobutrazol application. Correspondingly, Koutroubas et al. (Citation2015) reported that foliar application of paclobutrazol at 12.5 g a.i ha−1, under a single-application scheme reduced plant height of sunflowers without adverse effects on achene and oil yields, thus providing a basis for reducing the risk of plant lodging.
Figure 1. Growth of black rice plant treated with paclobutrazol (PBZ) at 6 weeks after planting.
Source; Dewi et al. (Citation2016)
2.2. Yield response
The positive effects of paclobutrazol on yield components such as greater fertile tillers, spike, fertile panicle or spikelet and in some cases mean grain weight has been shown in studies evaluating the production potential of cereals; however, numerous studies have revealed that the increased fertile tiller, altered phenology and better canopy have been the main important components that significantly associated with enhanced grain yield in response to paclobutrazol application (Tesfahun & Menzir, Citation2018).
Sumit et al. (Citation2012) reported that Camilina seed yield increased by 3.91%, 8.53%, 16.58% and 74.23%, respectively when compared to control with application of 25 mg L−1, 50 mg L−1, 75 g L−1 and 100 g L−1. The 2-year work of Kamran et al. (Citation2018) showed that average maize grain yields under seed soaking 200 mg L−1, 300 mg L−1, and 400 mg L−1 treatments were increased by 18.9%, 61.3% and 45.9%, respectively compared with control, whereas seed dressing of PBZ at rate of 1.5 g kg−1, 2.5 g kg−1 and 3.5 g kg−1 increased maize yield by 20.2%, 33.3% and 45.2%, respectively compared with control as indicated in Table .
Table 1. Effects of paclobutrazol on grain yield (kg ha−1) of maize in 2015–2016
One of the possible increments in grain yield is (i) the change in canopy coverage, in which the plant developed broader canopy this in turn facilitated improved light interception for better photosynthesis in leaves and stems of PBZ treated plants. Further, (ii) the leaves in PBZ treated plants were closely packed, dark green and remained on plants for a larger period than controls. This may explain increased dry matter accumulation in stem and root and simultaneous yield increments despite reduced plant height due to PBZ treatments. Pan et al. (Citation2013) linked the grain yield increment (iii) with slow senescence in leaves which prolong the phase of seed development and maturation and as a consequence, the yield can be increased, but the harvest time delayed. The other possible grain yield increment is closely related to (iv) the spread of roots, which determines the uptake and utilization of water and nutrients (Qi et al., Citation2012). In similar way, Zhang, Chen, Sun, Wang, and Shao (Citation2009) reported that greater root biomass is significantly and positively correlated with ear characteristics and enhanced biomass and grain yields. The increased in the grain yield is attributed partly to (v) decreased investment in above ground parts, due to a relatively stouter canopy of paclobutrazol treated plants, (vi) as well as enhanced grain filling in the treated plants due to the improved rooting system, which possibly increased the nutrients and water uptake (Kamran et al., Citation2018).
2.3. Physiological response
Chlorophyllis a critical component of the primary photosynthetic reaction has a dual function in photosynthesis. It captures light, and also serves as a medium for the light-driven charge separation and transport of electrons (Zhao et al., Citation2011). The biosynthesis of chloroplast pigments was significantly affected by paclobutrazol as indicated in Table . Several studies on tef (Tsegaw, Citation2007) and camilena (Sumit et al., Citation2012) showed that chlorophyll was higher on plants treated with paclobutrazol compared to control. The increased chlorophyll content treated with paclobutrazol might be from minimized damage caused by reactive oxygen and changes in the levels of carotenoids, ascorbate and the ascorbate peroxidase. The report of Nivedithadevi, Somasundaram and Pannerselvam (Citation2015) also showed that plants treated with paclobutrazol synthesized more cytokinin, which in turn enhanced chloroplast differentiation and chlorophyll biosynthesis, and prevented chlorophyll degradation. Furthermore, paclobutrazol appears to have delayed the onset of senescence, represented by the rate of chlorophyll degradation in attached mung bean leaves, which was probably due to the enhanced endogenous level of cytokinins through their secondary effect on plants (Fletcher, Gilley, & Sankhla, Citation2000). Paclobutrazol application in Camelina sativa L. Crantz also increased chlorophyll content which led to greater rate in photosynthesis and higher yield (Sumit et al., Citation2012). The results of Dewi et al. (Citation2016) showed that black rice plants treated with either 25 or 50 ppm paclobutrazol have greener leaves compared to control and the leaves also experienced late senescence. This could be due to an increase in the activity of oxidative enzymes that prevented cell maturation.
Table 2. Effects of paclobutrazol on leaf pigment content of Jatropha
2.4. Stress response
Since early migration from aquatic to terrestrial environments, plants have had to cope with periodic and unpredictable environmental stresses, such as drought and salinity. Crop production in arid or semi-arid regions is usually restricted by soil moisture deficit as well as soil salinity. Water deficit coupled with salinity in irrigation water is the major limiting factor in most regions where cereals are subjected to extreme water deficit during dry seasons. Enhanced stress tolerance in cereals can be achieved by exogenous application of some plant growth regulators, including paclobutrazol. Exogenous application of paclobutrazol can reduce some of the harmful effects of drought and salt stress and in some cases, compensate losses or damages caused by these stresses (Ashraf et al., Citation2011). Paclobutrazol increased stress tolerance of plants through the following methods.
2.4.1. Increasing root activity
Paclobutrazol are often referred as multi-stress protectants due to their innate potential of mitigating the negative effects of abiotic stresses had on plant growth and development, by regulating hormones level, enzymatic and non-enzymatic antioxidants and osmolytes (Kamran et al., Citation2018; Kuai et al., Citation2016). The 2-year results of Kamran et al. (Citation2018) showed that root activity and root-bleeding sap flow were significantly higher in paclobutrazol treatments than compared to control. As root-bleeding sap is the indicator of root pressure, therefore, the improved root-bleeding sap is attributed to higher root growth and root vigor in response to the paclobutrazol application. Also the study of Morita, Okamoto, Abe and Yamagishi (Citation2008) showed the presence of a close relationship between the bleeding rate and the root traits in maize. The rate of root bleeding sap is correlated to active water absorption of the root system and reflects the physiological root activity. Yan et al. (Citation2013) also observed that uniconazole, a triazole with a function similar to paclobutrazol promoted root activity, root bleeding sap and improved root growth in soybean. Previously, Zhao, Fang and Gao (Citation2006) also observed a higher root activity in rice and wheat treated with plant growth regulators. Thus the application of paclobutrazol may improve plant performance under stressful condition through stimulating root activity of the plant.
2.4.2. Submergence tolerance
Also paclobutrazol has a role on submergence stress. The long-time submergence is also detrimental to rice crop, and where this cannot be avoided some corrective measures are to be taken to exploit yield potential of rice crop. Under submerged conditions, 200 ppm paclobutrazol spray to rice seedlings resulted in 50% increase in percent survival over control. The increased seedling survival is presumably due to low energy use in elongation, while, the same was available for maintenance processes, for synthesis of anaerobic proteins and maintenance of membrane integrity essential for submergence tolerance (Chon, Koseki, Hirata, Saka, & Abe, Citation2000).
2.4.3. Increasing antioxidant enzyme
Increased the levels of antioxidant enzyme activities in plants under stress conditions are natural responses, which can help plants better tolerate the stress. Exogenous application of paclobutrazol enlarged these traits and enhanced stress tolerance in plants. Additionally, the enhanced antioxidant enzyme activities in response to paclobutrazol application may also protect their photosynthetic machineries against damages caused by Reactive oxygen species during water-deficit conditions (Jarunee et al., Citation2017).
Among these SOD and CAT are well-known antioxidative enzymes in cells, which can catalyze the poorly reactive oxygen species converting them to non-toxic substances. SOD constitutes the first line of defence against active oxygen species (AOS). This enzyme removes O2− by catalyzing its dismutation, wherein one O2_Mis reduced to hydrogenperoxide (H2O2) and another is oxidized to oxygen (Halliwell, Citation2006). CAT is an enzyme that can convert H2O2 directly into water and oxygen. This enzyme is present in every cell and in particular on peroxisome. SOD and CAT plays a significant role in defending against oxidative stress induced by abiotic stress in plant tissues (Finaud, Lac, & Filaire, Citation2006). Similarly, Rady and Gaballah (Citation2012) also found that the application of paclobutrazol on barley crop had a significant role in increasing CAT and SOD concentration (Table ). This compound reduced damage in plants grown under water stress conditions by enhancing the activity of these antioxidative enzymes. A number of studies showed that paclobutrazol minimizes the adverse effects of water-deficit stress by increasing the levels of the activities of antioxidative enzymes in many plants such as groundnuts, sesame seeds, mangos and tomatoes (Manivannan et al., Citation2008; Mohamed, Agamy, & Rady, Citation2011; Percival & Salim AlBalushi, Citation2007; Sankar et al., Citation2007; Somasundaram, Abdul Jaleel, Abraham, Azooz, & Panneerselvam, Citation2009; Srivastav, Kishor, Dahuja, & Sharma, Citation2010).
Table 3. Effect of paclobutrazol and water stress on superoxide dismutase (SOD) activity and catalase (CAT) activity of 10-week-old barley plants
2.4.4. Proline content
Proline is well-known as an osmotic regulator that can reduce osmotic damage (Reddy et al., Citation2015). It was reported that under non-water-stressed condition paclobutrazol does not have any significant effect; however, under water stress conditions, paclobutrazol (40 mg l−1) treatment resulted in a significant increase in proline content of barley plant as indicated in Table (Rady & Gaballah, Citation2012). Recent studies showed that paclobutrazol has effect in increasing free proline content of crops to protect from drought stress. However, the effect of paclobutrazol on proline content is still unclear. Supporting this idea Mohamed et al. (Citation2011) reported that free proline content in 50 mg L−1 paclobutrazol-treated tomato plants grown under 60% field capacity peaked at 54.56 mg g−1, which is 1.52-fold compared to control. In contrast, free proline content in 10 mgL−1paclobutrazol pretreated peanut under water deficit conditions (1.04-folds over control) was lower than non-treated plants (1.49-folds over control) (Sankar, Gopinathan, Karthishwaran, & Somasundaram, Citation2014).The accumulation of proline in leaves could possibly play a protection role apart from osmoregulation during drought stress. In sight of this sense we understand that paclobutrazol might act as a stress ameliorating agent crops, as this plant does not need to accumulate the proline content in the leaves. Previous studies have proved that proline accumulation was lower in tolerant plants when compared to sensitive plants during periods of drought stress (Jungklang, Usui, & Matsumoto, Citation2003). However, further study is needed in order to reach conclusive agreement on the effect of paclobutrazol on free proline content of crop leaves.
3. Future line of work
The first use of plant growth retardants such as paclobutrazol was for reduction in plant height to prevent stem lodging. However, nowadays, the effect of paclobutrazol on stem length reduction seems to be less important, due to the release of dwarf and semi dwarf cultivars. Irrespective of reduced lodging, the practice of paclobutrazol in crops with the aim of chemical regulation of growth and development to achieve higher grain yield needs advance research. It seems that the importance of paclobutrazol will be greater under stressful conditions, which draws the attention of researchers to paclobutrazol induced stress tolerance. Several studies only focused on physiological, morphological and biochemical response of crops to paclobutrazol. However, there is limited study in soil residual activity for succeeding crops. Thus, area needs further investigation because paclobutrazol is relatively immobile in soil and bound mainly by organic matter. A number of studies documented that chlorophyll content of crops increased due to paclobutrazol application but still there is doubt regarding to the mechanism of paclobutrazol effect on chlorophyll content. The effects of paclobutrazol in free proline content under water stress condition is still unclear, few study showed the proline content is raised while other literature showed the level of proline is decreased due to the role of paclobutrazol acting as a stress-ameliorating agent in plant, as the plant does not need to accumulate the proline content in the leaves. Therefore, the aforementioned gap shows still further study is need to increase our understanding about the effect of paclobutrazol on the mechanism of plant physiology, stress condition and soil residual activity for succeeding crops.
Additional information
Funding
Notes on contributors
Wakjira Tesfahun
Wakjira Tesfahun is a lecturer in the department of plant science at Raya University. Presently, he is teaching plant science courses and involved in research and community activities. His research interests mainly focus in physiology, agronomy, irrigation and crop protection.
References
- Ashraf, M., Akram, N. A., Al Qurainy, F., & Foolad, M. R. (2011). Drought tolerance: Roles of organic osmolytes, growth regulators, and mineral nutrients. Advances in Agronomy, 111, 249–296.
- Bayat, S., & Sepehri, A. (2012). Paclobutrazol and salicylic acid application ameliorates the negative effect of water stress on growth and yield of maize plants. Journal Researcher Agricultural Sciences, 8(2), 127–139.
- Chon, N. M., Koseki, N. N., Hirata, Y., Saka, H., & Abe, H. (2000). Effects of brassinolide on coleoptile and leaf growth in rice seedlings. Plant Production Science, 3(4), 360–365. doi:10.1626/pps.3.360
- Dewi, K., Agustina, R. Z., & Nurmalika, F. (2016). Effects of blue light and paclobutrazol on seed germination, vegetative growth and yield of black rice (Oryza Sativa L. ‘CempoIreng’). Biotropia, 23(2), 85–96. doi:10.11598/btb.2016.23.2.478
- Finaud, J., Lac, G., & Filaire, E. (2006). Oxidative stress: Relationship with exercise training. Sports Med, 36, 327–359. doi:10.2165/00007256-200636040-00004
- Fletcher, F. A., Gilley, A., & Sankhla, N. (2000). Triazoles as plant growth regulator and stress protectants. Horticultural Reviews, 24, 55–138.
- Ghosh, A., Chikara, J., Chaudhary, D. R., Prakash, A. R., Boricha, G., & Zala, A. (2010). Paclobutrazol arrests vegetative growth and unveils unexpressed yield potential of jatrophacurcas. Journal of Plant Growth Regulation. doi:10.1007/s00344-010-9137-0
- Hajihashemi, S., & Ehsanpour, A. A. (2014). Antioxidant response of Steviarebaudiana B. to polyethylene glycol and paclobutrazol treatments underin vitro culture. Applied Biochemistry and Biotechnology, 172, 4038–4052. doi:10.1007/s12010-014-0791-8
- Halliwell, B. (2006). Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology, 141, 312–322. doi:10.1104/pp.106.077693
- Jungklang, J., Saengnil, K., & Uthaibutra, J. (2016). Effects of water-deficit stressand paclobutrazol on growth, relative water content, electrolyte leakage, prolinecontent and some antioxidant changes in Curcuma alismatifoliaGagnep.cv.Chiang Mai Pink. SaudiJ Biologic Sciences, 24(7), 1505–1512.
- Jungklang, J, Saengnil, K, & Uthaibutra, J. (2017). Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in curcuma alismatifolia gagnep. cv. chiang mai pink. Saudi Journal Of Biological Sciences, 24(7), 1505–1512. doi: 10.1016/j.sjbs.2015.09.017
- Jungklang, J., Usui, K., & Matsumoto, H. (2003). Differences inphysiological responses to NaCl between salt-tolerant SesbaniarostrataBrem.and Oberm and non-tolerant Phaseolus vulgaris L. Weed Biologic Manage, 3, 21–27. doi:10.1046/j.1445-6664.2003.00077.x
- Kamran, M., et al. (2018). Effect of paclobutrazol, a potential growth regulator on stalk mechanical strength, lignin accumulation and its relation with lodging resistance of maize. Plant Growth Regulation, 84, 317–332. doi:10.1007/s10725-017-0342-8
- Kamran, M., Wennan, S., Ahmad, I., Xiangping, M., Wenwen, C., Xudong, Z., … Tiening, L. (2018). Application of paclobutrazol affect maize grain yield by regulating root morphological and physiological characteristics under a semi-arid region. Scientific Reports. doi:10.1038/s41598-018-23166-z
- Khan, M. S. H., Wagatsuma, T., Akhter, A., & Tawaraya, K. (2009). Sterol biosynthesis inhibition by paclobutrazol induces greater aluminum (Al) sensitivity in Al-tolerant rice. Amer Journal Plant Physiological, 4, 89–99. doi:10.3923/ajpp.2009.89.99
- Kim, J., Wilson, R. L., Case, J. B., & Binder, B. M. (2012). A comparative study of ethylene growth response kinetics in eudicots and monocots reveals a role for gibberellin in growth inhibition and recovery. Plant Physiology, 160, 1567–1580. doi:10.1104/pp.112.205799
- Kondhare, K. R., Hedden, P., Kettlewell, P. S., Farrell, A. D., & Monaghan, J. M. (2014). Use of the hormone-biosynthesisinhibitors fluridone and paclobutrazol to determine the effects of altered abscisic acid and gibberellin levelson pre-maturity amylase formation in wheat grains. Journal of Cereal Science, 60, 210–216. doi:10.1016/j.jcs.2014.03.001
- Kuai, J., Yingying S., Min Z., Peipei Z., Qingsong Z., Jiangsheng W., Guangsheng Z. (2016). The effect of nitrogen application and planting density on the radiation use efficiency and the stem lignin metabolism in rapeseed (Brassica napusL.). F Crop Researcher, 199, 89–98. doi:10.1016/j.fcr.2016.09.025
- Manivannan, P., AbdulJaleel, C., Kishorekumar, A., Sankar, B., Somasundaram, R., & Panneerselvam, R. (2008). Protection of Vignaunguiculata (L.)Walp. Plants form salt stress by paclobutrazol. Colloid Surf, 61, 315–318. doi:10.1016/j.colsurfb.2007.09.007
- Mohamed, G. F., Agamy, R. A., & Rady, M. M. (2011). Ameliorative effects of some antioxidants on water-stressed tomato (Lycopersicon esculentum Mill.) plants. Journal Applications Sciences Researcher, 7, 2470.
- Morita, S., Okamoto, M., Abe, J., & Yamagishi, J. (2008). Bleeding rate of field-grown maize with reference to root system development. Japanese Journal Crop Sciences, 69, 80–85. doi:10.1626/jcs.69.80
- Nivedithadevi, D., Somasundaram, R., & Pannerselvam, R. (2015). Effect of abscisic acid, paclobutrazol and salicylic acid on the growth and pigment variation in solanum trilobatum (l). International Journal Drug Developments Researcher, 4, 236–246.
- Orabi, S. A., Salman, S. R., & Shalaby, M. A. F. (2010). Increasing resistance to oxidative damage in cucumber (CucumissativusL.) plants by exogenous application of salicylic acid and paclobutrazol. World Journal Agricultural Sciences, 6(3), 252–259.
- Pan, S., Rasul, F., Li, W., Tian, H., Mo, Z., Duan, M., & Tang, X. (2013). Roles of plant growth regulators on yield, grainqualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice, 6, 9. doi:10.1186/1939-8433-6-9
- Percival, G. C., & Salim AlBalushi, A. M. (2007). Paclobutrazol-induced drought tolerance in containerized English and evergreen oak. Arboriculture Urban Forest, 33, 397–409.
- PGRSA. (2007). Plant growth regulation handbook of the Plant Growth Regulation Society of America (4th ed.). Athens: The Plant Growth Regulation Society of America.
- Qi, W. Z., Hui. H. L., Peng. L., Shu. T. D., Bing. Q. Z., Hwat. B. S., Geng L., Heng D.L., Ji. W. Z. and Bin Z. . (2012). Morphological and physiological characteristics of corn (Zea mays L.) roots from cultivars with different yield potentials. European Journal Agronomic, 38, 54–63. doi:10.1016/j.eja.2011.12.003
- Rady, M. M., & Gaballah, M. S. (2012). Improving barley yield grown under water stress conditions. Research Journal of Recent Sciences, 1(6), 1–6.
- Reddy Surender, P., Jogeswar, G., Rasineni, G.K., Varshney, R.K., #&. (2015). Prolineover-accumulation alleviates salt stress and protects photosyntheticand antioxidant enzyme activities in transgenic sorghum (sorghumbicolor (l.). Moench. Plant Physiol. Biochem., 94, 104–113.
- Sankar, B., Abdul Jaleel, C., Manivannan, P., Kishorekumar, A., Somasundaram, R., & Panneerselvam, R. (2007). Effect of paclobutrazol on water stress amelioration through antioxidants and free radical scavenging enzymes in Arachishypogaea L. Colloid Surf, B 60, 229–235. doi:10.1016/j.colsurfb.2007.06.016
- Sankar, B., Gopinathan, P., Karthishwaran, K., & Somasundaram, R. (2014). Biochemical content variation in Arachishypogaea under drought stress withor without paclobutrazol and abscisic acid. Journal Ecobiotechnol, 6, 9–14.
- Sankar, B., Karthishwaran, K., & Somasundaram, R. (2013). Leaf anatomychanges in peanut plants in relation to drought stress with or withoutpaclobutrazol and abscisic acid. Journal Phytol, 5, 25–29.
- Somasundaram, R., Abdul Jaleel, C., Abraham, S. S., Azooz, M. M., & Panneerselvam, R. (2009). Role of paclobutrazol and ABA in drought stress amelioration in Sesamumindicum L. Global Journal MolSci, 4, 56–62.
- Srivastav, M., Kishor, A., Dahuja, A., & Sharma, R. R. (2010). Effect of paclobutrazol and salinity on leakage, proline content and activities of antioxidant enzyme in mango (Mangiferaindica L.). Sciences Horticultural, 125, 785–788. doi:10.1016/j.scienta.2010.05.023
- Sumit, K., Ghatty, S., Satyanarayana, J., Guha, A., Chaitanya, B. S. K., & Reddy, A. R. (2012). Paclobutrazol treatment as a potential strategy for higher seed and oil yield in field-grown camelina sativa L. Crantz. BMC Research Notes, 5, 137. doi:10.1186/1756-0500-5-137
- Syahputra, B. A. S., Sinniah, U. R., Syed Rastan, S. O., & Ismail, M. R. (2013). Changes in gibberellic acid (GA) content in 3 Oryza sativa due to paclobutrazol treatment. Journal Food Pharmaceutical Sciences, 1, 14–17.
- Syahputra, B. S. A., Sinniah, U. R., Ismail, M. R., & Swamy, M. K. (2016). Optimization of paclobutrazol concentration and application time for increased lodging resistance and yield in field-grown rice. Philippine Agricultural Sciences, 99, 221–228.
- Tesfahun, W., & Menzir, A. (2018). Effect of rates and time of paclobutrazol application on growth, lodging, yield and yield components of Tef [Eragrostis Tef (Zucc.)Trotter] in Ada district, East Shewa, Ethiopia. Journal of Biology, Agriculture and Healthcare, 8(3), 104–117.
- Tsegaw, T. (2007). Growth, photosynthetic efficiency, rate of transpiration, lodging, and grain yield of Tef (Eragrostis Tef (Zucc.)Trotter) as influenced by stage and rate of paclobutrazol application. East African Journal of Sciences, 1(1), 35–44.
- Yan, W., et al. (2013). Responses of root growth and nitrogen transfer metabolism to uniconazole, a growth retardant, during the seedling stage of soybean under relay strip intercropping system. Communicable Soil Sciences Plant Analysis, 44, 3267–3280. doi:10.1080/00103624.2013.840838
- Zhang, X., Chen, S., Sun, H., Wang, Y., & Shao, L. (2009). Root size, distribution and soil water depletion as affected by cultivars and environmental factors. F Crop Researcher, 114, 75–83. doi:10.1016/j.fcr.2009.07.006
- Zhao, J., Zhang, W., Qiu, Q., Li, Z. P., Zhang, M. H., Yan, X. Y., & Du, D. H. (2011). Effects of PP333 spraying at different stages on soybean agronomic and physiological characters. Soybean Sciences, 30, 211–214.
- Zhao, X. F., Fang, Z. G., & Gao, Z. M. (2006). Effects of paclobutrazol (PP333) on root vigor and IAA oxidase and peroxidase activities in leaf of rice and wheat seedlings. Guangxi Agricultural Sciences, 37, 379–381.
- 'KOUTROUBAS, S.D, & Damlas, C.A. (2015). Sunflower response to repeated foliar applications of paclobutrazol. Planta Daninha, 33(1), 129-135. doi:10.1590/S0100-83582015000100015
