?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of this study was to investigate the optimal condition for extracting dragon fruit peel (DFP) pectin with the best physicochemical properties and high antioxidant activity by using conventional extraction (CE) and ultrasound-assisted extraction (UAE). DFP was extracted using CE and UAE method at 45, 60 and 75°C for 30 and 60 min. In this study, UAE significantly improved antioxidant activity of the extracted DFP pectin when comparing to CE. DFP extracted with UAE at 45°C for 30 min had the highest antioxidant activity (51.58 ± 0.30 by DPPH, 39.81 ± 1.43 mgGAE/100 g by ABTS) (p < 0.05). Moreover, UAE also increased the production yield of sample at 45°C for 30 min (9.38 ± 0.50%) (p < 0.05) which was higher than the treatments with CE. The chemical properties of DFP pectin including equivalent weight, degree of esterification, methoxyl content and total anhydroronic acid revealed that DFP pectin could be categorized as low-methoxyl pectin. Present study demonstrated that DFP pectin was an ideal alternative source of pectin with high antioxidant activity compared with commercial citrus pectin. The optimal condition for extraction of pectin by UAE was at 45°C for 30 min which could be established as a potential method to extract pectin from DFP for industrial scale.
PUBLIC INTEREST STATEMENT
Dragon fruit (Hylocereus undatus) is commonly grown in tropical climates in the Southeast Asia such as Thailand, Vietnam and Malaysia. It is often used to eat fresh or processed into other foods such as jam, wine or another beverage. Dragon fruit peel is always removed during processing. Pectin is a complex carbohydrate molecule and it is largely employed in numerous industrial applications such as gelling agent, thickener, stabilizer and emulsifier. Since the peels of dragon fruit are often discarded as waste, existing literature and studies have shown that it would be an advantage to convert it into a value-added product such as pectin with higher antioxidant levels than commercial citrus pectin. However, there are lack of studies on comparing the extraction of pectin by conventional extraction and UAE. In addition, UAE is considered as a useful method for extracting pectin and can improve the properties of pectin with shorter extraction time and less energy consumption than conventional extraction.
Competing interests
The author declares no competing interests.
1. Introduction
Pectins are complex carbohydrate molecules mainly used as gelling agents, thickener, emulsifier and stabilizer in the food and beverage industry (Vanitha & Khan, Citation2019). It consists of 1,4-linked α-d-galacturonic acid (Levigne, Ralet, & Thibault, Citation2002). In addition, pectin is used as a fat substitute in spreads, salad dressings and ice cream (Ismail, Ramli, Hani, & Meon, Citation2012). Pectin production has been extensively studied and this included recovery of pectin from food processing by-product such as fruit wastes, for example, pomaces of grape (Minjares-Fuentes et al., Citation2014), peach (Pagan, Ibarz, Llorca, Pagan, & Barbosa-Cánovas, Citation2001) and apple (Canteri-Schemin, Fertonani, Waszczynskyj, & Wosiacki, Citation2005). Fruit wastes in the form of peels were also studied in orange, passion fruit, grapefruit and banana (Emaga, Ronkart, Robert, Wathelet, & Paquot, Citation2008; Liu, Shi, & Langrish, Citation2006; Seixas et al., Citation2014; Xu et al., Citation2014). Dragon fruit contains an abundant of pectin (Tang, Wong, & Woo, Citation2011). There are many extraction methods that have been applied to extract pectin such as conventional extraction (CE) (Ismail et al., Citation2012), microwave-assisted extraction and ultrasound-assisted extraction (UAE) (Ali, Lim, Chong, Mah, & Chua, Citation2018). Ultrasound, as one of the green technologies, was investigated to degrade and modify biopolymers with high efficiency and low cost. In an aqueous system with pectin, ultrasonication creates localized high temperature and pressure spots and produces “microjets” and free radicals, which contribute to modify the structural, functional and bioactive properties of pectin. The factors influencing pectin modification include ultrasound frequency, power intensity, temperature, treatment time and duty cycle (Wang et al., Citation2018). Furthermore, previous studies showed that the application of ultrasound assistant method resulted in shorter extraction time and higher yield (Wang et al., Citation2015) and increased antioxidant activity in the product compared to other methods (Ma, Yang, Wang, & Guo, Citation2018). From extensive literature, it was found that only a few fruits have been studied in extracting pectin using UAE, for example grapefruit (Bagherian, Zokaee, Fouladitajar, & Mohtashamy, Citation2011), yellow passion fruit (Oliveira et al., Citation2016), banana peel (Lin & Cze, Citation2018) and apple (Zhang et al., Citation2013). To the best of our knowledge, no ultrasonic method has been performed for pectin extraction from white dragon fruit peels (DFPs).
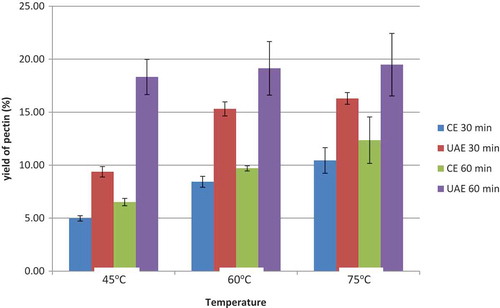
Figure 1. The yield content (%) of DFP pectin extracted with different extraction time (30 and 60 min) and temperature (45, 60 and 75°C) using CE and UAE method.
Dragon fruit (Hylocere usundatus) or white dragon fruit is widely cultivated in Asian countries such as Thailand, Vietnam, Philippine, Indonesia, Southern China and popular in Southeast Asia. Apart from being refreshing and tasty, it has high vitamin C content which contributes to the high antioxidant properties and water-soluble fiber (Jaafar, Ridhwan, Mahmod, & Vasudevan, Citation2009). Besides being consumed fresh, the fruit can be processed into beverages and other downstream products. However, the peel of the dragon fruit which makes up 20–25% of the fruit is normally discarded. This will create waste that could be transformed into a value-added product by using it as an alternative source to extract pectin. While available literature has established the beneficial properties of pectin yield and dragon fruit as its source, there are limited investigations on the effective extraction of the mentioned compound.
The objectives of this study were to evaluate the effects of CE and ultrasound-assisted extraction at different conditions on physiochemical properties and antioxidant activity of pectin from white DFP.
2. Materials and methods
2.1. Raw materials
Dragon fruits (Hylocereus undatus) were purchased from the local market in Bangkok, Thailand. It was cultivated in dry tropical climates with a moderate amount of rain in southern of Thailand. The fruits were washed with tap water and wiped to dry. The fresh peel was separated from the flesh fruit before cutting into small pieces (5 × 5 cm). After being milled, the peel was put on the tray and dried in hot air oven for 4 h at 50°C. Finally, the dried peel was ground into powder and kept at ambient temperature.
Citrus pectin bought from Thai Company (Bronson & Jacobs International Co., Ltd.) was used as control in this study.
2.2. Extraction of pectin
The extraction process was based on the method of Kratchanova, Pavlova and Panchev (Citation2004) considering several variables including time and temperature. Five gram of the peel powder was weighed and put into 250 ml conical flask, 150 ml distilled water was added (1:30 w/v) at different temperature (45, 60 and 75°C) for 30 and 60 min. CE was performed with continuous stirring in the shaking water bath (WNB 22, Becthai Bangkok Equipment & Chemical Co., Ltd.). Ultrasonic-assisted extraction (UAE) was performed by using ultrasonic equipment (GT SONIC-D6, China). Citric acid was used to maintain the pH at 2.0 during extraction process. Pectin was obtained by filtration through filter paper (Whatman No.113) and washed with 55% and then with 75% ethanol. Finally, the pectin produced was dried in an air-circulated oven at 50°C for 5 h.
2.3. Quality analysis of DFP pectin
2.3.1. Physical properties of DFP pectin
2.3.1.1. Pectin yield
Pectin yield was calculated as the ratio of the weight of dried pectin to the weight of powder taken for extraction for each extraction condition.
The pectin yield was calculated using the following equation:
where ypec (%) was the extracted pectin yield in percent (%), P was the amount of extracted pectin in g and Bi was the initial amount of dried dragon fruits peel powder (5 g).
2.3.1.2. Moisture and ash contents of DFP pectin
Moisture: To determine the moisture content, 1 g of pectin was weighed and dried at 100°C for 4 h to a constant weight (A.O.A.C, Citation1990). The moisture content (%) was calculated as follows:
Ash content: The ash content was determined using standard (AOAC Citation1990), by weighing 1 g of pectin sample into a tared crucible. Then, crucibles were ignited overnight at temperature of 550°C. Crucibles were cooled down before weighed the tared crucible with left matter. The ash content was calculated as follows:
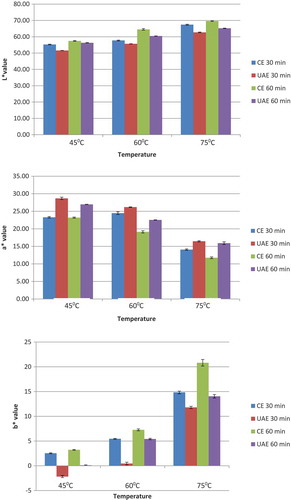
2.3.1.3. Color of DFP pectin
The color of samples expressed by the L*, a*, b* system was measured using a spectrophotometer (Minolta CM-3500d, Japan). A fixed amount of DFP pectin powder was poured into the measurement cell. Each sample was measured in triplicate.
2.3.2. Chemical properties of DFP pectin
2.3.2.1. Equivalent weight of DFP pectin
The equivalent weight (EW) was analyzed following the method of Owens et al. (Citation1952). An amount of 0.5 g of pectin was put in 250 ml conical flask and moistened it with 5 ml of ethanol for determination EW. One gram of sodium chloride was added to sharpen the endpoint. Free carbon dioxide distilled water (100 ml) and six drops of phenol red indicator were added. Titration was done slowly with 0.1 N standardized NaOH until the color of the indicator changed to pink (pH 7.5) and kept for at least 30 s. The neutralized solution was used to determine methoxyl. The following equation was used to calculate the EW:
2.3.2.2. The methoxyl content of DFP pectin
The methoxyl (MeO) content was determined by adding 25 ml of 0.25 N NaOH to the titrated solution from EW experiment (2.3.2.1), which were shaken thoroughly, and allowed to stand for 30 min at room temperature in the stoppered flask. Twenty-five milliliter of 0.25 N HCl will be added and titrated to the same endpoint pink as before. The following equation was used to calculate the methoxyl content (Owens et al., Citation1952):
where 31 is the molecular weight of the methoxyl group.
2.3.2.3. Total anhydrouronic acid content of DFP pectin
Estimation of anhydrouronic acid (AUA) content was essential to determine the purity and degree of esterification (DE), by using EW and methoxyl content value. Total AUA of pectin was obtained by the following formula (Mohamed & Hasan, Citation1995):
where molecular unit of AUA (1 U) = 176 g, z = ml of NaOH from equivalent weight determination, y = ml of NaOH from methoxyl content determination, w = weight of sample.
2.3.2.4. DE of DFP pectin
The DE was determined by using the method of Mizote, Odagir, Toei and Tanaka (Citation1975) with some modification. Fifty milligram of the DFP pectin powder was moistened with 65% isopropanol and dissolve in 10 ml of distilled water. Then, the resulting pectin was titrated with 0.1 N NaOH solution (a ml) to pH 7.5. The solution was added with 30 ml of 0.1 N NaOH and keep for 30 min, followed by the addition of 30 ml of 0.1 N HCl. The pectin solution was then titrated again with 0.1 N NaOH (b ml) to pH 7.5. DE of the each extracted pectin was calculated by using formula of DE (%) = (b/a + b) × 100% (Jiang et al., Citation2005).
2.4. Antioxidant activity of DFP pectin
2.4.1. DPPH radical scavenging assay
Antioxidant activity was determined by using DPPH (2,2-diphenyl-1-picrylhydrazyl) assay (Bedawey, Mansour, Zaky, & Hassan, Citation2010). The preparation of DPPH solution was adapted from Molyneux (Citation2003) with minor modification. DPPH 0.2 mM was prepared with methanol. Then, 1.5 ml of 0.1 g/ml of pectin extracted was pipetted into 1.5 ml DPPH solution and vortexed for 10 s. After storing at dark place for 30 min, the absorbance was read at wavelength 517 nm by using UV–vis spectrophotometer (BIOMATE 3S, Becthai Bangkok Equipment & Chemical Co., Ltd.). Calibration curve was plotted with % DPPH scavenged versus concentration of standard antioxidant (gallic acid).
2.4.2. ABTS radical scavenging assay
Free radical scavenging activity of pectin samples was determined by ABTS radical cation decolorization assay (Re et al., Citation1999) with some modifications. ABTS + cation radical was produced by the reaction between 7 mM ABTS in water and 2.45 mM potassium persulfate (1:1), stored in the dark at room temperature for 12–16 h before use. ABTS + solution was then diluted with ethanol to obtain an absorbance at 734 nm. After the addition of 1.5 ml of 0.1 g/ml of pectin extracted to 1.5 ml of diluted ABTS + solution, the absorbance was measured again at 6 min after the initial mixing. Gallic acid 10 µg/ml was used as standard substance.
3. Results and discussion
3.1. Physical properties of DFP pectin
3.1.1. Pectin yield
The yield of DFP pectin extracted with ultrasound-assisted extraction (UAE) and CE varied from 4.98% to 19.48% (dry basis), depending on the extraction conditions. The highest yield (19.48 ± 2.95%) was obtained when extracting with UAE at 75°C for 60 min while the lowest yield (4.98 ± 0.25%) was obtained with CE at 45°C for 30 min by CE (Figure ).
The yields of pectin extracted from DFP by CE for 30 min at 45, 60 and 75°C were 4.98%, 8.42% and 10.44%, respectively. Similarly, the yields when extracted by CE for 60 min at 45°C, and at 75°C were 6.51% and 12.35%, respectively. Pectin yield increased as extraction time and temperature increased. Schemin, Fertonani, Waszczynskyj and Wosiacki (Citation2005) have reported that the calcium–pectin linkage in protopectin was thermally reversible when the incubating temperature ranged from 46 to 74°C with an extrinsic calcium source (Fu & Rao, Citation1999) or from 5 to 85°C with an intrinsic calcium source (Gilsenan, Richardson, & Morris, Citation2000). The explanation is that when DFP was extracted at high temperature, the molecular motion of the pectin molecules was enhanced, resulting in structural tangling of molecular chains that would cause more junction zones to be exposed to the free calcium ions, thus have a greater effect on the release of soluble pectin from the insoluble pectin, by enhancing hydrolysis of pectin, in comparison with low temperature (Iijima, Hatakeyama, Nakamura, & Hatakeyama, Citation2002).
By UAE, the yields of pectin extracted from DFP for 30 min at 45, 60 and 75°C were 9.38%, 15.30% and 16.30%, respectively. Similar to CE, the yield of pectin increased when extraction time increased from 30 to 60 min and temperature increased from 45 to 75°C. However, the effect of time and temperature on the yield of pectin extraction by UAE was more significant than that of CE. The percentage of pectin extracted by UAE was higher than that of CE. The ultrasound has a high pressure which is throughout the solvent and effect to the tissue of DFP. Moreover, the ultrasound will destroy tissue on surface and inside of cell wall DFP so that it helps to release easier solubility substance. Some studies have explained the mechanism of ultrasound-assisted extraction through exploring the effect of ultrasound on the vegetal materials. These studies observed the disruption of cell structure of vegetal tissue exposed to ultrasound and concluded that ultrasound waves increase the accessibility of the solvent to the internal particle structure, thereby facilitating the release of the cell contents (Anese, Mirolo, Beraldo, & Lippe, Citation2013; Supardan, Fuadi, Alam, & Arpi, Citation2011). Moreover, it was reported that ultrasound also enhanced tissue hydration and swelling during steeping, which was deemed to be responsible for the increases of extraction yield in a short time (Toma, Vinatoru, Paniwnyk, & Mason, Citation2001).
3.1.2. Moisture and ash contents of DFP pectin
Table showed the moisture and ash content of DFP pectin extracted by CE and UAE. Moisture content ranged from 5.02 ± 0.65% to 7.83 ± 0.68%. The lowest moisture content by CE was 5.02% and the highest moisture content was 7.83%. The results indicated that moisture content increased as the extraction time increased from 30 to 60 min. In contrast, moisture decreased when upping the temperature from 45 to 75°C. The moisture contents of pectin samples were similar when comparing between CE, UAE and control sample. The obtained values of all samples (5.02–7.83%) were lower than 10% which can be considered as low moisture content pectin. According to Mohamadzadeh, Sadeghi-Mahoonak, Yaghbani and Aalami (Citation2010), it was noted that high moisture content could enhance the growth of microorganisms and produce pectinase enzymes that can affect pectin quality. The moisture contents found in the pectin samples were low; therefore, microorganism growth and enzyme production will be minimum.
Low ash content (below 10%) is one of the good criteria for gel formation (Ismail et al., Citation2012). The ash content indicates the purity of the pectin. The lower the ash content, the higher the level of purity pectin. Ash content of all samples was ranged from 2.66% to 4.17% which was two times lower than 10%. From that results, pectin extracted from both CE and UAE can be classified as high purity pectin.
3.1.3. Color of DFP pectin
Color of pectin is an important parameter as it affects the appearance of the gel produced. Furthermore, highly colored pectin may contain polyphenols (Baississe, Ghannem, Fahloul, & Lekbir, Citation2010) or other water-soluble pigments trapped inside the pectin during precipitation. Different extraction conditions (time and temperature) influence the pectin color. The results of the CIEL*, a* and b* parameters study are shown in Figure . As can be seen, L values of extracted pectin samples decreased in all extraction conditions, whereas a value significantly increased. The yellowness b value of all samples was significantly lower than control. These results indicated that pectin extracted from DFP contains a huge amount of pigment and polyphenols. Differences were observed particularly on the color of ultrasonic-extracted samples. Lightness increased when extraction temperature increased. It means that high temperature destroys the pigment and resulted in more purify pectin. Similarly, the increase of extraction time destroyed water-soluble components, thus increased lightness of pectin samples. a* value of all samples extracted by UAE was higher than samples extracted by CE at the same temperature and time. Overall, the a* values of citrus pectin control (9.47 ± 0.37) were lower than all DFP pectin extracted by both UAE and CE methods. This result coincided with Zahari et al. (Citation2016) who reported that DFP pectin showed greater values for redness compared to citrus pectin. This is because DFP, which has darker and redness appearance, causes a more redness pectin product to be produced.
3.2. Chemical properties of DFP pectin
3.2.1. EW
EW of pectin extracted from DFP using citric acid at various conditions was found to be range from 305.75 ± 6.43 to 446.41 ± 11.40 g. There was a significant difference between control and DFP pectin. All EW of DFP pectin samples was lower than that of citrus pectin control (445.59 ± 16.49) except sample at 45°C in 30 min by CE (446.41 ± 11.40). This result showed that the produced pectin was high partial degradation. Additionally, the results showed that extraction conditions (time, temperature) had no effect on EW of extracted samples. According to Norazelina, Ismail, Norziah, Hani and Zainudin (Citation2012), the EW of DFP pectin was range from 475.64 to 713.99 which was higher than all EW value in this research. However, pectin with the equivalent or combining weight in the range of 250–350 will form the gel in the presence of calcium or magnesium ion regardless of whether sugar is present or not. This low EW was an advantageous characteristic of pectin when applied in food. It does not need anymore the sugar in forming gelling (Aksel et al., Citation1941). This parameter as reported in the literature varies in a wide range depending on the method and the nature of the fruits used for extraction. The increase or decrease of the EW might be also dependent upon the amount of free acid (Wahengbam et al., Citation2014).
3.2.2. Methoxyl content
Methoxyl content is defined as the number of moles of methyl alcohol in 100 mol galacturonic acid. Methoxyl content of pectin is important to control the gel strength, the setting time, the sensitivity to metal ions and to determine the functional properties of pectin solutions and ability of the pectin to form gels (Constenla & Lozano, Citation2003). Spreading quality and sugar binding capacity of pectin are increased with increase methoxyl content (Madhav & Pushpalatha, Citation2002). Methoxyl content of pectin varies from 0.2% to 12% depending on the source and mode of extraction (Aina et al., Citation2012). Table showed that the methoxyl content of DFP pectin ranged from 3.69 ± 0.18% to 6.76 ± 0.03%. The methoxyl contents of DFP pectin extracted at various conditions by CE and UAE were similar to the study of Ismail et al. (Citation2012) who also reported methoxyl content of DFP pectin ranged from 2.98% to 4.34%. Methoxyl content of commercial pectins generally varies from 8% to 11% and can form high sugar gels (>65% sugar). On the other hand, low methoxyl pectins (LMPs) (less than 7.0%) can form gels with lower concentrations of sugars. The methoxyl content value from all samples in this study indicated as LMP.
Table 1. Moisture and ash content (%) of DFP pectin extracted by CE and UAE with various conditions
Table 2. Chemical composition of DFP pectin extracted by CE and UAE with various conditions
3.2.3. DE
Similar to methoxyl content, DE can be used to classify LMP with DE ≤50% and high methoxyl pectin with DE >50%. The DE of pectin extracted from DFP by CE and UAE was ranged from 26.57 ± 0.65% to 49.87 ± 0.48% and can be categorized as LMP. It is the same with citrus pectin control (39%). These results were consistent with the study from Ismail et al. (Citation2012) who reported DE from DFP pectin ranged from 31.05% to 46.96% as LMP.
3.2.4. Total AUA content
AUA content indicates the purity of the extracted pectin and is suggested to be not less than 65% (Food Chemical Codex, Citation1996). Table showed the AUA content obtained from all extraction conditions by both CE and UAE ranged from 65.29% to 87.19% which was higher than 65%. The results indicated that all extracted samples were higher in purity as compared with control citrus pectin. In this study, the results of AUA content were higher than pectin from DFP (45.25–52.45%) which was reported by Ismail et al. (Citation2012) and 39.11% reported by Muhammad, Zahari, Gannasin, Adzahan and Bakar (Citation2014).
3.3. Antioxidant activity of DFP pectin by DPPH and ABTS method
The results from Table showed that the antioxidant activity of DFP pectin extracted by CE and UAE was ranged from 12.52 ± 0.40 to 51.58 ± 0.30 mgGAE/100 g.
Table 3. Antioxidant activity of DFP pectin by DPPH and ABTS method with various conditions
DFP pectin contained the highest antioxidant activity when extracted by UAE at 45°C for 30 min while the lowest antioxidant was obtained at 75°C for 60 min by CE. When extraction time and temperature increased, the amount of antioxidant decreased. The amount of antioxidant activity of all DFP pectin samples was higher than citrus pectin (control) analyzed by DPPH and ABTS methods. The color of the dragon fruit comes from the betalain pigments, betacyanins and betaxanthins which are also known as antioxidants (Priatni & Pradita, Citation2015).
The results showed that antioxidant activity of DFP pectin extracted by UAE was higher than that of DFP pectin extracted by CE at the same extraction conditions. This study was aligned with the study of Annegowda, Anwar, Mordi, Ramanathan and Mansor (Citation2010), who reported that a higher amount of antioxidant constituents could be observed by using UAE. It was explained that the extract obtained of ultrasonic exhibits significant amounts of polyphenol and betacyanin contents. According to Ma et al. (Citation2018), UAE treatment can change the structure of betacyanin by altering beta structures. It also revealed that ultrasonicated betacyanin contained larger aggregates than untreated samples. UAE treatment can be used to improve antioxidant activities through varying the ultrasound treatment conditions. Moreover, CE using orbital shaker at high temperature involves longer extraction hours and hence resulted in degradation of heat-sensitive bioactive compounds (Ramli, Ismail, & Rahmat, Citation2014). This result also aligned with the results of color measurement which showed the redness (a*) of UAE was higher than CE, and the highest a* was also obtained by UAE at 45°C for 30 min.
4. Conclusion
In this study, UAE was a good approach to extract pectin from DFP. The result revealed that pectin yield was significantly affected by extraction temperature and time. Although the lowest yield of pectin extracted by UAE was obtained at 45°C for 30 min, it was insignificant compared with samples extracted by CE. The results from pectin physiochemical properties analysis including EW, methoxyl content and total AUA content indicated that pectin extracted from both CE and UAE was of LMP. Moreover, the extraction method and conditions did not make significant differences in structure compared with commercial pectin (control). Pectin extracted at 45°C for 30 min had the highest amount of antioxidant activity compared with other conditions. UAE was an innovative technology of high-efficiency, time-saving and low energy consumption for pectin extraction from DFP. With this study, despite dragon fruit could be an ideal pectin source with high antioxidant, there is still a lack of studies about the application of pectin in food product for using this high antioxidant pectin source in a good way.
Additional information
Funding
Notes on contributors
Bao Manh Ngoc Nguyen
Bao Manh Ngoc Nguyen was born on 20 August 1993 in Vietnam. He studied Advance Program at Nong Lam University in Ho Chi Minh city, Vietnam, from 2011 to 2015. After his graduation, he continued his Master Degree from Kasetsart University for studying in the Department of Product Development, Faculty of Agro-Industry, Bangkok, Thailand, since 2016.
Tantawan Pirak
Tantawan Pirak is an assistant professor at the Department of Product Development, Faculty of Agro-Industry, Kasetsart University, Bangkok, Thailand, since 2007 until now. She received her Ph.D. in Food Technology from Chulalongkorn University, Thailand, in 2007. Her research expertise is the development of functional ingredients and functional meat products, natural extracts and their utilization in food, chitosan and hydrocolloids in food, protein hydrolysate and functional peptides and interactions of food macromolecules with functional ingredients. Her research interest and future research is in the field of the study using in vitro gut model system.
References
- A.O.A.C. (1990). A.O.A.C. Official Methods of Analysis (14th ed.). Washington, D.C: Association of Official Analytical Chemists.
- Aina, V. O., Barau, M. M., Mamman, O. A., Zakari, A., Haruna, H., Hauwa Umar, M. S., & Abba, Y. B. (2012). Extraction and characterization of pectin from peels of lemon (Citrus limon), grape fruit (Citrus paradise) and sweet orange (Citrus sinensis). British Journal of Pharmacology and Toxicology, 3(6), 259–13.
- Ali, A., Lim, X. Y., Chong, C. H., Mah, S. H., & Chua, B. L. (2018). Ultrasound-assisted extraction of natural antioxidants from betel leaves (Piper betle): Extraction kinetics and modeling. Separation Science and Technology, 53(14), 2192–2205. doi:10.1080/01496395.2018.1443137
- Anese, M., Mirolo, G., Beraldo, P., & Lippe, G. (2013). Effect of ultrasound treatments of tomato pulp on microstructure and lycopene in vitro bio accessibility. Food Chemistry, 136, 458–463. doi:10.1016/j.foodchem.2012.08.013
- Annegowda, H. V., Anwar, L. N., Mordi, M. N., Ramanathan, S., & Mansor, S. M. (2010). Influence of sonication on the phenolic content and antioxidant activity of Terminalia catappa L. leaves. Pharmacognosy Research, 2(6), 368–373. doi:10.4103/0974-8490.75457
- Bagherian, H., Zokaee, A. F., Fouladitajar, A., & Mohtashamy, M. (2011). Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chemical Engineering and Processing: Process Intensification, 50(11–12), 1237–1243. doi:10.1016/j.cep.2011.08.002
- Baississe, S., Ghannem, H., Fahloul, D., & Lekbir, A. (2010). Comparison of structure and emulsifying activity of pectin extracted from apple pomace and apricot pulp. World Journal of Dairy & Food Sciences, 5(1), 79–84.
- Bedawey, A. A., Mansour, E. H., Zaky, M. S., & Hassan, A. A. (2010). Characteristics of antioxidant isolated from some plant sources. Food and Nutrition Sciences, 1(01), 5. doi:10.4236/fns.2010.11002
- Canteri-Schemin, M. H., Fertonani, H. C. R., Waszczynskyj, N., & Wosiacki, G. (2005). Extraction of pectin from apple pomace. Brazilian Archives of Biology and Technology, 48(2), 259–266. doi:10.1590/S1516-89132005000200013
- Constenla, D., & Lozano, J. E. (2003). Kinetic model of pectin demethylation. Latin American Applied Research, 33(2), 91–95.
- Emaga, T. H., Ronkart, S. N., Robert, C., Wathelet, B., & Paquot, M. (2008). Characterisation of pectins extracted from banana peels (Musa AAA) under different conditions using an experimental design. Food Chemistry, 108(2), 463–471. doi:10.1016/j.foodchem.2007.10.078
- Food Chemical Codex. (1996). IV monographs (p. 283). Washington, DC: National Academy Press.
- Fu, J. T., & Rao, M. A. (1999). The influence of sucrose and sorbitol on gel–Sol transition of low-methoxyl pectin+ Ca2+ gels. Food Hydrocolloids, 13(5), 371–380. doi:10.1016/S0268-005X(99)00022-3
- Gilsenan, P. M., Richardson, R. K., & Morris, E. R. (2000). Thermally reversible acid-induced gelation of low-methoxy pectin. Carbohydrate Polymers, 41(4), 339–349. doi:10.1016/S0144-8617(99)00119-8
- Iijima, M., Hatakeyama, T., Nakamura, K., & Hatakeyama, H. (2002). Effect of annealing on calcium pectin gel formation by thermo mechanical analysis. Journal of Thermal Analysis and Calorimetry, 70(3), 815.
- Ismail, N. S. M., Ramli, N., Hani, N. M., & Meon, Z. (2012). Extraction and characterization of pectin from dragon fruit (Hylocereuspolyrhizus) using various extraction conditions. Malaysiana Science (In Malay), 41(1), 41–45.
- Jaafar, R. A., Ridhwan, A., Mahmod, N. Z. C., & Vasudevan, R. (2009). Proximate analysis of dragon fruit (Hylecereus polyhizus). American Journal of Applied Sciences, 6(7), 1341–1346. doi:10.3844/ajassp.2009.1341.1346
- Jiang, C. M., Liu, S. C., Wu, M. C., Chang, W. H., & Chang, H. M. (2005). Determination of the degree of esterification of alkaline de-esterified pectins by capillary zone eletrophoresis. Food Chemistry, 91, 551–555.
- Kratchanova, M., Pavlova, E., & Panchev, I. (2004). The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydrate Polymers, 56(2), 181–185. doi:10.1016/j.carbpol.2004.01.009
- Levigne, S., Ralet, M. C., & Thibault, J. F. (2002). Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydrate Polymers, 49, 145–153. doi:10.1016/S0144-8617(01)00314-9
- Lin, C. B., & Cze, C. Y. (2018). Drying kinetics and optimisation of pectin extraction from banana peels via response surface methodology. In MATEC web of conferences (Vol. 152, p. 01002). EDP Sciences.
- Liu, Y., Shi, J., & Langrish, T. (2006). Water-based extraction of pectin from flavedo and albedo of orange peels. Chemical Engineering Journal, 120(3), 203–209. doi:10.1016/j.cej.2006.02.015
- Ma, S., Yang, X., Wang, C., & Guo, M. (2018). Effect of ultrasound treatment on antioxidant activity and structure of β-Lactoglobulin using the Box–Behnken design. CyTA - Journal of Food, 16(1), 596–606. doi:10.1080/19476337.2018.1441909
- Madhav, A., & Pushpalatha, P. B. (2002). Characterization of pectin extracted from different fruit wastes. Journal of Tropical Agriculture, 40(1–2), 53–55.
- Minjares-Fuentes, R., Femenia, A., Garau, M., Meza-Velázquez, J., Simal, S., & Rosselló, C. (2014). Ultrasound-assisted extraction of pectins from grape pomace using citric acid: A response surface methodology approach. Carbohydrate Polymers, 106, 179–189. doi:10.1016/j.carbpol.2014.02.013
- Mizote, A., Odagir, H., Toei, K., & Tanaka, K. (1975). Determination of residues of carboxylic acids (mainly galacturonic acid) and their degree of esterification in industrial pectins by colloid titration with Cat-Floc. Analysis Journal, 100, 822–827.
- Mohamadzadeh, J., Sadeghi-Mahoonak, A. R., Yaghbani, M., & Aalami, M. (2010). Extraction of pectin from sunflower head residues of selected iranian cultivars. World Applied Science Journal, 8(1), 21–24.
- Mohamed, S., & Hasan, Z. (1995). Extraction and characterization of pectin from various tropical agro wastes. ASEAN Food Journal, 10(2), 143–150.
- Molyneux, P. (2003). The use of stable free radical diphenyl-picrylhydrazyl (DPPH) for estimating antioxidant activity. Journal of Science and Technology, 26(2), 211–219.
- Muhammad, K., Zahari, N. I. M., Gannasin, S. P., Adzahan, N. M., & Bakar, J. (2014). High methoxyl pectin from dragon fruit (Hylocereus polyrhizus) peel. Food Hydrocolloid, 42, 289–297. doi:10.1016/j.foodhyd.2014.03.021
- Norazelina, S. M., Ismail, N., Norziah, R., Hani, M., & Zainudin, M. (2012). Extraction and Characterization of Pectin from Dragon Fruit (Hylocereus polyrhizus) using Various Extraction Conditions. Sains Malaysiana, 41(1), 41–45.
- Oliveira, C. D. E., Giordani, D., Lutckemier, R., Gurak, P. D., Cladera-Olivera, F., & Marczak, L. D. F. (2016). Extraction of pectin from passion fruit peel assisted by ultrasound. LWT - Food Science and Technology, 71, 110–115. doi:10.1016/j.lwt.2016.03.027
- Olsen, A. G., Summit, Fehlberg, E. R., & Rutherford, N. L. (1941), assignors to General Foods Corporation, New York Delaware N. Y., a corporation of No Drawing. Application January 14, 1941, Serial No. 374,400.
- Owens, H. S., McCready, R. M., Shepard, A. D., Schultz, T. H., Pippen, E. L., Swenson, H. A., … Erlandsen, R. F. (1952). Methods used at Western Regional Research Laboratory for Extraction of Pectic Materials (pp. 9). USDA Bur. Agric. Ind. Chem.
- Pagan, J., Ibarz, A., Llorca, M., Pagan, A., & Barbosa-Cánovas, G. (2001). Extraction and characterization of pectin from stored peach pomace. Food Research International, 34(7), 605–612. doi:10.1016/S0963-9969(01)00078-3
- Priatni, S., & Pradita, A. (2015). Stability study of betacyanin extract from red dragon fruit (Hylocereus polyrhizus) peels. Procedia Chemistry, 16, 438–444. doi:10.1016/j.proche.2015.12.076
- Ramli, N. S., Ismail, P., & Rahmat, A. (2014). Influence of conventional and ultrasonic-assisted extraction on phenolic contents, betacyanin contents, and antioxidant capacity of red dragon fruit (Hylocereus polyrhizus). The Scientific World Journal, 2014. doi:10.1155/2014/964731
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237.
- Schemin, M. H. C., Fertonani, H. C. R., Waszczynskyj, N., & Wosiacki, G. (2005). Extraction of pectin from apple pomace. Brazilian Archives of Biology and Technology, 48(2), 259–266. doi:10.1590/S1516-89132005000200013
- Seixas, F. L., Fukuda, D. L., Turbiani, F. R. B., Garcia, P. S., Petkowicz, C. L. D. O., Jagadevan, S., & Gimenes, M. L. (2014). Extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) by microwave-induced heating. Food Hydrocolloids, 38, 186–192. doi:10.1016/j.foodhyd.2013.12.001
- Supardan, M. D., Fuadi, A., Alam, P. N., & Arpi, N. (2011). Solvent extraction of Ginger Oleoresin using ultrasound. MAKARA of Science Series, 15(2), 163–167.
- Tang, P., Wong, C., & Woo, K. (2011). Optimization of pectin extraction from peel of dragon fruit (Hylocereus polyrhizus). Asian Journal of Biological Sciences, 4(2), 189–195. doi:10.3923/ajbs.2011.189.195
- Toma, M., Vinatoru, M., Paniwnyk, L., & Mason, T. J. (2001). Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrasonics Sonochemistry, 8(2), 137–142. doi:10.1016/S1350-4177(00)00033-X
- Vanitha, T., & Khan, M. (2019). Role of pectin in food processing and food packaging. In Pectins-Extraction, Purification, Characterization and Applications. IntechOpen.
- Wahengbam, E., Shukla, R., Bala, L., Kumar, K., Mishra, A., Chandra Yadav, K., & Professor, A. (2014). Extraction of pectin from citrus fruit peel and its utilization in preparation of jelly. International Journal of Engineering Research, 3(5).
- Wang, W., Chen, W., Zou, M., Lv, R., Wang, D., Hou, F., & Liu, D. (2018). Applications of power ultrasound in oriented modification and degradation of pectin: A review. Journal of Food Engineering, 234, 98–107. doi:10.1016/j.jfoodeng.2018.04.016
- Wang, W., Ma, X., Xu, Y., Cao, Y., Jiang, Z., Ding, T., & Liu, D. (2015). Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chemistry, 178, 106–114. doi:10.1016/j.foodchem.2015.01.080
- Xu, Y., Zhang, L., Bailina, Y., Ge, Z., Ding, T., Ye, X., & Liu, D. (2014). Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. Journal of Food Engineering, 126, 72–81. doi:10.1016/j.jfoodeng.2013.11.004
- Zahari, M., Izalin, N., Muhammad, S., Kharidah, S., Bakar, J., & MohdAdzahan, N. (2016). Functional properties of pectin from dragon fruit (Hylocereus polyrhizus) peel and its sensory attributes. Journal of Tropical Agriculture and Food Science, 44(1), 95–101. ISSN 1394-9829; ESSN: 2289-9650.
- Zhang, L., Ye, X., Ding, T., Sun, X., Xu, Y., & Liu, D. (2013). Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrasonics Sonochemistry, 20(1), 222–231. doi:10.1016/j.ultsonch.2012.07.021