?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In vitro cell viability tests are usually done using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) method. A new spectrofluorometry method was developed using acridine orange (AO) and propidium iodide (PI) using multi-label microplate reader. Nine biogenic amines (BAs) [histamine (HIM), putrescine (PUT), cadaverine (CAD), 2-phenylethylamine (PHM), tyramine (TYM), tryptamine (TPM), spermine (SPM), spermidine (SPD) and agmatine (AGM)] were exposed to RAW 264.7 macrophage in singles at 37°C with 5% carbon dioxide supplementation for 18–24 hours and cell viability was determined using MTS method and AO/PI developed method using dual-spectrofluorometry. Based on MTS assay, SPM and SPD were found to be cytotoxic and it was supported by AO/PI assay. The precedence of disintegration in the nucleus rather than mitochondria upon cell non-viability was also supported by transmission electron microscopy (TEM). The results showed that AO/PI method could be used as an alternative method to determine cytotoxicity besides usual usage in confocal microscopy.
PUBLIC INTEREST STATEMENT
Cell viability has been determined in various tests such as in studying the toxicity of a food compound to some cells grown in flasks in the laboratory. Acridine orange (AO) and propidium iodide (PI) have been used to color the live dan dead cells, respectively, in imaging the cells under the microscopes. In this study, we used AO and PI to determine the cell viability quantitatively by measuring the intensity of the fluorescence light they emit using the multi-label microplate reader. Thus, another application of their usage in measuring cell viability upon exposure to food-borne biogenic amines had been demonstrated in this article, with the support of evidence from the photographs of cells using the electron microscope.
Competing Interests
The authors declares no competing interests.
1. Introduction
In the study of cytotoxicity of food compounds on cell culture, the most common methods performed use the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) or 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagents. However, since both reagents produces final reaction colored product, the absorbance can only be read on one chromophore at a time using a microplate reader. Only %cell viability can be obtained from the reading. Mascotti, McCullough and Burger (Citation2000) had presented method comparison study on hematopoietic progenitor cell (HPC) viability measurement between trypan blue and acridine orange (AO)/propidium iodide (PI). Amount of trypan blue absorption by nonviable cells, amount of PI-binding to DNA or RNA in nonviable cells, and amount of AO binding to nucleic acids of viable cells were quantified through 100 cells scoring under compound light and fluorescence microscope observation.
Other than MTS assay, there is also need of in vitro methods which target the nucleic acids besides the mitochondria, since nucleic acids had been reported to be affected prior to mitochondria in apoptosis and necrosis upon cytotoxicity (Zamzami et al., Citation1996). Cytotoxicity studies are done to determine the safety threshold on compounds and are done in vitro in microtitre plates. In vitro cytotoxicity testing is becoming important in the drug discovery field due to its convenience and cost-effectiveness. In this study, a method to determine cell viability and cell non-viability using AO and PI were done on food-borne biogenic amines using RAW 264.7 macrophages. The main objective of this study was to compare correlation coefficients between cell viability measurement using AO assay and cell non-viability using PI assay to existing MTS cell viability assay.
Both AO and PI fluorophores which possessed different excitation and emission wavelengths were quantified simultaneously and method comparison and agreement with MTS assay was undertaken.
2. Materials and methods
2.1. Chemicals
Histamine dihydrochloride, putrescine dihydrochloride, cadaverine dihydrochloride, 2-phenylethylamine hydrochloride, tyramine hydrochloride, tryptamine hydrochloride, spermine trihydrochloride, spermidine tetrahydrochloride, agmatine sulphate, Escherichia coli 055: B5 lipopolysaccharide (LPS), interferon-gamma (IFN-γ) and L-NG-Nitroarginine methyl ester (L-NAME) used in the study were purchased from Sigma–Aldrich (St. Louis, MO, USA). Acridine orange/propidium iodide (AO/PI) stock solution was obtained from the Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia. All other chemicals were of analytical grade.
2.2. Cell culture
RAW 264.7 macrophages (ATCC®TIB-71TM; American Type Culture Collection, Manassas, VA, USA.) were grown in 30 ml of Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Life Technologies, NY, USA), added with 4 mM L-glutamine, 4.5 g/L glucose, 1.5 g/L sodium bicarbonate, 5.96 g/L HEPES [N-(2-hydroxyethyl)piperazine-N’-2-ethanesulfonic acid], 1 mM sodium pyruvate, 100 U/ml penicillin (Gibco, Life Technologies, NY, USA) and 100 µg/ml streptomycin (Gibco, Life Technologies, NY, USA) and supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Life Technologies, NY, USA) in 75 cm2 culture flasks. The cells were incubated at 37°C in humidified 5% carbon dioxide until 80% confluent. The cells were then dislodged from the flask inner surface using cell lifter (Corning, Sigma–Aldrich Co. LLC, St. Louis, Missouri, USA) and dispersed well. The cell suspension was centrifuged and the cell pellet was dispersed again in 1 ml growth medium. An aliquot of 10 µl cell suspension was mixed well with 10 µl dye solution, cells were stained using trypan blue dye exclusion method (Altman et al., Citation1993) and counted using an automated cell counter (Countess®, Life Technologies, NY, USA). A cell suspension of 1 × 104 cells/100 µl was prepared by adjusting the harvested cell suspension concentration with the addition of growth medium. The cell suspension was immediately pipetted into 96-well microtitre plates. These cells were then treated with various test compounds, and cell viability was determined using the MTS and AO/PI assays.
2.3. Developed method
2.3.1. Acridine orange/propidium iodide (AO/PI) assay
Aliquots (100 µl) of RAW 264.7 macrophages were seeded at 1 × 104 cells/well in a 96-well microtitre plate. The cells were then treated with the test compounds individually, namely, HIM, PUT, CAD, PHM, TYM, TPM, SPM, SPD or AGM at 1, 10 and 100 µg/ml and incubated in 5% humidified carbon dioxide at 37°C for 24 hr. An aliquot of 1 µl of 0.5 mg/ml acridine orange/propidium iodide (AO/PI) reagent was added to each well and incubated for 10 min at room temperature. Dual-fluorescence was measured using a multi-detection microplate reader (FLUOstar Omega, BMG LABTECH, Germany) with an excitation wavelength of 460 nm and an emission wavelength of 650 nm for AO and an excitation wavelength of 525 nm and an emission wavelength of 595 nm for PI. Assignment of blanks, control cells without treatment, positive control cells, and negative control cells were the same as in MTS assay. Cell viability determined using AO was calculated as a percentage of viable cells as follows:
Where, AOem = acridine orange emitted fluorescence
Cell non-viability determined using PI was calculated as a percentage of non-viable cells as follows:
Where, PIem = propidium iodide fluorescence emission
The experiment was carried out in triplicates on three independent batches of cells.
2.4. Method validation
2.4.1. Method comparison with MTS assay
RAW 264.7 macrophages in growth medium were seeded at 1 × 104 cells/100 µl/well in a sterile 96-well microtitre plate (TPP, Trasadingen, Switzerland). Cells were then incubated in 5% carbon dioxide at 37°C for 24 hr. The growth medium was discarded and replaced with 100 µl of fresh growth medium containing 1, 10 and 100 µg/ml of [histamine (HIM), putrescine (PUT), cadaverine (CAD), 2-phenylethylamine (PHM), agmatine (AGM), tyramine (TYM), tryptamine (TPM), spermine (SPM) or spermidine (SPD)] and further incubated for 18–24 hours. Positive control cells were obtained by treating RAW 264.7 macrophages with 10 µg/ml LPS and 100 U/ml IFN-γ using same treatment procedures as described for biogenic amines. Negative control cells were likewise treated with 250 µM L-NAME.
Untreated cells were assigned as controls and media without cells were assigned as blanks. The experiment was carried out in triplicates. At the end of incubation period, 20 µl of 3-,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent (CellTiter 96® AQueous One Solution Reagent; Promega, WI, USA) was added to each well, mixed and further incubated for 60 min. The absorbance (Abs) of each well was then measured at 492 nm using a microplate reader (Dynex, Model MRII; Dynex Technologies, VA, U.S.A.). The percentage of cell viability was calculated using the following formula:
The same procedures were performed on another six independent batches of RAW 264.7 macrophages i.e. the same procedures were repeated for another six times on six different batches of cells at a different day of cell culturing.
2.4.2. Cross-validation with transmission electron microscopy (TEM)
Approximately 1.6 × 105 cells/well of RAW 264.7 macrophages were placed in a six-well microtitre plate in a final volume of 1600 µl DMEM. The cells were then exposed to 1 and 100 µg/ml TYM, TPM, SPM or SPD and incubated in 5% humidified carbon dioxide at 37°C for 24 hr. Cells were dislodged using cell scraper (TPP®, Trasadingen, Switzerland) and cells were thoroughly dispersed in growth media using plastic Pasteur pipette. The cell suspension was centrifuged at 1,500 g for 10 min and growth media was discarded. The cell pellet was then fixed with 2% glutaraldehyde in 0.1 N phosphate buffered solution (Di Giorgio et al., Citation2011). The next experimental procedures were done at the Electron Microscopy Unit, Institute for Medical Research. The cells were washed with distilled water six times and fixing was done with 2% osmium tetra-oxide (Hyatt et al., Citation2000). The washing step was repeated, and the cells were dehydrated with 50%, 70%, and 90% acetone, for 5 min each. The cells were further dehydrated with 100% acetone three times. The cells were infiltrated with 100% acetone and epoxy-resin (1:1) before they were embedded in pure epoxy-resin (Agar Scientific Ltd., Essex, UK) for 10 min followed by incubation at 60°C overnight. Ultra-section of 90 nm was performed on the cells and was later viewed using a transmission electron microscope (Tecnai G2, Field Electron & Ion Co., Holland) at 2500× magnification.
2.5. Statistical analysis
The mean and standard error of means (SEMs) values in this study were calculated using Microsoft Excel (Microsoft Office Professional Plus 2010, Microsoft Corporation, Washington, USA). The comparison of means was determined by ANOVA with Bonferroni post-hoc using IBM SPSS Statistics version 22. These tests and inhibition concentration at 50% cell viability (IC50) determination were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California, USA; www.graphpad.com). p < 0.05 was accepted as statistically significant. Method comparison and method agreement between MTS and AO, and AO and PI assays were analyzed using Pearson’s correlation coefficient and Bland–Altman method (Bland & Altman, Citation1986).
3. Results and discussion
3.1. Method validation results
3.1.1. Method comparison with MTS assay
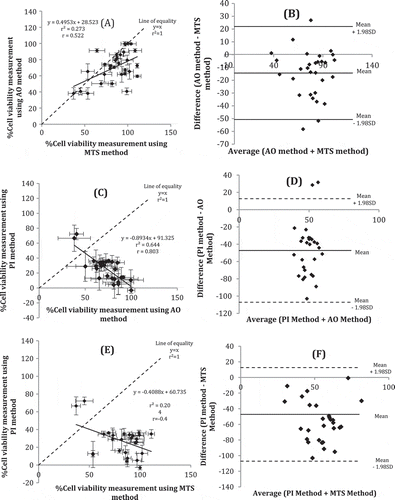
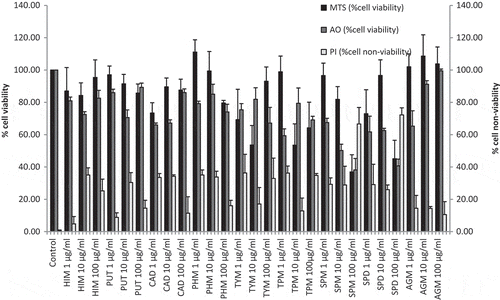
Percentage of cell viability (determined using MTS and AO methods) and percentage of cell non-viability (determined using PI method) results are as depicted in Figure . The results obtained from MTS and AO methods are not comparable even though both are quantifying percentage of cell viability. Both are significantly different (p = 0.011) and the difference from one another is observed since the target site for the former is mitochondria while the target site for the latter is nucleus. The results obtained using PI method is negatively correlated with the ones obtained from MTS and AO methods since PI method quantify the percentage of cell non-viability. If PI results is subtracted from 100% to derive percentage of cell viability, the results is not significantly different from results obtained using AO method (p = 1.000). This suggests that AO and PI complement each other. The same PI results are significantly different from MTS (p = 0.005).
Figure 1. Percentages of cell viability and cell non-viability (mean ± SEM) of RAW 264.7 macrophages upon exposure to biogenic amines (1, 10, 100 µg/ml) individually and assayed using MTS, acridine orange (AO) and propidium iodide (PI), respectively. The test was done on three to seven independent batches of cells.
Comparisons between AO versus MTS, PI versus AO and PI versus MTS showed that they showed moderate positive (r = 0.522), high negative (r = 0.803) and moderate negative (r = 0.452) correlations, respectively (Figure , and ). For the former comparison, the method agreement plot showed a mean ± 1.98SD was found to range between 20 and −50 µg/ml and only three data points were outside the range (Figure ). For the latter comparison, AO showed cell viability while PI showed non-viability, thus, their correlation slope (m) was −4.893. In method agreement plot, mean ± 1.98SD ranged from 12 to −105 µg/ml and only two data were above 20 µg/ml (Figure ). For comparison between MTS and PI method (Figure ) with negative slope (m) value of −0.409; the trend is the same as between AO and PI method. These trends depict that the higher the percentage of cell viability determined through MTS assay, the lower the percentage of cell non-viability determined through PI method; they are inversely correlated. Method agreement showed good agreement with all data are plotted within ± 1.98SD (Figure ). Determination of viable nucleic acids as fast as after 10 min incubation at RT and rapid fluorescence detection makes this AO/PI assay an alternative to its common usage in microscopy imaging. Previous research showed that upon necrosis and apoptosis, disintegration in nucleus happens earlier than in mitochondria (Zamzami et al., Citation1996). Furthermore, an apoptogenic protease is excreted from mitochondria when the permeability changes (Zamzami et al., Citation1996). The release of protease leads to nuclear changes (Leist, Single, Castoldi, Kühnle, & Nicotera, Citation1997). Necrosis will then occur when cells are void of ATP upon the presence of oligomycin which blocks the mitochondrial ATP synthesis (Leist et al., Citation1997). These processes explained the results obtained from the method comparison study between MTS and AO where more cytotoxicity detected using PI assay (nucleic acid-based) as compared to MTS assay (mitochondria-based). AO has different excitation and emission wavelengths upon binding to DNA or RNA. In this study, we applied the excitation wavelength for AO-RNA binding which is suitable for parallel usage of PI in a mixture. The emission wavelength of AO-RNA molecules did not coincide with PI excitation wavelength, hence, enables both AO and PI to be added together and their fluorescence is measured at the same time. It is important to note that in apoptotic cells, stainability with DNA-specific fluorochromes may decrease due to endonuclease activity which produces low molecular weight DNA (Darzynkiewicz et al., Citation1992). Necrotic cells lose cell membrane integrity, however, apoptotic cells do not. PI is used to stain the non-viable cells through exclusion process (Darzynkiewicz et al., Citation1992).
3.1.2. Cross-validation between in vitro cell viability methods
MTS method is based on mitochondria reductase activity (Kupcsik, Citation2011), AO is bound to viable nucleic acids (Bank, Citation1988) while PI is bound to the non-viable nucleic acids (Bank, Citation1988). These are different from TEM microscopy which visualizes ultra section of the cells (Burghardt & Droleskey, Citation2006). The methods using AO and PI dual-spectrofluorometry was newly developed. Method comparison and method agreement between MTS and AO, and AO and PI assays were analyzed using Pearson’s correlation coefficient and Bland–Altman method (Figure ).
3.1.3. Cross-validation with TEM
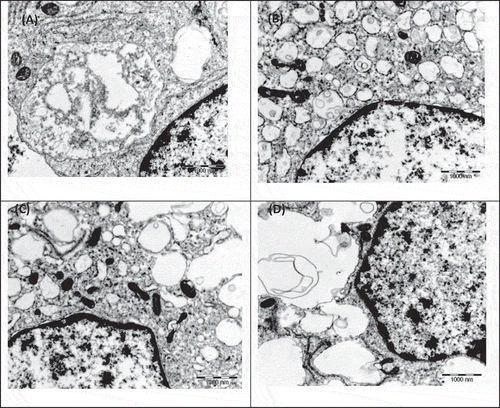
The results from method comparison between AO and MTS which depicted that non-viability detected from nucleus disintegration preceded non-viability detected from mitochondria disintegration are supported by TEM images (Figures and ). Early apoptosis still exhibited both intact nucleus and mitochondria (Figure ); early necrosis exhibited disintegrated nucleus with intact mitochondria and late necrosis exhibited disintegration of both nucleus and mitochondria (Figure ).
Figure 3. TEM electron micrograph of RAW 264.7 macrophage control cells upon exposure to (A) 1 ppm tyramine and (B) 1 ppm spermidine shows early apoptosis with apoptotic bodies and intact mitochondria.
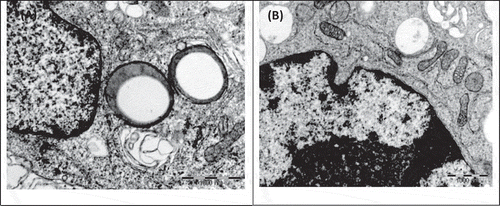
Figure 4. TEM electron micrograph of RAW 264.7 macrophage control cells upon exposure to (A) 1 ppm tryptamine shows early necrosis with nucleus disintegration, vacuolization and intact mitochondria; (B) 1 ppm spermine shows late necrosis with nucleus disintegration and mitochondria disintegration; (C) 100 ppm spermine shows early necrosis with nucleus disintegration, vacuolization and intact mitochondria and (D) 1 ppm spermidine shows late necrosis with nucleus disintegration and there are still some intact mitochondria.
3.1.4. Differences among methods compared
There are differences in the four methods used for determining cell viability in this study in terms of the cell target site for measurement and application. MTS (Kupcsik, Citation2011), AO (Bank, Citation1988) and PI (Bank, Citation1988) methods are chemical reaction-based while TEM microscopy (Burghardt & Droleskey, Citation2006) is an imaging method.
It is practical to know the degree of cell viability and cell non-viability quantitatively using the AO/PI method through the usage of multi-detection microplate reader. Percentage of cell viability measurement through MTS method is not similar with the percentage of cell viability measurement through AO method since the target sites are different, i.e. mitochondria versus nucleus. Choice of methods depends on the target site of interest for cell death activity. PI method can be used in hand with AO method to determine both quantitative percentage of cell viability and percentage of cell non-viability simultaneously.
3.2. Limitations of study
Among the limitations of this study were (i) the cell line was only macrophages from mouse ascites, thus, it does not represent the results from other organs of mouse body; (ii) TEM microscopy was only done on 1 and 100 µg/ml of TYM, TPM, SPM and SPD based on the frequencies of cytotoxic results from MTS results. Among the assumptions that we used in this study were (i) the percentage of cell viability of control untreated cells were 100%, disregarding the possibility that very small number of cells may be non-viable and (ii) the percentage of cell non-viability of control cells was 0%, disregarding the possibility that very small number of cells may be viable.
4. Conclusions
Different excitation and emission wavelength of AO and PI enable simultaneous dual-fluorescence measurement using multi-label microplate reader, enabling (i) determination of cell viability along with cell non-viability as counter-checking, and (ii) AO/PI (targeting the nucleus), when compared to MTS (targeting mitochondrial activity), precedent process effecting cytotoxicity could be determined, either in the nucleus or mitochondria.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was funded by the National Institutes of Health (NIH), Ministry of Health Malaysia (Project No. 06-057) and Universiti Putra Malaysia (Grant No.: FRGS 01-01-13-1217FR). The authors would like to thank the Director General of Health, Deputy Director-General of Health, Malaysia and Director of the Institute for Medical Research for giving the permission to publish this paper. Sincere gratitude to the staff of Nutrition Unit, Institute for Medical Research, the Faculty of Food Science & Technology, and Faculty of Biotechnology & Biomolecular Sciences (Assoc. Prof. Madya Dr. Noorjahan Banu Mohamed Alitheen), Universiti Putra Malaysia for their continuous support.
Additional information
Funding
Notes on contributors
Husniza Hussain
Our research activities under the Biogenic Amines project covers the determination of the chemical and microbiological profiles of fish-based food, and the effects of biogenic amines and the fish-based food extracts to the RAW 264.7 macrophage cell line as an in vitro model for inflammation. Along the way, two methods for quantification of biogenic amines and urocanic acid using the Ultra-High Performance Liquid Chromatography and the Liquid Chromatography-Mass Chromatography were developed and validated, one screening method for determining the biogenic amines-microbial producers, and the method to determine cell viability and non-viability upon exposure to the biogenic amines using the acridine orange and propidium iodide were developed, as reported in this paper.
References
- Altman, S. A., Randers, L., & Rao, G. (1993). Comparison of trypan blue dye exclusion and fluorometric assays for mammalian cell viability determinations. Biotechnology Progress, 9(6), 671-674.
- Bank, H. L. (1988). Rapid assessment of islet viability with acridine orange and propidium iodide. In Vitro Cellular & Developmental Biology, 24(4), 266–273. doi:10.1007/BF02628826
- Bland, J. M., & Altman, D. (1986). Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet, 327(8476), 307–310. doi:10.1016/S0140-6736(86)90837-8
- Burghardt, R. C., & Droleskey, R. (2006). Transmission electron microscopy. Current protocols in microbiology, 3(1), 2B-1.
- Darzynkiewicz, Z., Bruno, S., Del Bino, G., Gorczyca, W., Hotz, M. A., Lassota, P., & Traganos, F. (1992). Features of apoptotic cells measured by flow cytometry. Cytometry, 13(8), 795–808. doi:10.1002/cyto.990130802
- Di Giorgio, M. L., Di Bucchianico, S., Ragnelli, A. M., Aimola, P., Santucci, S., & Poma, A. (2011). Effects of single and multi walled carbon nanotubes on macrophages: Cyto and genotoxicity and electron microscopy. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 722(1), 20–31. doi:10.1016/j.mrgentox.2011.02.008
- Hyatt, A. D., Gould, A. R., Zupanovic, Z., Cunningham, A. A., Hengstberger, S., Whittington, R. J., … Coupar, E. H. (2000). Comparative studies of piscine and amphibian iridoviruses. Archives of Virology, 145(2), 301–331.
- Kupcsik, L. (2011). Estimation of cell number based on metabolic activity: The MTT reduction assay. In Mammalian cell viability (pp. 13–19). Humana Press.
- Leist, M., Single, B., Castoldi, A. F., Kühnle, S., & Nicotera, P. (1997). Intracellular adenosine triphosphate (ATP) concentration: A switch in the decision between apoptosis and necrosis. The Journal of Experimental Medicine, 185(8), 1481–1486.
- Mascotti, K., McCullough, J., & Burger, S. R. (2000). HPC viability measurement: Trypan blue versus acridine orange and propidium iodide. Transfusion, 40(6), 693-696.
- Zamzami, N., Susin, S. A., Marchetti, P., Hirsch, T., Gómez-Monterrey, I., Castedo, M., & Kroemer, G. (1996). Mitochondrial control of nuclear apoptosis. The Journal of Experimental Medicine, 183(4), 1533–1544.