Abstract
Consumers demand “fresh-like” and convenient products with preserved nutritional and organoleptic characteristics that can be kept for extended periods of time without compromising safety. This has posed greater challenges to food industries. High-pressure processing (HPP) can improve food safety by destroying the microorganisms that cause food-borne illness and spoilage that cause diseases. It also leads to the reduction of fungal toxic metabolites in food products when combined with moderate temperature. HPP holds promise as an emerging food treatment to process premium value food products while retaining food quality, maintaining natural freshness, and extends microbiological shelf life. This review presents current research findings associated with applying HPP as an emerging technology for microbial and mycotoxin control in the food industry and suggests future work.
PUBLIC INTEREST STATEMENT
Most conventional food preservation methods often result in a number of undesirable changes in foods. Thus, there is a need for alternative food processing technologies, which effectively inactivate the microorganisms, while at the same time maintaining the quality of food. Due to the rise in consumers demand high quality and safe food products, novel processes such as high-pressure processing (HPP) are continuously explored to control microbial and mycotoxin contamination in food products. Immense research works have revealed the potential benefits of HPP as an alternative to conventional treatments. These benefits are apparent in various areas of food processing, and the present review provides an update on recent research findings for microbial and mycotoxin reduction and control in foods.
Competing Interests
The authors declare no competing interests.
1. Introduction
There is a rising consumer demand for fresh and minimally processed foods with preserved nutritional and sensory qualities that can be kept for extended periods of time without compromising safety. Novel and alternative food processing methods, as well as combinations of existing methods, are continually being sought by the food industry in pursuit of producing better quality foods in an efficient and economical manner. Emerging non-thermal processes such as application of high pressure, pulsed electric field, ultrasound, cold plasma, and ultraviolet light are such alternatives for processing foods with maximum safety and quality. Taking advantage of specific potentials and opportunities, these new processes offer the possibility for a science-based development of tailor-made foods (Knorr et al., Citation2011).
High-pressure processing (HPP) is a technology that potentially addresses many, if not all, of the most recent challenges faced by the food industry. These include increasing consumers’ preference for high-quality convenient food products with natural flavour and taste, free from additives and preservatives, the drive towards health and wellness and concerns about fresh produce safety in an ever-competitive global environment. This innovation comprises the subjection of food to high hydrostatic pressure normally around 100 and 1000 MPa (Bárcenas, Altamirano-Fortoul, & Rosell, Citation2010). HPP retains food quality, maintains natural freshness, and extends the microbiological shelf-life of the food (Gupta & Balasubramaniam, Citation2012; Ramaswamy, Ahn, Balasubramaniam, & Yousef, Citation2013; Ştefãnoiu, Tãnase, Mitelut, & Popa, Citation2015). The pioneering application of HPP of foods was reported by Hite (Citation1899) for the preservation of milk, and the scope of this technology was extended for processing and preservation of fruits and vegetables (Hite, Citation1914). However, the late commercial success of the technology is mainly as a result of the high cost of the equipment (Balci & Wilbey, Citation1999; Galazka & Ledward, Citation1995; Gould, Citation1995).
Higher pressures (>1,200 MPa) are required to inactivate bacterial spores (Knorr, Citation1995). HPP has very little effect on spores if used alone, but it has great potential to sterilize food products only when combined with other treatments (Daryaei & Balasubramaniam, Citation2012; Kimura, Ida, Yosida, Ohki, & Onomoto, Citation1996; Tao, Sun, Hogan, & Kelly, Citation2014; Wilson, Dabrowski, Stringer, Moezelaar, & Brocklehurst, Citation2008). A combined treatment of high pressure and temperature is frequently considered most suitable for both pasteurization and sterilization processes (Farr, Citation1990; Patterson, Quinn, Simpson, & Gilmour, Citation1995). Microbial death at high pressures is considered to be due to permeabilization of cell membranes. The structure and cytoplasmic organelles were grossly deformed leading to the leakage of large quantities of intracellular material at a pressure of about 400 MPa, whilst the nucleus could not be recognized, and intracellular material was completely lost at 500 MPa (Farr, Citation1990; Osumi et al., Citation1996). Even though HPP is originally designed for the inactivation of microorganisms, it has also been studied as a means of reducing mycotoxins in foods (Avsaroglu, Bozoglu, Alpas, Largeteau, & Demazeau, Citation2015). However, mycotoxins are highly stable and moderately heat-resistant compounds that remain almost intact after food processing (Vidal, Sanchis, Ramos, & Marin, Citation2016). The removal of mycotoxins from food is a challenging task and requires a lot of efforts and so often the decontamination techniques are pricier than the food (Stoev, Citation2016). Conventional methods used for inactivation of fungi are not always effective or ecologically friendly. This suggests that there is a need to look into efficient mitigation strategies to control fungal growth and mycotoxins in food products to replace traditional methods or to include in the production process as part of a hurdle approach.
This review summarizes recent research findings and practical concepts associated with applying HPP as an emerging technology for microbial and mycotoxins control in the food industry while maintaining safety, high quality, and an extended shelf life of foods and provides research gaps and limitations for future work.
2. HPP equipment and processes
2.1. Principle of HPP
The governing principles of HPP are based on the assumption that foods which experience HP in a vessel follow the isostatic rule regardless of the size or shape of the food. The isostatic rule states that pressure is instantaneously and uniformly transmitted throughout a sample whether the sample is in direct contact with the pressure medium or hermetically sealed in a flexible package. Therefore, in contrast to thermal processing, the time necessary for HPP should be independent of the sample size (Rastogi, Raghavarao, Balasubramaniam, Niranjan, & Knorr, Citation2007).
The effect of HP on food chemistry and microbiology is governed by Le Chatelier’s principle. This principle states that when a system at equilibrium is disturbed, the system then responds in a way that tends to minimize the disturbance (Pauling, Citation1964). In other words, HP stimulates some phenomena (e.g. phase transition, chemical reactivity, change in molecular configuration, chemical reaction) that are accompanied by a decrease in volume, but opposes reactions that involve an increase in volume. The effects of pressure on protein stabilization are also governed by this principle, i.e. the negative changes in volume with an increase in pressure cause an equilibrium shift towards bond formation. Alongside this, the breaking of ions is also enhanced by HP, as this leads to a volume decrease due to the electrostriction of water. Moreover, as hydrogen bonds are stabilized by high pressure, as their formation involves a volume decrease, pressure does not generally affect covalent bonds. Consequently, HP can disrupt large molecules of or microbial cell structures, such as enzymes, proteins, lipids, and cell membranes, and leave small molecules such as vitamins and flavour components unaffected (Linton, Patterson, & Patterson, Citation2000).
Due to the work of compression, HPP causes temperatures to rise inside the HP vessel. This is known as adiabatic heating and should be given due consideration during the preservation process. The value of the temperature increments in the food and pressure transmitting medium will be different, as they depend on food composition as well as processing temperature and pressure and the rate of pressurization (Otero, Ramos, De Elvira, & Sanz, Citation2007). In food sterilization, adiabatic heating can be used advantageously to provide heating without the presence of sharp thermal gradients at the process boundaries (Toepfl, Mathys, Heinz, & Knorr, Citation2006). Moreover, the efficiency of HPP treatment may be affected by pressure’s level, time at pressure, time to achieve treatment pressure, adiabatic heating, decompression time, treatment temperature and product’s initial temperature, the foods’ intrinsic factors: pH, composition and aw (water activity), packaging materials and extrinsic factors prior to processing, during storage and distribution (Bilbao-Sáinz, Younce, Rasco, & Clark, Citation2009; Kadam, Jadhav, Salve, & Machewad, Citation2012; Perera, Gamage, Wakeling, Gamlath, & Versteeg, Citation2010).
2.2. Work inputs and mode of operations
In the basic HPP operation of foods, product to be treated will be loaded to a high-pressure chamber, and it will be filled with the pressure-transmitting fluid. The product receives high-pressure treatment by pressurizing the pressure-transmitting fluid either by a pump or by reducing the volume of the pressure chamber by a piston. The pressure-transmitting fluid can be water, castor oil, silicon benzoate, ethanol, or glycol. Generally, water is used as the pressurizing medium, which can be compressed by up to 15% volume at pressure above 600 MPa. Once the desired pressure is reached, the food material is held at the desired pressure for the required time period. During the hold time, the valves are closed, and pressure is maintained inside the chamber without any further energy input. After receiving the pressure treatment for the required time, the system is depressurized by releasing the pressure, and the samples are taken out from the chamber. The system is then ready for the next batch of the fresh product (Ting and Marshall, Citation2002). The time including the process of pressurization, holding and depressurization is referred to as the “cycle time”. Thus, the throughput of the system is determined by the cycle time and the loading factor (i.e., the percentage of the vessel volume actually used for holding packaged product, primarily a factor of package shape) (Hogan, Kelly, & Sun, Citation2005).
Currently, in the food industry, batch or semi-continuous HPP systems are being used depending on the type of food material to be processed. Solid food products or products with large solid particles are treated by the batch system only; whereas liquids and other pumpable products have the flexibility of receiving treatment by semi-continuous methods (Ting, E. & Marshall, R, Citation2002).
3. Microbial and mycotoxins control using HPP
3.1. Microbial inactivation
The inactivation of microorganisms by HPP is the result of a combination of factors (Simpson & Gilmour, Citation1997) including changes in the cell membranes, cell wall, proteins and enzyme-mediated cellular functions. Cell membranes are the primary sites of pressure-induced damage, with consequent alterations of cell permeability, transport systems, loss of osmotic responsiveness, organelle disruption and inability to maintain intracellular pH. In a model system of protein and lipid membrane, Kato, Hayashi, Tsuda, and Taniguchi (Citation2002) observed a decrease in the lipid bilayer fluidity and a reversible conformational change in transmembrane proteins at pressures of 100 MPa or lower, leading to a functional disorder of membrane-bound enzymes. At pressures of 100–220 MPa, there was a reversible phase transition in parts of the lipid bilayer, which passed from the liquid crystalline to gel phase; there was also dissociation and/or conformational changes in the protein subunits, which could cause the separation of protein subunits and gaps between protein and lipid bilayer, creating transmembrane tunnels. A pressure of 220 MPa or higher irreversibly destroyed and fragmented the gross membrane structure due to protein unfolding and interface separation, which was amplified by the increased pressure.
The presence of a cell wall does not mean that pressure resistance is enhanced; indeed, Ludwig, van Almsick, and Schreck (Citation2002) suggested that pressure may induce mechanical stresses on the microbial cell wall which, in turn, may interact with inactivation mechanisms. Bud scars, nodes to the cell wall and separation of the cell wall from the membrane were observed by Ritz, Tholozan, Federighi, and Pilet (Citation2001) and Park, Sohn, Shin, and Lee (Citation2001) by electronic microscopy. Moreover, models proposed to define the mechanical behaviour of cells under pressure predicted heterogeneous mechanical stresses under high hydrostatic pressure (Hartmann & Delgado, Citation2004; Hartmann, Mathmann, & Delgado, Citation2006). Protein denaturation and changes in the active centres have also been observed, together with changes in enzyme-mediated genetic mechanisms such as replication and transcription, although DNA itself is highly stable due to the fact that α-helical structures are supported by hydrogen bonds. The factors determining the efficiency of microbial inactivation by HPP include the type and number of microorganisms, the magnitude of pressure, treatment time, time to achieve pressure (come-up time), time of decompression, process temperature, pH, water activity, package integrity, product temperature, vessel temperature, and composition of the foods (Barbosa-Cánovas, Pothakamury, Palou, & Swanson, Citation1998) (Hoover, Citation1989; Morris, Brody, & Wicker, Citation2007). Generally, bacteria are more resistant to HPP than are yeasts and moulds. Spores of bacteria are extremely resistant to HPP (Nakayama, Yano, Kobayashi, Ishikawa, & Sakai, Citation1996). The resistances of spores are different even among the same species (Wilson et al., Citation2008). It is believed that the inactivation can be achieved by combining HPP with subsequent heat treatment (Paidhungat et al., Citation2002; Wuytack, Soons, Poschet, & Michiels, Citation2000). It is assumed that spores will germinate under moderate pressure condition (the germination pressure depends on the types of spores), then the germinated spores will be thermally inactivated (Vercammen, Vivijs, Lurquin, & Michiels, Citation2012; Wilson et al., Citation2008; Wimalaratne & Farid, Citation2008).
There are very few studies dealing with the mechanism by which high pressure inactivates microorganisms. One of the main reasons for this is the difficulty of carrying out in situ studies, that is, studying microbial cells in real time while they are being pressurized or depressurized, and not just before and after processing. The application of high pressure to cells triggers a series of events in the cells, not all of them necessarily lethal. Among the events studied, membrane damage has been observed in numerous studies, and it has been suggested that this is an important trigger of cell death during high-pressure processing (Michiels, Bartlett, & Aertsen, Citation2008).
In general, HPP above 200 MPa inactivates vegetative bacteria, yeast, and moulds. In practice, pressures up to 700 MPa and treatment times from a few seconds to several minutes are used to inactivate microbial cells. Bacterial spores, on the other hand, are highly resistant to pressure, showing a remarkable tolerance to pressures above 1000 MPa near room temperature. Nevertheless, sterilization of low-acid foods, as some fruit derivatives, is possible through combined high pressure (500–900 MPa) and relatively mild temperature (90–120°C) processing for about 5 min (Terefe, Buckow, & Versteeg, Citation2014). Bacterial spores are very resistant to pressure (Shigehisa, Ohmori, Saito, Taji, & Hayashi, Citation1991). Butz, Ries, Traugott, Weber, and Ludwig (Citation1990) investigated the effects of pressures between 150 and 400 MPa at temperatures of 25°C to 40°C on bacterial spores and showed that pretreatment at relatively low pressures (60–100 MPa) led to accelerated inactivation of spores at high pressure. Several papers on the use of HPP to inactivate spores have made similar suggestions for a two-exposure treatment with HPP to enhance the inactivation of spores. The first exposure germinates or activates the spores, and the second exposure at a higher pressure inactivates the germinated spores and vegetative cells (Heinz & Knorr, Citation1998). Some reviews have analysed the inactivation in vegetative cells, virus and spores (Basak, Ramaswamy, & Piette, Citation2002).
For pasteurization, treatment is in the range of 300–600 MPa for a short period of time, which inactivates the vegetative pathogenic and spoilage microorganisms (4-log units). Nevertheless, the response of pathogenic bacteria to HP treatments is variable and depends on the temperature applied. In fact, it has been observed that bacteria exhibit the biggest pressure resistance at temperatures between 20°C and 30°C. For example, studies on the inactivation of E. coli O157:H7 in poultry meat showed a 1-log decimal reduction when the product is treated at 400 MPa and 20°C for 15 min. The same results as for 50°C heat treatment alone. When treatments at 400 MPa are combined with a temperature of 50°C, a 6-log reduction was achieved (Patterson & Kilpatrick, Citation1998). The greatest challenge in the use of high pressure is the inactivation of bacterial spores. Differences in response to pressure between different species, and between strains of the same species, are frequent (Heinz & Knorr, Citation2002). For examples, spores of Clostridium sporogenes in fresh chicken breast required a pressure of 680 MPa to 1 h to achieve a relevant (5-log) inactivation (Crawford, Murano, Olson, & Shenoy, Citation1996), while other workers found that a 1,500- MPa treatment of C. Sporogenes in liquid media led only to a 1.5-log reduction (Maggi et al., Citation1996).
Bacterial spores represent a challenge for high-pressure technology, and more information about their resistance is required. Microbial spores suspended in foods or laboratory model system could be inactivated by high-pressure treatment but compared to vegetative cells the treatment conditions must be extreme: higher pressure and long exposure time at elevated temperature (Hoover, Citation1993). When pressure-temperature is combined at 690 MPa and 80°C for 20 min, the treatments were effective with a significant reduction in the Clostridium sporogenes spore count (Crawford et al., Citation1996). Successful treatment of Bacillus stearothermophilus is observed where pressure treatments are combined with moderate temperature (70°C) (Hayakawa, Kanno, Yoshiyama, & Fujio, Citation1994). There are no published reports on the high-pressure resistance of Clostridium botulinum spores, and their ability to withstand high pressure at low or high temperatures is unknown (Lechowich, Citation1993). Pressure inactivation of yeast and moulds has been reported in citrus juices. Juices pressurized at 400 MPa for 10 min at 40°C did not spoil during 2–3 months of storage (Ogawa, Fukuhisa, Kubo, & Fukumoto, Citation1990; Olsson, Citation1995).
The effect of HPP pressure (200, 400 and 600 MPa) for up to 40 min at 70°C on B. cereus spores was investigated (Evelyn & Silva, Citation2017). A pressure increment from 200 to 600 MPa slightly reduced the spore numbers in the reconstituted milk. Then, the influence of temperature at 600 MPa on spore inactivation for up to 40 min was studied. Increasing the HPP temperature from 38°C to 70°C increased the spore inactivation in milk by 3.5 log. The 600 MPa combined with heat enhanced the spore inactivation in milk, requiring a temperature 20°C lower to achieve the same spore inactivation. However, for a 5 log spore inactivation, the pressure–thermal process required higher specific energy than the thermal processing (Silva, Citation2015).
Concerning yeast and mould, these are less resistant than bacteria (Georget et al., Citation2015). Indeed, they are inactivated by pressure between 200 and 400 MPa. Most yeast and mould spores are destroyed by a pressure of 400 MPa. Yeasts and mould spores have been previously shown to be readily inactivated at 400 MPa (Smelt, Citation1998), while Ogawa et al. (Citation1990) reported a 5-log-unit reduction when nine species of yeasts and moulds in fruit juice were treated at 350 MPa for 30 min or 400 MPa for 5 min. These results are in agreement with results obtained in this study for P. roqueforti in cheese slurry at temperatures of 20 and 30°C. The pattern of inactivation by HP observed for the mould spores was different from that obtained with the bacterial species. However, it has been demonstrated that eukaryotic microorganisms are generally more sensitive to pressure than prokaryotic microorganisms (Hoover, Citation1989). Inactivation of moulds was not evident until a pressure of 300 MPa was reached, after which cell numbers decreased rapidly. A summary of recent applications of HPP for inactivation microorganisms is presented in Table .
Table 1. Microbial inactivation by HPP in different food products
3.2. Mycotoxin control
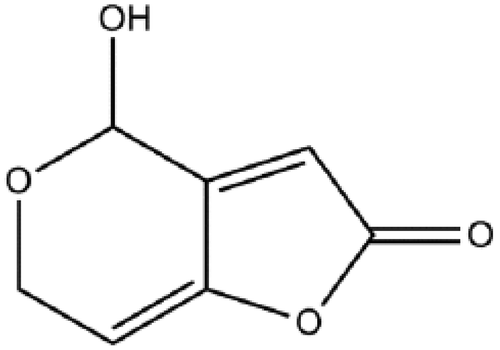
Mycotoxins are toxic secondary metabolites produced by fungi that present a potential hazard regarding food safety. Patulin (Figure ) is a mycotoxin and is known to be produced by more than 60 species of fungi belonging to greater than 30 genera (Barug et al., Citation2006; Lai, Fuh, & Shih, Citation2000).
Although typically associated with Penicillium expansum, patulin is also known to be produced by other fungi, including P. claviforme, P. urticae, P. patulum, Aspergillus clavatus, A. giganteus, Byssoclamys fulva, B. nivea, and Alternaria alternata (Drusch & Ragab, Citation2003). Due to the potential negative health effects of consuming patulin, regulatory agencies from around the world have instituted limits regarding the maximum amount of patulin that can be in food products. Many organizations such as Health Canada, The United States Food and Drug Administration, and the Codex Committee on Food Additives and Contaminants have all set limits of 50 μg/kg patulin (CODEX, Citation2003; FDA, Citation2004). The World Health Organization has suggested a limit of 0.4 μg/kg body weight, and the European Union has set a much lower maximum limit of 25 μg/kg for solid products and 10 μg/kg for any food marketed towards infants (EC, Citation2006; WHO, Citation1995).
Conventional processing has been shown to have an effect on patulin content in food products (Acar, Gökmen, & Taydas, Citation1998; De Souza Sant’Ana, Rosenthal, & de Massaguer, Citation2008). The extent to which the patulin content can be reduced by these means is unclear, with results dependent on the parameters and the initial patulin content (Funes & Resnik, Citation2009; Spadaro, Ciavorella, Frati, Garibaldi, & Gullino, Citation2007). Patulin is known to be resistant to degradation by heat treatment (Lovett & Peeler, Citation1973). Furthermore, some of the treatments used in apple processing that have been suggested as possibilities for the reduction of patulin are known to have a negative effect on some of the quality characteristics of the food product such as pH, clarity, colour, sugars, and °Brix (Gökmen, Artık, Acar, Kahraman, & Poyrazoğlu, Citation2001; Janotová, Čížková, Pivoňka, & Voldřich, Citation2011; Kadakal & Nas, Citation2002). Development in the field of non-thermal food processing techniques has opened up the potential for unconventional processing methods to play a role in apple processing and the reduction of patulin.
HPP treatment has been found to reduce up to 56.24% of patulin in apple juice contaminated with 100 ppb of the mycotoxin, depending on the operating conditions (Avsaroglu et al., Citation2015). Pressures ranged from 30 to 500 MPa, and temperatures ranged from (30–50°C). A higher pressure at 600 MPa for 300 s was found to reduce patulin in juice by 31% (Hao, Zhou, Koutchma, Wu, & Warriner, Citation2016). Bruna, Voldrich, Marek, and Kamarád (Citation1997) achieved up to 62% reduction of patulin at 800 MPa in apple juice. It has also been reported that HPP treatment can also degrade patulin levels in apple juice and concentrate. Under commercial practice, the treatments applied for HPP juice is 180 s at 600 MPa to achieve the 5 log reduction of relevant vegetative pathogens (Rastogi et al., Citation2007; Rendueles et al., Citation2011). No clear trend as to the optimal pressure/temperature combination has been concluded, suggesting that further study is required to refine this technology. It has been shown that HPP primarily works on hydrophobic and electrostatic interactions, not the covalent ones found in patulin molecules (Patterson, Citation2005). The reductions in patulin content have been attributed to the formation of adducts with compounds containing sulphhydryl groups such as glutathione or cysteine (Avsaroglu et al., Citation2015; Schebb et al., Citation2009). These adducts have been shown to be 100 times less toxic than patulin itself (Lindroth & von Wright, Citation1990).
Different pressure levels (300–500 MPa) in combination with temperature (20–50°C) were applied to artificially contaminated clear apple juice. Patulin decrease was ranged from 0% to 51.16%. There were not a linear decrease in patulin concentrations for different pressure/temperature applications, and the highest reduction was obtained at 400 MPa/30°C. There was not any statistically significant difference between different pressure applications including 400 MPa/30 °C (p >0.05) (Avsaroglu et al., Citation2015). Bruna et al. (Citation1997) studied the reduction of patulin in apple juice by high-pressure application (300, 500 and 800 MPa) at room temperature for 1 h. They obtained 42%, 53% and 62% reductions, respectively. Authors indicated that the exposure time of pressure was a parameter to reach higher reductions of patulin. The results obtained by Avsaroglu et al. (Citation2015) were in agreement with those obtained by Bruna et al. (Citation1997) if pressure-holding times were considered. For 5 min pressure application, 5.7% (5 ppb), 14.06% (50 ppb) and 24.66% (100 ppb) reductions were observed at 500 MPa/20°C. Test juices were spiked with 200 ppb (1.29 µM) patulin and then subjected to different HPP regimes. The extent of patulin degradation was dependent on the applied pressure and duration of treatment. With the apple spinach combination (AS juice) the highest level of patulin degradation (43 ppb, 0.28 µM) was recorded for treatments at 600 MPa for 300 s (Hao et al., Citation2016).
Fusarium graminearum is a recognized as one of the most devastating plant pathogens which produces hazardous mycotoxins named deoxynivalenol (DON), nivalenol (NIV), and zearalenone (ZEA) (Zhou et al., Citation2017). The HPP was also effective in minimizing the levels of DON and ZEA in maize grains, and complete reduction in DON and ZEA were achieved at 550 MPa of pressure, 45°C of temperature and 20 min of pressure holding time (Kalagatur et al., Citation2018). On the other hand, Tokuşoğlu, Alpas, and Bozoğlu (Citation2010) have applied HPP and successfully reduced the level of citrinin mycotoxin in black table olives at 250 MPa/35°C for 1 min. Overall, the obtained results in these studies showed that HPP was very effective in controlling the level of DON and ZEA in agricultural commodities.
The 600 MPa pressure is considered by many authors as threshold value and also is considered to be economical and microbiologically safe for achieving the pasteurization level if it is combined with temperatures in the range 35–55ºC (Aymerich, Picouet, & Monfort, Citation2008; Garriga, Grebol, Aymerich, Monfort, & Hugas, Citation2004; Perera et al., Citation2010). A summary of recent applications of HPP for mycotoxin control is presented in Table .
Table 2. Mycotoxins control by HPP in different food products
4. HPP integrated technologies
In general, the safety and stability of food are not based on one factor only but on a combination of several factors. In addition to the preservation factors applied to foods, both microbial growth and survival are influenced by different intrinsic factors characteristic of the food (Gould, Citation2000). These multiple intrinsic factors are part of a dynamic system that changes from the moment of application to when the food reaches the consumer. During this process, each factor plays a role of different magnitude and such magnitude changes over time. It has been known for many centuries that a combination of preservation factors can influence the microbial stability and safety of foods, and this concept has been used empirically for many years.
Studies on new inactivation technologies that offer alternatives to heat have also contributed to the development of hurdle technology in the last few years. Some microorganisms, especially bacterial spores and enzymes related to food quality, are very resistant to nonthermal treatments. To extend the use of nonthermal technologies in food preservation, combinations of such with other preservation factors have been investigated.
4.1. Combination of HPP with moderate temperatures
The aim of nonthermal technologies is to destroy the microorganisms in foods and at the same time produce a product that is safe and stable with the least amount of damage to the sensory and nutritional properties. To achieve this goal, food is held below the temperatures normally used in thermal processing (Barbosa-Cánovas et al., Citation1998). However, temperature during nonthermal treatments is an important factor that influences microbial destruction. A higher microbial inactivation rate is usually observed when nonthermal treatments are applied at temperatures above room temperature. The combination of moderate temperatures with nonthermal processes is of great practical interest, because application of nonthermal treatments at temperatures not affecting food properties may cause equivalent microbial inactivation at lower nonthermal treatment intensities and/or for shorter periods. In order to enhance the lethal effects of nonthermal technologies, in addition to applying a moderate heat treatment during the nonthermal process, the effect of heat treatment before or after the nonthermal treatment has been investigated.
High-pressure processing (HPP) increases the inactivation of vegetative microorganisms at temperatures below or above ambient temperature. It has been demonstrated that application of HPP at refrigeration and even freezing temperatures is more effective for microbial inactivation than pressurization at room temperature (Carlez, Rosec, Richard, & Cheftel, Citation1993; Gervilla, Capellas, Ferragut, & Guamis, Citation1997a; Gervilla, Felipe, Ferragut, & Guamis, Citation1997b; Ponce, Pla, Capellas, Guamis, & Mor-Mur, Citation1998a; Ponce, Pla, Mor-Mur, Gervilla, & Guamis, Citation1998b). This effect has been attributed to the higher susceptibility of some proteins to HPP denaturation at low temperatures instead of room temperatures (Patterson, Citation1999).
Despite the higher inactivation observed at low temperatures, to obtain microbial inactivation that ensures food safety it is more efficient to combine HPP with temperatures above room temperature. By combining moderate HPP treatments with moderate temperatures, an inactivation higher than 5 log10 cycles can be obtained in different pathogenic microorganisms suspended in several types of laboratory media or foods (Table ).
Table 3. Combinations of HPP and moderate temperatures to reach an inactivation of at least 5 log10 cycles in different pathogenic vegetative microorganisms
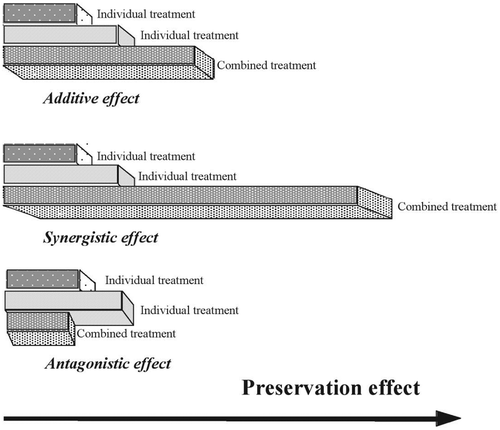
When HPP is applied above room temperature, microbial inactivation increases at both nonlethal and lethal temperatures for the microorganisms. At moderate lethal temperatures, the simultaneous application of high pressure and heat is more lethal than the addition of either treatment alone; therefore, the effect of the combination is synergistic (Figure ).
In general, the kinetics of inactivation of most vegetative cells by HPP at low temperatures show an initial exponential rate followed by pronounced tailing (Smelt, Citation1998). This tail tends to disappear when HPP is combined with heat (Kalchayanand, Sikes, Dunne, & Ray, Citation1998). Another additional advantage to this combination is that although at room temperature microbial resistance to HPP is very variable, the variation in pressure resistance between different microbial species or between different strains of the same species is much lower when HPP is combined with temperature (Alpas et al., Citation1999). At room temperature, bacterial spores are resistant to pressures as high as 1200 MPa; however, pressures as low as 10 MPa have been found to trigger spores to germinate (Clouston & Wills, Citation1969; Gould & Sale, Citation1970). Several theories have been postulated to explain the pressure-induced germination, but the mechanism has not been fully elucidated (Gould & Sale, Citation1970; Wuytack, Boven, & Michiels, Citation1998; Wuytack et al., Citation2000). Both initiation of germination and inactivation of bacterial spores by HPP are greatly enhanced at raised temperatures (Gould, Citation1973; Murrell & Wills, Citation1977; Raso, Barbosa-Canovas, & Swanson, Citation1998a). As germinated spores lose their exceptional resistance to physico-chemical agents, the pressure-germinated spores may be inactivated by HPP treatment or by some other treatments such as heat or irradiation at moderate intensities. In order to inactivate bacterial spores at moderate temperatures, the application of a pasteurization treatment subsequent to HPP has been proposed (Cheftel, Citation1995). Appreciable inactivation of bacterial spores can be obtained by combining HPP with heat in continuous or cycling treatments (Table ).
Table 4. Combinations of HPP and moderate temperatures to reach an inactivation of at least 5 log10 cycles in different bacterial spores
Inactivation of vegetative forms of yeast and moulds with HPP in combination with moderate temperatures has been scarcely studied because of their low pressure resistance at room temperature (Ogawa et al., Citation1990; Palou, Lopez-Malo, Barbosa-Canovas, Welti-Chanes, & Swanson, Citation1997; Pandya, Jewett, & Hoover, Citation1995; Parish, Citation1998; Raso, Calderón, Góngora, Barbosa‐Cánovas, & Swanson, Citation1998b). However, inactivation of ascospores of moulds requires combining HPP and moderate temperatures (60–70°C) (Butz, Funtenberger, Haberditzl, & Tauscher, Citation1995). It has been demonstrated at laboratory scale that the combination of HPP with moderate temperatures is an effective means to increase microbial inactivation. However, application of this combination at an industrial scale could encounter some technical limitations. One of the major advantages of the HPP process is that the treatment is homogeneous because the pressure is transmitted uniformly and instantaneously inside the pressure vessel. As heat transmission does not involve these properties, the application of uniform treatments when HPP treatments are combined with moderate heating is a technical challenge.
4.2. Combination of HPP with pulsed electric fields
Pulsed electric fields (PEF) have been reported as a promising technology for cold pasteurization of liquid products; however, spore inactivation using this technology is not feasible so far. Nevertheless, combining HPP and PEF can lead to notable spore inactivation. In a study conducted using spores of Bacillus subtilis in different chemical solutions such as 0.1% NaCl solution and buffers, HPP and PEF were used alone and together to explore the spore death. High pressure was tested at 700 MPa and 55°C; meanwhile, PEF were conducted at 12 kV, 50 Hz, in a treatment chamber containing two acrylic resin pipes (55 mm length, 19 mm outside diameter for the inner pipe, inserted in the outer pipe with a gap of 3.5 mm) keeping the sample at 55°C. Results showed that PEF by itself was not able to inactivate spores, but high pressure was able to reduce some spore counts. However, when the treatments were combined, two different trends were observed. First, when PEF was applied followed by high pressure, the reduction of spores was possible up to 7 log, especially in the buffer solution. However, when the treatment was initiated by high pressure followed by PEF, the reduction was not observed, even though the reactivation of spores was reported. The efficacy of PEF followed by high pressure is related to the presence of cracks on the spore surface generated by the electric field and the subsequent rupture of the cell due to the elevated pressure. In the opposite case (high pressure followed by PEF), some spore inactivation was observed because of the elevated pressure, but when PEF was applied, the electric fields favoured the spore germination and promoted the growth of H-spores. The H-spores were found during high-pressure treatment at low pH, in which the process replaced the ions of the spores (Ca+2, Na+1, Mg+2, Mn+2) by hydrogen (Sasagawa et al., Citation2006). It is interesting to see that even the order of the processes could not have a significant effect on cell inactivation; the mechanisms of inactivation need to be understood and taken into account in order to successfully combine two or more processes.
5. Conclusion and future outlook
Application of high-pressure processing (HPP) has shown significant potential and realized success as an emerging technology in the food industry in terms of assuring safety and quality attributes to that of thermal treatment, while at the same time maintains food freshness, taste, flavour and nutrition which produce greater quality products. HPP application can inactivate microorganisms and mycotoxins and modify structures, with little or no effect on the nutritional and sensory quality of foods. As summarized in this review, HPP can be an effective means of degradation of some type of mycotoxins such as patulin and citrinin; however, the optimal conditions have yet to be determined and may depend in part on the properties of the food product. Future development should focus on determining the nature and safety of chemicals produced from the breakdown of these mycotoxins in treatment techniques. Despite all the advantages, food processors still face challenges in the form of extremely resistant bacterial spores. However, immense possibilities have been shown by combining HPP with other alternative treatments for use as a hurdle technology to increase inactivation effects.
Even though opportunities clearly exist for innovative applications, further and complementary research related to the above HPP issues would undoubtedly contribute to continued development and innovation in this technology where conventional methods fail to yield satisfying results. Information regarding the success of current food manufacturers and companies employing HPP technology can encourage other companies to realize the potential of HPP and the many benefits it can provide to both the consumer and industry, either alone or in combination with other processes as an alternative to thermal processing. It is considered that a special stress ought to be provided to HPP conditions (pressure, holding time, and temperature) to optimize in each case in order to enhance sensory and nutritional values, rather than extrapolating the findings made with any one species for all others. HPP has relatively better preservation of nutrients, but there still needs further investigation to clearly understand its effect at different operating conditions. It is also necessary to compile data in order to clarify the role of HP towards toxicity, allergenicity, loss of digestibility and the eating quality of foods. Furthermore, most researches are limited only to very few kinds of mycotoxins decontamination and future investigations could be carried out on the effect of HPP on other toxic metabolites and their degradation mechanisms.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Funding
Notes on contributors
Henock Woldemichael Woldemariam
Henock Woldemichael Woldemariam is a PhD candidate at the department of Chemical Engineering, Addis Ababa Institute of Technology with expertise in non-thermal food processing technologies with specific interest in high pressure processing and pulsed electric field processing technologies.
Shimelis Admassu Emire
Shimelis Admassu Emire is an associate professor in Addis Ababa Institute of Technology and does research in Food Processing and Agro-food industry business development. He has a great aspiration to work in the cutting-edge-research areas of Agro-food and Bio-System Engineering including Emerging Technologies and Food Quality Assurance Management Systems.
References
- Acar, J., Gökmen, V., & Taydas, E. E. (1998). The effects of processing technology on the patulin content of juice during commercial apple juice concentrate production. Zeitschrift Für Lebensmitteluntersuchung und-Forschung A, 207(4), 328–21.
- Alpas, H., Kalchayanand, N., Bozoglu, F., Sikes, A., Dunne, C., & Ray, B. (1999). Variation in resistance to hydrostatic pressure among strains of food-borne pathogens. Applied and Environmental Microbiology, 65(9), 4248–4251.
- Ananta, E., Heinz, V., Schlüter, O., & Knorr, D. (2001). Kinetic studies on high-pressure inactivation of Bacillus stearothermophilus spores suspended in food matrices. Innovative Food Science & Emerging Technologies, 2(4), 261–272. doi:10.1016/S1466-8564(01)00046-7
- Andrés, V., Villanueva, M.-J., & Tenorio, M.-D. (2016). Influence of high pressure processing on microbial shelf life, sensory profile, soluble sugars, organic acids, and mineral content of milk-and soy-smoothies. LWT-Food Science and Technology, 65, 98–105. doi:10.1016/j.lwt.2015.07.066
- Avsaroglu, M., Bozoglu, F., Alpas, H., Largeteau, A., & Demazeau, G. (2015). Use of pulsed-high hydrostatic pressure treatment to decrease patulin in apple juice. High Pressure Research, 35(2), 214–222. doi:10.1080/08957959.2015.1027700
- Aymerich, T., Picouet, P. A., & Monfort, J. M. (2008). Decontamination technologies for meat products. Meat Science, 78(1–2), 114–129. doi:10.1016/j.meatsci.2007.07.007
- Balci, A. T., & Wilbey, R. A. (1999). High pressure processing of milk-the first 100 years in the development of a new technology. International Journal of Dairy Technology, 52(4), 149–155. doi:10.1111/idt.1999.52.issue-4
- Barbosa-Cánovas, G., Pothakamury, U., Palou, E., & Swanson, B. (1998). Nonthermal preservation of foods. New York: Macel Dekker. Inc.
- Bárcenas, M. E., Altamirano-Fortoul, R., & Rosell, C. M. (2010). Effect of high pressure processing on wheat dough and bread characteristics. LWT-Food Science and Technology, 43(1), 12–19. doi:10.1016/j.lwt.2009.06.019
- Barug, D., Bhattnagar, D., Van Egmond, H. P., van der Kamp, J. W., Van Osenbruggen, W. A., & Visconti, A. (2006). The mycotoxin factbook. Wageningen, The Netherlands: Wageningen Academic Publishers.
- Basak, S., Ramaswamy, H., & Piette, J. (2002). High pressure destruction kinetics of Leuconostoc mesenteroides and Saccharomyces cerevisiae in single strength and concentrated orange juice. Innovative Food Science & Emerging Technologies, 3(3), 223–231. doi:10.1016/S1466-8564(02)00008-5
- Bilbao-Sáinz, C., Younce, F. L., Rasco, B., & Clark, S. (2009). Protease stability in bovine milk under combined thermal-high hydrostatic pressure treatment. Innovative Food Science & Emerging Technologies, 10(3), 314–320. doi:10.1016/j.ifset.2009.01.003
- Bruna, D., Voldrich, M., Marek, M., & Kamarád, J. (1997). Effect of high pressure treatment on patulin content in apple concentrate. High Pressure Research in the Biosciences, 335–338.
- Butz, P., Funtenberger, S., Haberditzl, T., & Tauscher, B. (1995). High pressure inactivation of Byssochlamys nivea Ascospores and other heat resistant moulds. LWT-Food Science and Technology, 29(5–6), 404–410. doi:10.1006/fstl.1996.0062
- Butz, P., Ries, J., Traugott, U., Weber, H., & Ludwig, H. (1990). The high-pressure inactivation of bacteria and bacterial-spores. Pharmazeutische Industrie, 52(4), 487–491.
- Carlez, A., Rosec, J.-P., Richard, N., & Cheftel, J.-C. (1993). High pressure inactivation of Citrobacter freundii, Pseudomonas fluorescens and Listeria innocua in inoculated minced beef muscle. LWT-Food Science and Technology, 26(4), 357–363. doi:10.1006/fstl.1993.1071
- Chai, C., Lee, J., Lee, Y., Na, S., & Park, J. (2014). A combination of TiO2–UV photocatalysis and high hydrostatic pressure to inactivate Bacillus cereus in freshly squeezed Angelica keiskei juice. LWT-Food Science and Technology, 55(1), 104–109. doi:10.1016/j.lwt.2013.08.015
- Cheftel, J. C. (1995). Review: High-pressure, microbial inactivation and food preservation. Food Science and Technology International, 1(2–3), 75–90. doi:10.1177/108201329500100203
- Clouston, J., & Wills, P. A. (1969). Initiation of germination and inactivation of Bacillus pumilus spores by hydrostatic pressure. Journal of Bacteriology, 97(2), 684–690.
- CODEX. (2003). Maximum level for Patulin in apple juice and apple juice ingredients and other beverages. In Codex standard 235. Rotterdam: Joint FAO/WHO Food Standards Programme.
- Crawford, Y. J., Murano, E. A., Olson, D. G., & Shenoy, K. (1996). Use of high hydrostatic pressure and irradiation to eliminate Clostridium sporogenes spores in chicken breast. Journal of Food Protection, 59(7), 711–715. doi:10.4315/0362-028X-59.7.711
- Daryaei, H., & Balasubramaniam, V. (2012). Microbial decontamination of food by high pressure processing. In Microbial decontamination in the food industry (pp. 370–406). Woodhead Publishing.
- De Souza Sant’Ana, A., Rosenthal, A., & de Massaguer, P. R. (2008). The fate of patulin in apple juice processing: A review. Food Research International, 41(5), 441–453. doi:10.1016/j.foodres.2008.03.001
- Devatkal, S., Somerville, J., Thammakulkrajang, R., & Balasubramaniam, V. (2015). Microbiological efficacy of pressure assisted thermal processing and natural extracts against Bacillus amyloliquefaciens spores suspended in deionized water and beef broth. Food and Bioproducts Processing, 95, 183–191. doi:10.1016/j.fbp.2015.05.007
- Drusch, S., & Ragab, W. (2003). Mycotoxins in fruits, fruit juices, and dried fruits. Journal of Food Protection, 66, 1514–1527. doi:10.4315/0362-028X-66.8.1514
- EC. (2006). Commission regulation (EC) No 1881/2006 of 19 december 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal European Union, 364, 5–24.
- Evelyn, M. E., & Silva, F. V. M. (2017). Comparing high pressure thermal processing and thermosonication with thermal processing for the inactivation of bacteria, moulds, and yeasts spores in foods. Journal of Food Engineering, 214, 90–96. doi:10.1016/j.jfoodeng.2017.06.027
- Farr, D. (1990). High pressure technology in the food industry. Trends in Food Science & Technology, 1, 14–16. doi:10.1016/0924-2244(90)90004-I
- FDA. (2004). Compliance policy guidance for fda staff. Sec. 510.150 Apple juice, apple juice concentrates, and apple juice products-Adulteration with patulin. In compliance policy guide. Silver Spring, MD, USA: U.S. Food and Drug Administration
- Fornari, C., Maggi, A., Gola, S., Cassara, A., & Manachini, P. L. (1995). Inactivation of Bacillus endospores by high-pressure treatment. Industria Conserve, 70(3), 259–265.
- Funes, G., & Resnik, S. (2009). Determination of patulin in solid and semisolid apple and pear products marketed in Argentina. Food Control, 20(3), 277–280. doi:10.1016/j.foodcont.2008.05.010
- Galazka, V., & Ledward, D. (1995). Developments in high pressure food processing. Food Technology International Europe, 12, 123–125.
- Gao, Y., Qiu, W., Wu, D., & Fu, Q. (2011). Assessment of Clostridium perfringens spore response to high hydrostatic pressure and heat with nisin. Applied Biochemistry and Biotechnology, 164(7), 1083–1095. doi:10.1007/s12010-011-9196-0
- Garriga, M., Grebol, N., Aymerich, M., Monfort, J., & Hugas, M. (2004). Microbial inactivation after high-pressure processing at 600 MPa in commercial meat products over its shelf life. Innovative Food Science & Emerging Technologies, 5(4), 451–457. doi:10.1016/j.ifset.2004.07.001
- Georget, E., Sevenich, R., Reineke, K., Mathys, A., Heinz, V., Callanan, M., … Knorr, D. (2015). Inactivation of microorganisms by high isostatic pressure processing in complex matrices: A review. Innovative Food Science & Emerging Technologies, 27, 1–14. doi:10.1016/j.ifset.2014.10.015
- Gervilla, R., Capellas, M., Ferragut, V., & Guamis, B. (1997a). Effect of high hydrostatic pressure on Listeria innocua 910 CECT inoculated into ewe’s milk. Journal of Food Protection, 60(1), 33–37. doi:10.4315/0362-028X-60.1.33
- Gervilla, R., Felipe, X., Ferragut, V., & Guamis, B. (1997b). Effect of high hydrostatic pressure on Escherichia coli and Pseudomonas fluorescens strains in ovine milk. Journal of Dairy Science, 80(10), 2297–2303. doi:10.3168/jds.S0022-0302(97)76179-4
- Gökmen, V., Artık, N., Acar, J., Kahraman, N., & Poyrazoğlu, E. (2001). Effects of various clarification treatments on patulin, phenolic compound and organic acid compositions of apple juice. European Food Research and Technology, 213(3), 194–199. doi:10.1007/s002170100354
- Gould, G. (1973). Inactivation of spores in food by combined heat and hydrostatic pressure. Acta Aliment, 2, 377–383.
- Gould, G. (2000). Strategies for food preservation. The Microbiological Safety and Quality of Food, 1, 19–35.
- Gould, G., & Sale, A. (1970). Initiation of germination of bacterial spores by hydrostatic pressure. Microbiology, 60(3), 335–346.
- Gould, G. W. (1995). The microbe as a high pressure target. In D. A. Ledward, D. E. Johnston, R. G. Earnshaw, & A. P. M. Hasting (Eds.), High pressure processing of foods (pp. 27–35). Loughborough, Nottingham: University Press.
- Gupta, R., & Balasubramaniam, V. (2012). High-pressure processing of fluid foods. In Novel thermal and non-thermal technologies for fluid foods (pp. 109–133). Academic Press.
- Hao, H., Zhou, T., Koutchma, T., Wu, F., & Warriner, K. (2016). High hydrostatic pressure assisted degradation of patulin in fruit and vegetable juice blends. Food Control, 62, 237–242. doi:10.1016/j.foodcont.2015.10.042
- Hartmann, C., & Delgado, A. (2004). Numerical simulation of the mechanics of a yeast cell under high hydrostatic pressure. Journal of Biomechanics, 37(7), 977–987. doi:10.1016/j.jbiomech.2003.11.028
- Hartmann, C., Mathmann, K., & Delgado, A. (2006). Mechanical stresses in cellular structures under high hydrostatic pressure. Innovative Food Science & Emerging Technologies, 7(1–2), 1–12. doi:10.1016/j.ifset.2005.06.005
- Hayakawa, I., Kanno, T., Yoshiyama, K., & Fujio, Y. (1994). Oscillatory compared with continuous high pressure sterilization on Bacillus stearothermophilus spores. Journal of Food Science, 59(1), 164–167. doi:10.1111/jfds.1994.59.issue-1
- Heinz, V., & Knorr, D. (1998). High pressure germination and inactivation kinetics of bacterial spores. Special Publication-Royal Society of Chemistry, 222, 435–441.
- Heinz, V., & Knorr, D. (2002). Effects of high pressure on spores. In Ultra high pressure treatments of foods (pp. 77–113). Boston, MA: Springer.
- Hite, B. H. (1899). The effect of pressure in the preservation of milk: A preliminary report. West Virginia Agricultural Experiment Station.
- Hite, B. H. (1914). The effects of pressure on certain microorganisms encountered in the preservation of fruits and vegetables. Bull West Virginia University Agricultural Experiment Station, 146, 3–67.
- Hogan, E., Kelly, A. L., & Sun, D.-W. (2005). High pressure processing of foods: An overview. InEmerging technologies for food processing (pp. 3–32). Academic Press.
- Hoover, D. (1993). Pressure effects on biological systems. Food Technology (USA), 47(6), 150–155.
- Hoover, D. G. (1989). Biological effects of high hydrostatic pressure on food microorganisms. Food Technology, 43, 99–107.
- Janotová, L., Čížková, H., Pivoňka, J., & Voldřich, M. (2011). Effect of processing of apple puree on patulin content. Food Control, 22(6), 977–981. doi:10.1016/j.foodcont.2010.12.005
- Ju, X.-R., Gao, Y.-L., Yao, M.-L., & Qian, Y. (2008). Response of Bacillus cereus spores to high hydrostatic pressure and moderate heat. LWT-Food Science and Technology, 41(10), 2104–2112. doi:10.1016/j.lwt.2007.11.011
- Kadakal, C., & Nas, S. (2002). Effect of activated charcoal on patulin, fumaric acid and some other properties of apple juice. Food/Nahrung, 46(1), 31–33. doi:10.1002/1521-3803(20020101)46:1<31::AID-FOOD31>3.0.CO;2-D
- Kadam, P., Jadhav, B., Salve, R., & Machewad, G. (2012). Review on the high pressure technology (HPT) for food preservation. Journal Food Process Technol, 3(1), 135.
- Kalagatur, N. K., Kamasani, J. R., Mudili, V., Krishna, K., Chauhan, O. P., & Sreepathi, M. H. (2018). Effect of high pressure processing on growth and mycotoxin production of Fusarium graminearum in maize. Food Bioscience, 21, 53–59. doi:10.1016/j.fbio.2017.11.005
- Kalchayanand, N., Sikes, A., Dunne, C., & Ray, B. (1998). Interaction of hydrostatic pressure, time and temperature of pressurization and pediocin AcH on inactivation of foodborne bacteria. Journal of Food Protection, 61(4), 425–431.
- Kato, M., Hayashi, R., Tsuda, T., & Taniguchi, K. (2002). High pressure‐induced changes of biological membrane: Study on the membrane‐bound Na+/K+‐ATPase as a model system. European Journal of Biochemistry, 269(1), 110–118.
- Kimura, K., Ida, M., Yosida, Y., Ohki, K., & Onomoto, M. (1996). Application of high pressure for sterilization of low acid food. In Progress in biotechnology (Vol. 13, pp. 429–432). Elsevier.
- Knorr, D. (1995). Hydrostatic pressure treatment of food: Microbiology. In G. Gw (Ed.), New methods for food preservation (pp. 159–175). London: Blackie Academic and Professional.
- Knorr, D., Froehling, A., Jaeger, H., Reineke, K., Schlueter, O., & Schoessler, K. (2011). Emerging technologies in food processing. Annual Review of Food Science and Technology, 2, 203–235. doi:10.1146/annurev.food.102308.124129
- Lai, C.-L., Fuh, Y.-M., & Shih, D. Y.-C. (2000). Detection of mycotoxin patulin in apple juice. Journal of Food and Drug Analysis, 8, 2.
- Lechowich, R. (1993). Food safety implications of high hydrostatic pressure as a food processing method. Food Technology (USA), 47(6), 170–172.
- Li, R., Wang, Y., Wang, S., & Liao, X. (2015). A comparative study of changes in microbiological quality and physicochemical properties of N 2-infused and N 2-degassed banana smoothies after high pressure processing. Food and Bioprocess Technology, 8(2), 333–342. doi:10.1007/s11947-014-1401-z
- Lindroth, S., & von Wright, A. (1990). Detoxification of patulin by adduct formation with cysteine. Journal of Environmental Pathology, Toxicology and Oncology, 10(4–5), 254–259.
- Linton, M., Patterson, M. F., & Patterson, M. (2000). High pressure processing of foods for microbiological safety and quality. Acta Microbiologica Et Immunologica Hungarica, 47(2–3), 175–182. doi:10.1556/AMicr.47.2000.2-3.3
- Liu, F., Li, R., Wang, Y., Bi, X., & Liao, X. (2014). Effects of high hydrostatic pressure and high-temperature short-time on mango nectars: Changes in microorganisms, acid invertase, 5-hydroxymethylfurfural, sugars, viscosity, and cloud. Innovative Food Science & Emerging Technologies, 22, 22–30. doi:10.1016/j.ifset.2013.11.014
- Lovett, J., & Peeler, J. (1973). Effect of pH on the thermal destruction kinetics of patulin in aqueous solution. Journal of Food Science, 38(6), 1094–1095. doi:10.1111/jfds.1973.38.issue-6
- Ludwig, H., van Almsick, G., & Schreck, C. (2002). The effect of hydrostatic pressure on the survival of microorganisms. In Biological systems under extreme conditions (pp. 239–256). Heidelberg, Berlin: Springer.
- Maggi, A., Gola, S., Rovere, P., Miglioli, L., Dall’Aglio, G., & Loenneborg, N. (1996). Effects of combined high pressure-temperature treatments on Clostridium sporogenes spores in liquid media. Industria Conserve, 71(1), 8–14.
- Michiels, C., Bartlett, D. H., & Aertsen, A. (2008). High-pressure microbiology. ASM Press.
- Morris, C., Brody, A. L., & Wicker, L. (2007). Non‐thermal food processing/preservation technologies: A review with packaging implications. Packaging Technology and Science: an International Journal, 20(4), 275–286. doi:10.1002/(ISSN)1099-1522
- Moussa-Ayoub, T. E., Jäger, H., Knorr, D., El-Samahy, S. K., Kroh, L. W., & Rohn, S. (2017). Impact of pulsed electric fields, high hydrostatic pressure, and thermal pasteurization on selected characteristics of Opuntia dillenii cactus juice. LWT-Food Science and Technology, 79, 534–542. doi:10.1016/j.lwt.2016.10.061
- Mukhopadhyay, S., Sokorai, K., Ukuku, D., Fan, X., & Juneja, V. (2017). Effect of high hydrostatic pressure processing on the background microbial loads and quality of cantaloupe puree. Food Research International, 91, 55–62. doi:10.1016/j.foodres.2016.11.029
- Murrell, W., & Wills, P. A. (1977). Initiation of Bacillus spore germination by hydrostatic pressure: Effect of temperature. Journal of Bacteriology, 129(3), 1272–1280.
- Nakayama, A., Yano, Y., Kobayashi, S., Ishikawa, M., & Sakai, K. (1996). Comparison of pressure resistances of spores of six bacillus strains with their heat resistances. Applied and Environmental Microbiology, 62(10), 3897–3900.
- O’Reilly, C. E., O’Connor, P. M., Kelly, A. L., Beresford, T. P., & Murphy, P. M. (2000). Use of hydrostatic pressure for inactivation of microbial contaminants in cheese. Applied and Environmental Microbiology, 66(11), 4890–4896. doi:10.1128/aem.66.11.4890-4896.2000
- Ogawa, H., Fukuhisa, K., Kubo, Y., & Fukumoto, H. (1990). Pressure inactivation of yeasts, molds, and pectinesterase in Satsuma mandarin juice: Effects of juice concentration, pH, and organic acids, and comparison with heat sanitation. Agricultural and Biological Chemistry, 54(5), 1219–1225.
- Olsson, S. (1995). Production equipment for commercial use. In D. A. Ledward, D. E. Johnston, R. G. Earnshaw, & A. P. M. Hasting (Eds.), High pressure processing of foods (pp. 167). Nottingham: Nottingham University Press.
- Osumi, M., Sato, M., Kobori, H., Feng, Z. H., Ishizima, S. A., Hamada, K., & Shimada, S. (1996). Morphological effects of pressure stress on yeast. In R. Hayashi & C. Balny (Eds.), High pressure bioscience and biotechnology (Vol. 13, pp. 37–46). The Netherlands: Progress in biotechnology. Elsevier Science B.V.
- Otero, L., Ramos, A., De Elvira, C., & Sanz, P. (2007). A model to design high-pressure processes towards an uniform temperature distribution. Journal of Food Engineering, 78(4), 1463–1470. doi:10.1016/j.jfoodeng.2006.01.020
- Paidhungat, M., Setlow, B., Daniels, W. B., Hoover, D., Papafragkou, E., & Setlow, P. (2002). Mechanisms of induction of germination of Bacillus subtilis spores by high pressure. Applied and Environmental Microbiology, 68, 3172–3175. doi:10.1128/aem.68.6.3172-3175.2002
- Palou, E., Lopez-Malo, A., Barbosa-Canovas, G., Welti-Chanes, J., & Swanson, B. (1997). Combined effect of high hydrostatic pressure and water activity on Zygosaccharomyces bailii inhibition. Letters in Applied Microbiology, 24, 417–420.
- Pandya, Y., Jewett, F. F., Jr, & Hoover, D. G. (1995). Concurrent effects of high hydrostatic pressure, acidity and heat on the destruction and injury of yeasts. Journal of Food Protection, 58(3), 301–304. doi:10.4315/0362-028X-58.3.301
- Parish, M. E. (1998). High Pressure Inactivation of Saccharomyces Cerevisiae, Endogenous Microflora and Pectin methylesterase in Orange Juice 1. Journal of Food Safety, 18(1), 57–65. doi:10.1111/jfs.1998.18.issue-1
- Park, S. W., Sohn, K. H., Shin, J. H., & Lee, H. J. (2001). High hydrostatic pressure inactivation of Lactobacillus viridescens and its effects on ultrastructure of cells. International Journal of Food Science & Technology, 36(7), 775–781. doi:10.1046/j.1365-2621.2001.00518.x
- Patterson, M. (2005). Microbiology of pressure‐treated foods. Journal of Applied Microbiology, 98(6), 1400–1409. doi:10.1111/j.1365-2672.2005.02564.x
- Patterson, M. F. (1999). High-pressure treatment of foods. In R. K. Robinson, C. A. Batt, & P. D. Patel (Eds.), Encyclopedia of food microbiology (pp. 1051–1065). New York: Academic Press.
- Patterson, M. F., & Kilpatrick, D. J. (1998). The combined effect of high hydrostatic pressure and mild heat on inactivation of pathogens in milk and poultry. Journal of Food Protection, 61(4), 432–436.
- Patterson, M. F., Quinn, M., Simpson, R., & Gilmour, A. (1995). Effect of high pressure on vegetable pathogens. In D. A. Ledward, D. E. Johnston, R. G. Earnshaw, & A. P. M. Hasting (Eds.), High pressure processing of foods (pp. 47–63). Loughborough: Nottingham University Press.
- Pauling, L. (1964). College chemistry: An introductory textbook of general chemistry. New York, NY: WH Freeman and Company.
- Perera, N., Gamage, T., Wakeling, L., Gamlath, G., & Versteeg, C. (2010). Colour and texture of apples high pressure processed in pineapple juice. Innovative Food Science & Emerging Technologies, 11(1), 39–46. doi:10.1016/j.ifset.2009.08.003
- Ponce, E., Pla, R., Capellas, M., Guamis, B., & Mor-Mur, M. (1998a). Inactivation of Escherichia coli inoculated in liquid whole egg by high hydrostatic pressure. Food Microbiology, 15(3), 265–272. doi:10.1006/fmic.1997.0164
- Ponce, E., Pla, R., Mor-Mur, M., Gervilla, R., & Guamis, B. (1998b). Inactivation of Listeria innocua inoculated in liquid whole egg by high hydrostatic pressure. Journal of Food Protection, 61(1), 119–122. doi:10.4315/0362-028X-61.1.119
- Rajan, S., Pandrangi, S., Balasubramaniam, V., & Yousef, A. E. (2006). Inactivation of Bacillus stearothermophilus spores in egg patties by pressure-assisted thermal processing. LWT-Food Science and Technology, 39(8), 844–851. doi:10.1016/j.lwt.2005.06.008
- Ramaswamy, R., Ahn, J., Balasubramaniam, V., & Yousef, A. (2013). Food safety engineering. In Myer Kutz (Ed.), Handbook of farm, dairy and food machinery engineering (pp. 43–66). Ohio State University.
- Raso, J., Barbosa-Canovas, G., & Swanson, B. (1998a). Sporulation temperature affects initiation of germination and inactivation by high hydrostatic pressure of Bacillus. Journal of Applied Microbiology, 85, 17–24. doi:10.1046/j.1365-2672.1998.00460.x
- Raso, J., Calderón, M. L., Góngora, M., Barbosa‐Cánovas, G. V., & Swanson, B. G. (1998b). Inactivation of Zygosaccharomyces bailii in fruit juices by heat, high hydrostatic pressure and pulsed electric fields. Journal of Food Science, 63(6), 1042–1044. doi:10.1111/j.1365-2621.1998.tb15850.x
- Rastogi, N., Raghavarao, K., Balasubramaniam, V., Niranjan, K., & Knorr, D. (2007). Opportunities and challenges in high pressure processing of foods. Critical Reviews in Food Science and Nutrition, 47(1), 69–112. doi:10.1080/10408390600626420
- Reddy, N., Solomon, H., Fingerhut, G., Rhodehamel, E., Balasubramaniam, V., & Palaniappan, S. (1999). Inactivation of Clostridium botulinum type E spores by high pressure processing. Journal of Food Safety, 19(4), 277–288. doi:10.1111/jfs.1999.19.issue-4
- Rendueles, E., Omer, M., Alvseike, O., Alonso-Calleja, C., Capita, R., & Prieto, M. (2011). Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT-Food Science and Technology, 44(5), 1251–1260. doi:10.1016/j.lwt.2010.11.001
- Ritz, M., Tholozan, J., Federighi, M., & Pilet, M. (2001). Morphological and physiological characterization ofListeria monocytogenes subjected to high hydrostatic pressure. Applied and Environmental Microbiology, 67(5), 2240–2247. doi:10.1128/AEM.67.5.2240-2247.2001
- Sale, A., Gould, G., & Hamilton, W. (1970). Inactivation of bacterial spores by hydrostatic pressure. Microbiology, 60(3), 323–334.
- Sasagawa, A., Yamazaki, A., Kobayashi, A., Hoshino, J., Ohshima, T., Sato, M., … Yamada, A. (2006). Inactivation of Bacillus subtilis spores by a combination of hydrostatic high-pressure and pulsed electric field treatments. The Review of High Pressure Science and Technology, 16(1), 45–53. doi:10.4131/jshpreview.16.45
- Schebb, N. H., Faber, H., Maul, R., Heus, F., Kool, J., Irth, H., & Karst, U. (2009). Analysis of glutathione adducts of patulin by means of liquid chromatography (HPLC) with biochemical detection (BCD) and electrospray ionization tandem mass spectrometry (ESI-MS/MS). Analytical and Bioanalytical Chemistry, 394(5), 1361–1373. doi:10.1007/s00216-009-2765-1
- Shahbaz, H. M., Yoo, S., Seo, B., Ghafoor, K., Kim, J. U., Lee, D.-U., & Park, J. (2016). Combination of TiO 2-UV photocatalysis and high hydrostatic pressure to inactivate bacterial pathogens and yeast in commercial apple juice. Food and Bioprocess Technology, 9(1), 182–190. doi:10.1007/s11947-015-1614-9
- Shao, Y., Zhu, S., Ramaswamy, H., & Marcotte, M. (2010). Compression heating and temperature control for high-pressure destruction of bacterial spores: An experimental method for kinetics evaluation. Food and Bioprocess Technology, 3(1), 71. doi:10.1007/s11947-008-0057-y
- Shigehisa, T., Ohmori, T., Saito, A., Taji, S., & Hayashi, R. (1991). Effects of high hydrostatic pressure on characteristics of pork slurries and inactivation of microorganisms associated with meat and meat products. International Journal of Food Microbiology, 12(2–3), 207–215.
- Silva, F. V. (2015). High pressure processing of milk: Modeling the inactivation of psychrotrophic Bacillus cereus spores at 38–70° C. Journal of Food Engineering, 165, 141–148. doi:10.1016/j.jfoodeng.2015.06.017
- Simpson, R., & Gilmour, A. (1997). The resistance of Listeria monocytogenes to high hydrostatic pressure in foods. Food Microbiology, 14(6), 567–573. doi:10.1006/fmic.1997.0117
- Smelt, J. (1998). Recent advances in the microbiology of high pressure processing. Trends in Food Science & Technology, 9(4), 152–158. doi:10.1016/S0924-2244(98)00030-2
- Spadaro, D., Ciavorella, A., Frati, S., Garibaldi, A., & Gullino, M. (2007). Incidence and level of patulin contamination in pure and mixed apple juices marketed in Italy. Food Control, 18(9), 1098–1102. doi:10.1016/j.foodcont.2006.07.007
- Ştefãnoiu, A., Tãnase, E. E., Mitelut, A. C., & Popa, M. E. (2015). Unconventional antimicrobial treatments for food safety and preservation. Scientific Bulletin Series F Biotechnologies, 12, 324–336.
- Stoev, S. D. (2016). Food security and foodborne mycotoxicoses, risk assessment, preventive measures, and underestimated hazard of masked mycotoxins or joint mycotoxin interaction. Food Toxicology, 9, 169–199.
- Tao, Y., Sun, D.-W., Hogan, E., & Kelly, A. L. (2014). High-pressure processing of foods: An overview. In Da-Wen Sun (Ed.), Emerging technologies for food processing (pp. 3–24). Academic Press.
- Terefe, N. S., Buckow, R., & Versteeg, C. (2014). Quality-related enzymes in fruit and vegetable products: Effects of novel food processing technologies, part 1: High-pressure processing. Critical Reviews in Food Science and Nutrition, 54(1), 24–63. doi:10.1080/10408398.2011.566946
- Ting, E. & Marshall, R. (2002). Production issues related to UHP food. In Jorge Welti-Chanes, Gustavo V. Barbosa-Cánovas, & Jose Miguel Aguilera (Eds.), Engineering and food for the 21st century. CRC Press. 722–733.
- Toepfl, S., Mathys, A., Heinz, V., & Knorr, D. (2006). Potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Reviews International, 22(4), 405–423. doi:10.1016/j.ijfoodmicro.2011.02.019
- Tokuşoğlu, Ö., Alpas, H., & Bozoğlu, F. (2010). High hydrostatic pressure effects on mold flora, citrinin mycotoxin, hydroxytyrosol, oleuropein phenolics and antioxidant activity of black table olives. Innovative Food Science & Emerging Technologies, 11(2), 250–258. doi:10.1016/j.ifset.2009.11.005
- Vercammen, A., Vivijs, B., Lurquin, I., & Michiels, C. W. (2012). Germination and inactivation of Bacillus coagulans and Alicyclobacillus acidoterrestris spores by high hydrostatic pressure treatment in buffer and tomato sauce. International Journal of Food Microbiology, 152(3), 162–167. doi:10.1016/j.ijfoodmicro.2011.02.019
- Vidal, A., Sanchis, V., Ramos, A. J., & Marin, S. (2016). The fate of deoxynivalenol through wheat processing to food products. Current Opinion in Food Science, 11, 34–39. doi:10.1016/j.cofs.2016.09.001
- WHO. (1995). Evaluation of certain food additives and contaminants. Technical Report Series, 859, 36–38.
- Wilson, D. R., Dabrowski, L., Stringer, S., Moezelaar, R., & Brocklehurst, T. F. (2008). High pressure in combination with elevated temperature as a method for the sterilisation of food. Trends in Food Science & Technology, 19(6), 289–299. doi:10.1016/j.tifs.2008.01.005
- Wimalaratne, S., & Farid, M. (2008). Pressure assisted thermal sterilization. Food and Bioproducts Processing, 86(4), 312–316. doi:10.1016/j.fbp.2007.08.001
- Wuytack, E. Y., Boven, S., & Michiels, C. W. (1998). Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Applied and Environmental Microbiology, 64(9), 3220–3224.
- Wuytack, E. Y., Soons, J., Poschet, F., & Michiels, C. W. (2000). Comparative study of pressure-and nutrient-induced germination of Bacillus subtilis spores. Applied and Environmental Microbiology, 66(1), 257–261. doi:10.1128/aem.66.1.257-261.2000
- Zhou, H., George, S., Hay, C., Lee, J., Qian, H., & Sun, X. (2017). Individual and combined effects of Aflatoxin B1, Deoxynivalenol and Zearalenone on HepG2 and RAW 264.7 cell lines. Food and Chemical Toxicology, 103, 18–27. doi:10.1016/j.fct.2017.02.017