Abstract
Despite maize’s current high productivity, higher than other major cereal crops, it is still below its potential, mainly due to many biotic and abiotic factors causing yield losses. The fall armyworm (FAW, Spodoptera frugiperda) is among the major factors which have contributed to the low productivity of maize in Ethiopia since its introduction in February, 2017. Now its infestation covers about 642.2 thousand hectares of which, 640.8 thousand hectares are in 144 districts of six major maize-growing regional states, namely Benishangul-Gumuz, Amhara, Tigray, Gambella, Oromia, and SNNPs (Southern Nations Nationalities and Peoples). FAW, a new destructive insect pest, is one of the major problems for agricultural crop production, especially maize in Ethiopia under warm and humid conditions. This is due to its ability to breed rapidly, migrate, and feed on a wide range of host plants, all of which makes it very difficult to control. Nonetheless, there are several ways of managing the pest as reported in other parts of the world that can potentially be adapted and/or validated and used in Ethiopia. Hence, to manage this pest we have to use different management options. Integrated pest management (cultural, chemical, and biological) is commonly used for controlling FAW infestations. Therefore, in this paper, we review the status and control measures of the fall armyworm, which could be useful to improve its management in maize fields in Ethiopia.
PUBLIC INTEREST STATEMENT
Current maize productivity is below its potential, despite being higher than that of other major cereal crops. The low yield is attributed to a combination of several production constraints mainly lack of improved production technologies such as varieties and pest management practices, moisture stress, low fertility and poor cultural practices. Fall armyworm (FAW) is among the key factors that cause serious damage to maize crop production under warm and humid condition today. Therefore, effective control should focus since it is impossible to avoid this pest unless developing sustainable management.
Competing Interests
The authors declares no competing interests.
1. Introduction
Maize (Zea mays L.) is one of the main and popular cereal crops due to its high value as a stable food, as well as its stover demand for animal feed and fuel and even for construction purposes (Abebe & Feyisa, Citation2017). Maize is also the most important staple crop in terms of calorie intake in Ethiopian rural families. Approximately 88% of maize produced in Ethiopia is used as food, in both green cobs and grain (Nigussie, Tanner, & Twumasi-Afriyie, Citation2002). Because of its multiple advantages, it ranks second in production area, next to teff, but first in its productivity among major cereal crops (Abate et al., Citation2015). Therefore, it is one of the high priority crops to feed the ever-increasing Ethiopian population (Nigussie et al., Citation2002)
Current maize productivity is below its potential, although still higher than that of other major cereal crops. The low yield is attributed to a combination of several production constraints mainly lack of improved production technologies such as pest management practices, moisture stress, low fertility and poor cultural practices (Tufa & Ketema, Citation2016). Arthropod pests are among the key factors contributing to low yields facing maize production today. More than 40 species of insects have been recorded on maize in the field. Of these pests, the maize stalk borer (Busseola fusca), spotted stalk borer (Chilo partellus), and various termite species (Macrotermes and Microtermes spp.) have long been recognized as key pests, but a more recent invasive species, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), commonly named fall armyworm (FAW), is now the major insect pest causing substantial yield losses of maize in Ethiopia.
FAW is a polyphagous lepidopteran pest that is indigenous throughout the Americas (Blanco et al., Citation2016; Cruz et al., Citation2012; Prasanna, Huesing, Eddy, & Peschke, Citation2018). It can be very destructive during (June through August) when minimum average temperature exceeds 10°C, feeding in large numbers on the leaves and stems of more than 100 plant species (Pogue, Citation2002). Economically important cultivated crops include maize, millet, wheat, potato, soybean, cowpea, peanuts, sorghum, rice, sugarcane, even vegetables and cotton (CABI, Citation2017c; Pogue, Citation2002).
Even though it has been regularly intercepted in intercontinental trade (CABI, Citation2017a; Jeger et al., Citation2017), it has not previously become established outside the Americas. However, it has now invaded Africa (Day et al., Citation2017; Goergen, Kumar, Sankung, Togola, & Tamo, Citation2016) and is rapidly spreading throughout tropical and subtropical regions of the continent. It was first detected in 2016, in Nigeria, Sao Tome and Principe, Benin, and recently in Togo. The FAW was intercepted on a few hectares of irrigated maize fields in southern Ethiopia in February, 2017, and is now distributed over about 640.8 thousand hectares in 144 districts in six of the major maize-growing regional states, namely Benishangul-Gumuz, Amhara, Tigray, Gambella, Oromia and SNNPs (Tesfaye, Personal Communication, 16 October 2017).
FAW is a migratory insect pest known to cause serious damage to maize crops under warm and humid conditions in the Americas (Ayala, Navarro, & Virla, Citation2013; Clark et al., Citation2007; Luginbill, Citation1928b). According to Zebdewos Salato (Personal communication on 30 January 2017), Director of the Plant Protection Directorate at the Ethiopian Ministry of Agriculture and Natural Resources, planting maize fields during the warmer and moister summer season provides a favourable environment for the insect to quickly multiply and spread to more areas. Aggregations of moths from a single generation can spread quickly more than 500 km away from the point of emergence aided by wind fronts (FAO, Citation2017; Pogue, Citation2002) until they are sexually mature and ready to mate (Rose, Silversides, & Lindquist, Citation1975). Accordingly, the main purpose of this review is to highlight the status and control measures of this new devastating and challenging insect pest in maize fields of Ethiopia.
1.1. Biology and distribution of fall armyworm in Ethiopia
1.1.1. Biology
The fall armyworm, Spodoptera frugiperda (J.E. Smith), is a moth in the noctuid family indigenous to the Western Hemisphere where it has long been a major agricultural problem for both continents (North and South America) (Nagoshi, Meagher, & Hay‐Roe, Citation2012). It is primarily a pest of maize but has a wide host range and is capable of feeding on over 80 plant species, periodically causing significant economic damage to maize, rice, sorghum, millet, soybean, wheat, alfalfa, cotton, turf, and fodder crops (CABI, Citation2017c; Pogue, Citation2002)
Warm, humid growing seasons with heavy rainfall favour its survival and populaiton buildup, because it cannot develop at temperatures below about 10°c (Stokstad, Citation2017). Adults of FAW are nocturnal (CABI, Citation2017b). After a pre-oviposition period of three to four days, the female normally deposits most of her eggs during the first four to five days of life, but some oviposition occurs for up to three weeks (Prasanna et al., Citation2018). Duration of adult life averages about 10 days, with a range of about 7 to 21 days (Capinera, Citation2000) and due to the duration of the lifecycle, 2 to 10 generations can be completed in each cropping cycle depending on climate. It is reproductively efficient in tropical areas, where the warmer temperature allows more generations per year compared to temperate areas that may have two or fewer generations in a year. In some tropical and subtropical regions (areas without frost) fall armyworms can produce up to 10 generations during a year (Metcalf, Flint, & Metcalf, Citation1965). The number of eggs per female during its lifetime ranged from 1,342 up to 1,844 when larvae were fed millet or corn and soybean leaves, respectively, and 1,839 eggs when fed on cotton (Barros, Torres, & Bueno, Citation2010). Like all holometabolous, it has four stages to complete its lifecycle namely, egg, larva, pupa and adult (Figure ).
Figure 1. The lifecycle of fall armyworm—length of time in each life stage depends on temperatures. For green caption blurb—female can lay up to a total of 2,000 eggs.
Source: photo by (FAO, Citation2017) (African Department of Agriculture, Forestry, and Fisheries).
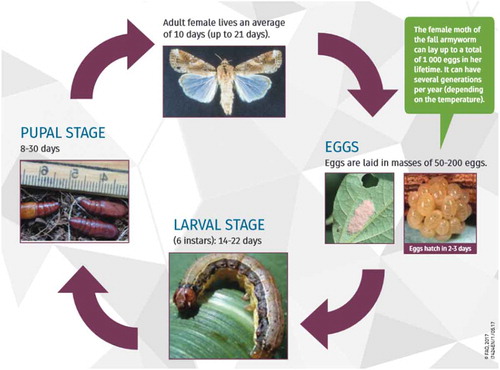
Eggs are usually laid on the upper surface of leaves but occasionally may be deposited on other parts of the host plants. The number of eggs per mass can vary from 100 to 200 (Prasanna et al., Citation2018). Eggs hatch in two to four days at temperatures ranges of 21–27 °C. The larvae develop through six developmental instars (Table ), with the last instar causing over 70% of the overall damage. A single larva can consume about 140cm2 of maize leaf area to complete the larval development period. Duration of the larval stage is about 14 days during the summer or 30 days during cool weather (Capinera, Citation2000). Pupation normally takes place in the soil at a depth 2 to 8 cm (CABI, Citation2017b; Capinera, Citation2000). The larva constructs a loose cocoon, oval in shape and 20 to 30 mm in length, by tying together particles of soil with silk. If the soil is too hard, larvae may web together leaf debris and other material to form a cocoon on the soil surface. The pupal stage of FAW cannot enter a diapause period to withstand protracted periods of cold weather or a dry season without host plants (Sparks, Citation1979). Duration of the pupal stage is about eight to nine days during the summer, but reaches 20 to 30 days during the winter in Florida (Silva et al., Citation2017).
The older larvae of FAW exhibit a cannibalistic behaviour on other smaller larvae, when they co-occur. Cannibalism was found to account for approximately 40% mortality when maize plants were infested with two or four fourth-instar larvae over a three-day period (Chapman et al., Citation2000). This behaviour, which is different from that of African armyworm (Spodoptera exempta), is accentuated when food is limited and larvae are crowded (Chapman et al., Citation2000). The role of this density-dependent mortality in the overall population dynamics is unclear (Chapman et al., Citation1999) but could be an important factor that may reduce the intensity of some outbreaks, although the experience in Africa shows clearly that it does not prevent outbreaks. The high capacity for dispersal of the newly hatched larvae (Pannuti et al., Citation2016; Rojas, Kolomiets, & Bernal, Citation2018) should minimize cannibalism when population levels are low.
Table 1. Different larval stages of fall armyworm
1.1.2. Distinguishing features of fall armyworm
The egg of FAW is dome-shaped with a flattened base that measures about 0.4 mm in diameter and 0.3 mm in height (Prasanna et al., Citation2018). There are six instars in fall armyworm as it is described above in Table . For instars 1–6, head capsule widths are about 0.35, 0.45, 0.75, 1.3, 2.0, and 2.6 mm, and body lengths are about1.7, 3.5, 6.4, 10.0, 17.2, and 34.2 mm, respectively (Capinera, Citation2000). First instar larvae are greenish with a black head, the head-turning orangish in the second instar. During the third instar, the dorsal surface of the body becomes brownish, and lateral white lines begin to form. In the fourth to the sixth instars, the head is reddish brown, mottled with white, and the brownish body bears white subdorsal and lateral lines. The face of the mature larva is also marked with a white inverted “Y” and the epidermis of the larva is rough or granular in texture when examined closely (Prasanna et al., Citation2018) and the four black dots arranged in a square on the back of the last abdominal segment are also distinctive to FAW larvae (CABI, Citation2017b). Elevated spots occur dorsally on the body, which is usually dark in colour, and bear spines (CABI, Citation2017b; Capinera, Citation2000). Newly hatched larvae are burrowing and feed on the leaves of the host plant on which the eggs were deposited, but when they grow larger they will disperse to other plants (CABI, Citation2017c). The first and second instars feed on one side of the leaf and skeletonize it, but as they grow they feed, making holes on the leaf. Adult moths of FAW are variable in colour and wingspan (32 to 40 mm). Male moths have a shaded grey and brown forewing with triangular white spots at the tip and near the centre of the wing. Forewings of females are less distinctly marked, ranging from a uniform greyish brown to a fine mottling of grey and brown. The hind wing of both sexes is shining silver-white with a narrow dark border (Prasanna et al., Citation2018).
1.1.3. Distribution of fall armyworm in Ethiopia
Ethiopia is perhaps one of the recent African countries to recently confirm the presence of FAW, in 2017. The country has been severely affected by the pest in this short time of its introduction. As of early June 2017, FAW was confirmed in six major maize-producing regions, as indicated above, including the remote rural areas of the Gamo-gofa zone of south-western Ethiopia, where Nuru Ethiopia (NE), an international agriculture project operates. FAW is a tropical species adapted to the warmer climates with average temperatures between 10.9–30°C. Stages of larvae are killed at lower temperatures, while the wings of the adult FAW tend to be deformed at temperatures above 30°C (Simmons, Citation1993). In most parts of Ethiopia, there are lower temperatures during summer (June–August) and winter (October–January). Nonetheless, except in the extreme highlands where the temperature can sometimes fall below 0°C (and where maize is not widely grown), the minimum average temperature remains above 10°C throughout the year, which creates favourable conditions for the development of FAW. According to a recent media briefing by the Ethiopian Ministry of Agriculture and Natural Resources, FAW infestations were first reported in February, 2017, in irrigated maize fields in the Bench Maji Zone of southern Ethiopia. Later, at the beginning of the summer (the main rainy season which is June to September in Ethiopia) in 2017, the pest had spread out to six administrative regions mentioned below (Figure ) which is a maximum distance of 940 km to Tigray region, and a minimum of 480 km to Oromia. The FAW is still challenging to maize farmers and investors in Oromia, Amahara, Tigray, Gambella, Beninshangul and SNNPs.
Figure 2. Distribution of Fall Army Worm in Ethiopia on 16 June 2017.
Source: http://www.agri-learning-ethiopia.org/wpcontent/uploads/2015/10/AKLDP_Armyworm_brief_online.pdf
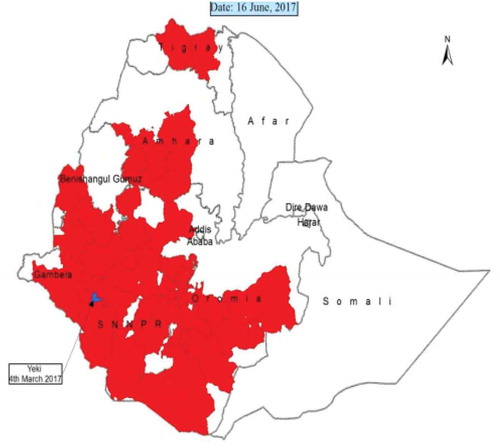
In another report made by the Ministry of Agriculture and Natural Resources (Citation2017), maize cultivated in 145 hectares of land in Somali and 1,224 hectares of land in Afar has been infested by the FAW. Currently, 342,708 hectares of maize in Oromia; 133,705 hectares in the SNNPs; 36,677 hectares in Binishangul Gumuz; 122,520 hectares in Amhara and 5,230 hectares in Tigray regions have also been infested by the armyworm (Tesfaye, personal Communication, 16 October 2017).
Using wind currents and storm fronts, FAW’s of a single generation can rapidly spread over 500 kilometers (Nagoshi, Meagher, & Jenkins, Citation2010; Westbrook, Nagoshi, Meagher, Fleischer, & Jairam, Citation2016). It has been reported that the pest spread over 46,320 hectares in three months since its first entrance to Ethiopia’s southern part which is on the border of Kenya
1.2. Damage of maize crop by fall armyworm
Continental research centers and developmental organizations have indicated that fall armyworms have become the most destructive pest in reducing maize production in Africa (Abrahams et al., Citation2017).
In recent years, Ethiopia has been faced with different disasters, including diseases, insect pests, invasive weed species and drought, all of which have had a negative impact on crop productivity.
FAW’s cause significant damage to a number of economically important cultivated grasses including maize, rice, sorghum and sugarcane, but also to vegetables and cotton. Previous damage evaluations (Hruska & Gould, Citation1997) have shown that infestations during mid-to-late maize growth stages can result in yield losses of 15–73% (as 55–100% of the plants are infested) and similarly an evaluation in Ethiopia indicated that FAW caused up to 30% loss at the late whorl stage unless the pest is timely controlled (Fentahun, Personal Communication, 26 October 2017). Depending on the growth stage of maize, fall armyworm larvae are found on young leaves, leaf whorls, tassels or cobs (Goergen et al., Citation2016). Therefore, infestation levels of the pest on these parts of the plants were assessed, non-destructively, using methodologies adapted from (Midega, Bruce, Pickett, & Khan, Citation2015). Marenco, Foster, and Sanchez (Citation1992) also indicated that infestation by FAW on sweet corn causes more injury at the late whorl stage compared to early and mid-whorl stages. But, better plant recovery happens during early developmental stages (Hanway, Citation1969). Pannuti, Baldin, Hunt, and Paula-Moraes (Citation2015) studied larval feeding behaviour, and reported that although young (vegetative stage) leaf tissue is suitable for growth and survival, on more mature plants the leaf tissue is unsuitable, and the larvae tend to settle and feed in the ear zone, and particularly on the silk tissues. As shown in Figure ) below, FAW caterpillars appear to be much more damaging than African armyworms (Spodoptera exempta) to maize in late whorl stages relative to early in the mid-stage (IITA, Citation2016).
Figure 3. (a) Corn ear damaged by caterpillar of Spodoptera frugiperda (CABI, Citation2017a) and (b) Fifth instar larva of S. frugiperda (Benin, Calavi Akassato, 2.vi.2016, G. Goergen)Photo by GG. https://doi.org/10.1371/journal.pone.0165632.g003.

This is due to (a) African armyworms first build up dense populations on wild grasses before older larvae move onto cultivated graminaceous crops (Rose, Dewhurst, & Page, Citation2000), while adult females of FAW directly oviposit on maize. (b) Unlike African armyworm (Spodoptera exempta), the mandibles of caterpillars of the fall armyworm have comparatively stronger, serrated cutting edges, which better allows the feeding on plants with high silica content (Brown & Dewhurst, Citation1975; Pogue, Citation2002). (c) Older larvae (Figure )) become cannibalistic and have the ability to dominate interspecific competitors and reduce intraspecific rivals (Chapman et al., Citation2000).
According to Roger Day, Coordinator of the Center for Agriculture and Biosciences International (CABI, Citation2017a) a conservative estimation indicates that maize losses could amount to $ 3 billion for the African continent in the coming year because of FAW that threatens the food security of millions of households and for years to come. If not controlled, FAW can potentially devastate thousands of hectares of crop farmland, especially maize. FAW damage to maize may be observed in all plant parts depending on the development stages of the pest. Larger caterpillars act as cutworms by entirely sectioning the stem base of maize seedlings. Following hatching, neonate larvae usually bore into the host plant and develop under protected conditions.
1.3. Control measures for fall armyworm
Detecting fall armyworm infestations before they cause economic damage is the key to their management. On maize, if 5% of seedlings are cut or 20% of whorls of small plants (during the first 30 days) are infested with FAW, it is recommended to apply an effective control measure to prevent further damage (Fernández, Citation2002). In America and Brazil, different strategies have been used to manage FAW including cultural practices, biological control using [parasitoids (Cotesia marginiventris (Cresson), Chelonus texanus (Cresson) and Archytas marmoratus (Townsend))] (Luginbill, Citation1928a; Vickery, Citation1929), predators (birds, rodents, beetles, earwigs (Pair & Gross, Citation1984); and pathogens [(Spodoptera frugiperda nuclear polyhedrosis virus (NPV), Entomophaga aulicae, Nomuraea rileyi, and Erynia radicans (Gardner & Fuxa, Citation1980) and botanicals (Gross & Pair, Citation1986)].
The proper timing for applying the above management options is very important for effective pest control, also taking into consideration the FAW larval stages cycle and the time of day for application.
1.4. Cultural control
Cultural control is an important component of a pest management strategy for FAW. Maize only cropping systems offer a favourable environment for FAW to spread fast. According to Bahiru Setegna (personal communication on 18 June 2017), control of armyworm is possible through a combination of cultural and chemical control methods. The cultural control includes avoiding late planting since the maize ears would be heavily attacked by a higher FAW infestation than those of the early plantings. Also, intercropping and rotating maize with non-host crops like sunflower and bean may be useful to minimize the invasion of FAW (FAO, Citation2018). Most subsistence farmers in Africa also do not apply pesticides to maize to control pests; nevertheless, they do practice cultural control methods which deter or kill pests, such as maize intercropping, handpicking and killing of caterpillars, application of wood ashes and soils to leaf whorls (Abate et al., Citation2000). A survey conducted in Ethiopia and Kenya showed that 14% and 39% of the farmers practiced cultural methods (such as handpicking), respectively, for FAW management (Kumela et al., Citation2018). The farmers who adopt a mechanical method are controlling the pest up to 54% (Fentahun, Personal Communication, 26 October 2017).
1.5. Biological control
Biological control can be considered as a powerful tool and one of the most important alternative control measures providing environmentally safe and sustainable plant protection. The success of biological control depends on understanding the adaptation and establishment of applied biological control agents in agricultural ecosystems. Microbial pathogens and arthropod biocontrol agents have been successfully used in agricultural systems (Pilkington, Messelink, van Lenteren, & Le Mottee, Citation2010). Their production costs have been significantly reduced in recent times as they are mass produced in liquid media (such as; bacteria, fungi and nematodes) (Mahmoud, Citation2016). Even though biological control may not replace conventional insecticides, a number of parasitoids, predators and pathogens readily attack larval and adult stages of FAW.
The migratory behavior of the FAW makes the natural enemies less efficient. Various insects have been reported parasitizing FAW larvae and eggs. Ashley (Citation1979) listed 53 species of parasitoids reared from FAW eggs and larvae. Only 18 of these are common to the continental United States, while 21 are present in South America and Central America, including Mexico. Ashley (Citation1986) studied the impact on FAW population of eight native and one imported parasite in south Florida. These included: Apanteles marginiventris, Campoletis grioti, Chelonus insularis, Meteorus autographae, Ophion spp., Rogus laphygmae, Ternelucha spp. and Eiphosoma vitticole (imported). Although 63% of the first four larval instars were parasitized by these species, they concluded that FAW has the reproductive potential to increase its population beyond regulation by native parasites.
In Kenya, a tachinid fly, Archytas marmoratus was the main parasitoid with 12.5% parasitism. Charops ater and Coccygidium luteum were commonly encountered parasitoids in Kenya and Tanzania where parasitism ranged from 6–12% and 4–8.3%, respectively (Birhanu et al., Citation2018).
According to Birhanu (Citation2018); three species of parasitoids namely, Cotesia icipe (Hymenoptera: Braconidae), Palexorista zonata (Diptera: Tachinidae) and Charops ater (Hymenoptera: Icheneumonidae) were recovered from FAW larvae in eleven districts of Ethiopia. Cotesia icipe was the most common parasitoid that emerged in Hawassa, Jimma (Southwestern Ethiopia) and Awash Melkassa surveyed areas. The parasitism ranged from 33.8 to 45.3% in Awash Melkassa and Jimma, respectively. On the other hand, parasitism by a tachinid fly, Palexorista zonata and Charops ater was relatively low (6.4%) (Table ). Recruitment and host adaptation of native parasitoids to FAW suggests the potential for biological control of the pest. Other studies have reported invasive pest situations whereby native parasitoids have shifted eventually to the new host, resulting in increased mortality and population suppression (Matošević & Melika, Citation2013; Vercher, Costa-Comelles, Marzal, & García-Marí, Citation2005).
Table 2. List of identified FAW parasitoids collected from some parts of Ethiopia and their mean per cent parasitism
The predators of FAW are generalists that attack larvae of other lepidopterans. In the Americas, the most important predators of FAW that have been reported include various ground beetles (Coleoptera: Carabidae); the striped earwig, Labidura riparia (Pallas) (Dermaptera: Forficulidae, Labiduridae), Doru luteips, D. lineare, and other earwigs (Jones, Gilstrap, & Andrews, Citation1988; Romero Sueldo, Dode, & Virla, Citation2014; Silva et al., Citation2018); the spined soldier bug, Podisus maculiventris (Hemiptera: Pentatomidae); and the insidious flower bug, Orius insidiosus (Hemiptera: Anthocoridae) (Capinera, Citation2001). Among the vertebrate predators, birds, skunks, and rodents also feed on larvae and pupae of FAW (Capinera, Citation2000).
The FAW is reported to be susceptible to at least 16 species of entomopathogens including viruses, fungi, protozoa, bacteria, and nematodes (Agudelo-Silva, Citation1986; Fuxa, Citation1982; Gardner & Fuxa, Citation1980; Molina Ochoa et al., Citation1996; Richter & Fuxa, Citation1990), but their occurrence and distribution may vary with their habitat. Moreover, geographical location, agricultural practices, and insecticides use have an impact on the occurrence of natural control agents that potentially help to manage fall armyworm populations (Fargues & Rodriguez-Rueda, Citation1980; Miętkiewicz, Dzięgielewska, & Janowicz, Citation1998; Sosa-Gomez & Moscardi, Citation1994; Vänninen, Citation1996).
Among the pathogens, Bacillus thuringiensis, Metarhizium anisopliae and Beauveria bassiana can cause significant mortality in FAW populations and help to reduce leaf defoliation in crops (Molina-Ochoa et al., Citation2003). These authors reported 3.5 % FAW larval mortality in Mexico due to naturally occurring entomopathogens and parasitic nematodes. Hence,1.5% microbial insecticides are only used in of the Mexican agricultural land (Blanco et al., Citation2014)
Several microbial pathogens have been studied in hopes of utilizing them to control fall armyworm populations. Viruses have demonstrated limited efficacy against fall armyworm, which is not temporally effective, allowing for significant damage prior to insect mortality (Sparks, Citation1986).
1.6. Botanical control
The use of botanical pesticides is recommended as a substitute to hazardous synthetic insecticides, such as pyrethroids and organophosphorus which may result in disturbances in the environment, increasing user cost, pest resurgence and pest resistance to insecticides (Arya & Tiwari, Citation2013). Because of affordability and availability of botanical insecticides, farmers in developing countries have used these safer and more environmentally friendly tools for centuries to control insect pests of both field crops and stored products (Schmutterer, Citation1985). Among many other botanicals, extracts of plants such as Azadirachta indica, Milletia ferruginea, Croton macrostachyus, Phytolacea docendra, Jatropha curcas, Nicotina tabacum and Chrysanthemum cinerariifollium have been used successfully to control insect pests (Jirnmci, Citation2013; Schmutterer, Citation1985). Silva et al. (Citation2015) reported high larval mortality of FAW using seed cake extract of A. indica. In recent studies, ethanolic extracts of Argemone ochroleuca (Papaveraceae) caused FAW larval mortality due to a reduction in feeding and slowed larval growth (Martínez et al., Citation2017).
Extracts of many other plants show insecticidal activity against FAW (Batista-Pereira et al., Citation2006), but relatively few have been successfully commercialized. Azadirachtin (from neem) and pyrethrins (from pyrethrum) are the most widely used products throughout Latin America. Some products based on rotenone, garlic, nicotine, rianodine, quassia and other extracts have been registered worldwide (Isman, Citation1997). Those products may be formulated to be diluted with water and sprayed in the same way as chemical pesticides, although dust formulations are also available.
For neem-based sprays, a major problem is the high photosensitivity of azadirachtin, which breaks down or isomerizes under sunlight; thus, neem has a low residual life under field conditions. Moreover, the lack of standardization and quality control in neem-based formulations affect the reproducibility of the insecticide effect (Forim et al., Citation2010). Viana and Prates (Citation2003) reported that using an aqueous extract from neem leaves at 1%, the mortality level of S. frugiperda caterpillars was low during the first three days, after initial feeding, and high by 10 days, indicating that protocols for testing the efficacy of conventional pesticides may not be suitable for testing neem extracts.
1.6.1. Chemical control
Control of FAW is usually achieved through the application of synthetic insecticides (Blanco et al., Citation2014, Citation2010; Hruska & Gould, Citation1997), but it involves high cost, potential environmental contamination, and development of resistance to chemicals, and often pest resurgence (Colborn, Citation1995; Crowe & Booty, Citation1995). There are no registered chemicals for FAW in Ethiopia at this point, but various general pesticides (such as pyrethroids, carbamates and organophosphates) are being used. The exact timing for applying chemicals is very important for effective pest control; both the life cycle and the time of day matter i.e. spraying when larvae are deeply embedded inside the whorls and ears of maize is ineffective; and spraying during the day is ineffective because larvae only come to feed on plants at night, dawn or dusk (Day et al., Citation2017). Threshold levels are not being used in determining the need for chemicals, thus there are concern that inappropriate use of chemical controls could lead to resistance development, plant damage, and risks to human health and the environment (Togola et al., Citation2018).
Seed treatment with thiamethoxam did not prevent FAW infestation (de Albuquerque, Borges, Iacono, Crubelati, Singer, Citation2006). Thrash et al. (Citation2013) reported that the use of chlorantraniliprole and cyantraniliprole as seed treatments reduced the need for foliar sprays against FAW in soya. In laboratory tests, thiodicarb and clothianidin reduced the number of plants cut or injured by FAW, but chlorpyrifos, fipronil and thiamethoxam (Camillo, Di Oliveira, de Bueno, & Bueno, Citation2005) and kerosene (Portillo, Meckenstock, & Gómez, Citation1994) were not effective. Another approach is to apply pesticide to the soil at planting, though this is likely to be less efficient than seed treatments. van Huis (Citation1981) concluded that in experiments in Nicaragua, soil treatment did not exert any control on FAW.
Dry mixtures of sand with trichlorfon, formulated as granules or powder, applied into the whorls with a plastic bottle are considered effective and widely used by smallholder farmers in Ethiopia and Kenya (Kumela et al., Citation2019), while mixtures of chlorpyrifos with sawdust reduced the amount of pesticide needed by 20%, without loss of control (van Huis, Citation1981).
1.6.2. FAW management options in Ethiopia
At present, the major problems affecting FAW management efforts in Ethiopia are lack of adequate knowledge of the pest and its management options in the Ethiopian context; lack of sound contingency and long-term plans; lack of coordinated research and development interventions; scarcity of financial and material resources.
FAW’s ability to breed rapidly, migrate, and feed on a wide range of host plants makes it very difficult to control. Nonetheless, there are several ways of managing the pest as reported in other parts of the world, which can potentially be adapted and/or validated and used in Ethiopia (Table ). In Ethiopia, 26% of the farmers combined handpicking larvae with insecticide sprays, while 15% of the farmers practiced only handpicking for FAW management (Kumela et al., Citation2019).
Table 3. FAW management options in Ethiopia
Table suggests that all options have their own advantages and disadvantages. Therefore, there is a need to use the combination of different options judiciously including cultural, chemical, and biological ones as integrated pest management (IPM) for controlling FAW effectively. This helps to minimize the use of insecticides and tackle resistance development challenges. Besides, it reduces also socioeconomic and environmental impacts. Most importantly, maize is a cheaper-priced commodity in Ethiopia, so only reduced use of an efficient application of chemicals is economically viable.
2. Conclusion
The fall armyworm (Spodoptera frugiperda) has recently been introduced into the African continent and has already moved to many countries, including Ethiopia. In Ethiopia, the pest caused huge damage to maize crop in 2017/18 cropping season. Because in Ethiopia, planting of maize during the warmer and moister summer season provides a favorable environment for the insect to quickly multiply and spread to more areas. Therefore, effective control should focus since it is impossible to avoid this pest unless developing sustainable management. Furthermore, there is an urgent need to increase awareness among the farming communities about the life stages of the pest, scouting for the pest (as well as its natural enemies), understanding the right stages of the crop on which high economical damage may occur by FAW, and the time for management application and implementing low-cost agronomic practices and other landscape management practices for sustainable management of the pest. At the same time, it is important to introduce, validate, and deploy low-cost, environmentally safer, and effective technological interventions (like single and pyramided-gene Bt maize) over the short, medium and long-term for sustainable management of FAW in Ethiopia, especially keeping in view that a huge majority of Ethiopian farmers are low-resource smallholders.
2.1. Future line of work
As the FAW is a new invasive pest in Ethiopia, there are many information gaps about its biology, ecology, dispersal mechanisms and effective preventive actions that need to be investigated before science-based, effective management strategies can be recommended for small farmers. Hence, it is necessary to;
Conduct a research program on its management options in Ethiopia.
Examine its ability to reproduce, build up populations and overwinter in different Ethiopian agro-ecological zones.
Create awareness about the pest and its management options in the Ethiopian context;
Aggressively outreach and educate maize growers about the FAW
Explore and introduce natural enemies of FAW from its native distribution range like Cotesia
Explore the mechanisms to enhance Cotesia as a biocontrol agent
Speedy registration of soft insecticides in Ethiopia
Explore the introduction of GE Bt maize in Ethiopia
Additional information
Funding
Notes on contributors
Fenta Assefa
Fenta Assefa is an M.sc student in Horticulture program and staff in Department of plant sciences, College of agriculture and environmental sciences, Bahir Dar University, Ethiopia. Currently, I am teaching several practical courses in Plant sciences and engaged in research and community works. Basically his research interest focuses on agronomy, plant entomology and pathology.
Dereje Ayalew (PhD) is an Associate professor in the field of Agronomy, especially in seed sciences and technology and Head of the seed sciences and technology Research Group in College of Agriculture and Environmental Sciences, Bahir Dar University, Ethiopia. His key research interest is on seed multiplication, field crop production and quality seed production
References
- Abate, T., Shiferaw, B., Menkir, A., Wegary, D., Kebede, Y., Tesfaye, K., … Keno, T. (2015). Factors that transformed maize productivity in Ethiopia. Food Security, 7(5), 965–16. doi:10.1007/s12571-015-0488-z
- Abate, T., van Huis, A., & Ampofo, J. (2000). Pest management strategies in traditional agriculture: An African perspective. Annual Review of Entomology, 45(1), 631–659. doi:10.1146/annurev.ento.45.1.631
- Abebe, Z., & Feyisa, H. (2017). Effects of nitrogen rates and time of application on yield of maize: Rainfall variability influenced time of N application. International Journal of Agronomy, 2017. doi:10.1155/2017/1545280
- Abrahams, P., Bateman, M., Beale, T., Clottey, V., Cock, M., Colmenarez, Y., … Godwin, J. (2017). Fall armyworm: Impacts and implications for Africa. Evidence Note, (2). Retrived from https://www.invasive-species.org/wp-content/uploads/sites/2/2019/02/FAW-Evidence-Note-October-2018.pdf
- Agudelo-Silva, F. (1986). Naturally occurring pathogens of Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae collected on corn in Venezuela. Florida Entomologist (USA). doi:10.2307/3495228
- Arya, M., & Tiwari, R. (2013). Efficacy of plant and animal origin bioproducts against lesser grain borer, rhyzopertha dominica (fab.) in stored wheat. International Journal of Recent Scientific Research, 4(5), 649–653.
- Ashley, T. R.; Ashley. (1979). Classification and distribution of fall armyworm parasites. The Florida Entomologist, 62, 114–123. doi:10.2307/3494087
- Ashley, T. R.; Ashley. (1986). Geographical distributions and parasitization levels for parasitoids of the fall armyworm, Spodoptera frugiperda. The Florida Entomologist, 69, 516–524. doi:10.2307/3495384
- Ayala, O. R., Navarro, F., & Virla, E. G. (2013). Evaluation of the attack rates and level of damages by the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), affecting corn-crops in the northeast of Argentina. Revista de la Facultad de Ciencias Agrarias, 45(2). Retrived from http://bdigital.uncu.edu.ar/app/navegador/?idobjeto=6006
- Barros, E. M., Torres, J. B., & Bueno, A. F. (2010). Oviposition, development, and reproduction of Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) fed on different hosts of economic importance. Neotropical Entomology, 39(6), 996–1001.
- Batista-Pereira, L. G., Castral, T. C., Da Silva, M. T., Amaral, B. R., Fernandes, J. B., Vieira, P. C., … Corrêa, A. G. (2006). Insecticidal activity of synthetic amides on Spodoptera frugiperda. Zeitschrift für Naturforschung C, 61(3–4), 196–202. doi:10.1515/znc-2006-3-408
- Birhanu, S. (2018). Evaluation of different management options of fall armyworm, Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) and assessment of its parasitoids in some parts of Ethiopia. Dire Dawa: Haramaya University.
- Birhanu, S., Simiyu, J., Malusi, P., Likhayo, P., Mendesil, E., Elibariki, N., … Tefera, T. (2018). First report of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. Journal of Applied Entomology, 142(8), 800–804. doi:10.1111/jen.12534
- Blanco, C. A., Chiaravalle, W., Dalla-Rizza, M., Farias, J., García-Degano, M., Gastaminza, G., … Pieralisi, B. (2016). Current situation of pests targeted by Bt crops in Latin America. Current Opinion in Insect Science, 15, 131–138. doi:10.1016/j.cois.2016.04.012
- Blanco, C. A., Pellegaud, J. G., Nava-Camberos, U., Lugo-Barrera, D., Vega-Aquino, P., Coello, J., … Vargas-Camplis, J. (2014). Maize pests in Mexico and challenges for the adoption of integrated pest management programs. Journal of Integrated Pest Management, 5(4), E1–E9. doi:10.1603/IPM14006
- Blanco, C. A., Portilla, M., Jurat-Fuentes, J. L., Sánchez, J. F., Viteri, D., Vega-Aquino, P., … Arias, R. (2010). Susceptibility of isofamilies of Spodoptera frugiperda (Lepidoptera: Noctuidae) to Cry1Ac and Cry1Fa proteins of Bacillus thuringiensis. Southwestern Entomologist, 35(3), 409–416. doi:10.3958/059.035.0325
- Brown, E., & Dewhurst, C. (1975). The genus Spodoptera (Lepidoptera, Noctuidae) in Africa and the near east. Bulletin of Entomological Research, 65(2), 221–262. doi:10.1017/S0007485300005939
- CABI. (2017a). Fall armyworm: Impacts and implications for Africa. Retrieved from https://www.cabi.org/isc/datasheet/29810
- CABI. (2017b). How to identify … fall armyworm. A4 flyer. Plantwise. Retrieved from http://www.plantwise.org/FullTextPDF/2017/20177800462.pdf
- CABI. (2017c). Spodoptera frugiperda (fall army worm) invasive species compendium. Retrieved from http://www.cabi.org/isc/datasheet/29810
- Camillo, M. F., Di Oliveira, J. R. G., de Bueno, A. F., & Bueno, R. C. O. D. F. (2005). Seeds treatment on maize for Spodoptera frugiperda control. Ecossistema, 30(1/2), 59–63.
- Capinera. (2001). Handbook of vegetable pests (4th ed.). University of Florida. Retrieved from http://creatures.ifas.ufl.edu
- Capinera, J. L. (2000). Fall armyworm, Spodoptera frugiperda (JE Smith)(Insecta: Lepidoptera: Noctuidae). University of Florida IFAS Extension.
- Chapman, J. W., Williams, T., Escribano, A., Caballero, P., Cave, R. D., & Goulson, D. (1999). Age‐related cannibalism and horizontal transmission of a nuclear polyhedrosis virus in larval Spodoptera frugiperda. Ecological Entomology, 24(3), 268–275. doi:10.1046/j.1365-2311.1999.00224.x
- Chapman, J. W., Williams, T., Martínez, A. M., Cisneros, J., Caballero, P., Cave, R. D., & Goulson, D. (2000). Does cannibalism in Spodoptera frugiperda (Lepidoptera: Noctuidae) reduce the risk of predation? Behavioral Ecology and Sociobiology, 48(4), 321–327. doi:10.1007/s002650000237
- Clark, P. L., Molina-Ochoa, J., Martinelli, S., Skoda, S. R., Isenhour, D. J., Lee, D. J., … Foster, J. E. (2007). Population variation of the fall armyworm, Spodoptera frugiperda, in the Western Hemisphere. Journal of Insect Science, 7(1). doi:10.1673/031.007.0501
- Colborn, T. (1995). Pesticides–How research has succeeded and failed to translate science into policy: Endocrinological effects on wildlife. Environmental Health Perspectives, 103(Suppl 6), 81.
- Crowe, A., & Booty, W. G. (1995). A multi-level assessment methodology for determining the potential for groundwater contamination by pesticides. Environmental Monitoring and Assessment, 35(3), 239–261. doi:10.1007/BF00547635
- Cruz, I., Figueiredo, M. D. L. C., Silva, R. B. D., Silva, I. F. D., Paula, C. D., & Foster, J. E. (2012). Using sex pheromone traps in the decision-making process for pesticide application against fall armyworm (Spodoptera frugiperda [Smith][Lepidoptera: Noctuidae]) larvae in maize. International Journal of Pest Management, 58(1), 83–90. doi:10.1080/09670874.2012.655702
- Day, R., Abrahams, P., Bateman, M., Beale, T., Clottey, V., Cock, M., … Godwin, J. (2017). Fall armyworm: Impacts and implications for Africa. Outlooks on Pest Management, 28(5), 196–201. doi:10.1564/v28_oct_02
- de Albuquerque, F., Borges, L., Lacono, T. D. O., Crubelati, N. D. S., & Singer, A. D. C. (2006). Efficiency of insecticides applied in seed treatment and pulverization, in the control of maize initial pests. Revista Brasileira de Milho e Sorgo (brazil),5(1), 15–25.
- FAO. (2017). Fall armyworm continues to spread in Ethiopia’s maize fields. Facilitates national awareness training for key partners and field offices. Retrieved from http://www.fao.org/food-chain-crisis/howwe-work/plant-protection/fall-armyworm/en/
- FAO. (2018). Integrated management of the fall armyworm on maize a guide for farmer field schools in Africa. Retrieved from http://www.fao.org/faostat/en/.
- Fargues, J., & Rodriguez-Rueda, D. (1980). Sensibilité des larves deSpodoptera littoralis [Lep.: Noctuidae] aux hyphomycètes entomopathogènesNomuraea rileyi etPaecilomyces fumoso-roseus. Entomophaga, 25(1), 43–54. doi:10.1007/BF02377521
- Fernández, J. (2002). Nota corta: Estimación de umbrales económicos para Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) en el cultivo del maíz. Invest. Agric. Prod. Prot. Veg, 17, 467–474.
- Forim, M. R., Matos, A. P., Silva, M. F. D. G. F., Cass, Q. B., Vieira, P. C., & Fernandes, J. B. (2010). The use of HPLC in the control of neem commercial products quality: Reproduction of the insecticide action. Quimica Nova, 33(5), 1082–1087. doi:10.1590/S0100-40422010000500014
- Fuxa, J. (1982). Prevalence of viral infections in populations of fall armyworm, Spodoptera frugiperda, in southeastern Louisiana. Environmental Entomology, 11(1), 239–242. doi:10.1093/ee/11.1.239
- Gardner, W. A., & Fuxa, J. R. (1980). Pathogens for the suppression of the fall armyworm. The Florida Entomologist, 63, 439–447. doi:10.2307/3494527
- Goergen, G., Kumar, P. L., Sankung, S. B., Togola, A., & Tamo, M. (2016). First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith)(Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PloS One, 11(10), e0165632. doi:10.1371/journal.pone.0165632
- Gross, H., Jr, & Pair, S. (1986). The fall armyworm: Status and expectations of biological control with parasitoids and predators. The Florida Entomologist, 69, 502–515. doi:10.2307/3495383
- Hanway, J. (1969). Defoliation effects on different corn (Zea mays, L.) hybrids as influenced by plant population and stage of development 1. Agronomy Journal, 61(4), 534–538. doi:10.2134/agronj1969.00021962006100040016x
- Hruska, A. J., & Gould, F. (1997). Fall armyworm (Lepidoptera: Noctuidae) and Diatraea lineolata (Lepidoptera: Pyralidae): Impact of larval population level and temporal occurrence on maize yield in Nicaragua. Journal of Economic Entomology, 90(2), 611–622. doi:10.1093/jee/90.2.611
- IITA. (2016). First report of outbreaks of the “Fall Armyworm” on the African continent. Bulletin no. 2330. Retrieved from http://bulletin.iita.org/index.php/2016/06/18/first-reportof-outbreaks-of-the-fall-armyworm-on-the-african-continent/
- Isman, M. B. (1997). Neem and other botanical insecticides: Barriers to commercialization. Phytoparasitica, 25(4), 339–344. doi:10.1007/BF02981099
- Jeger, M., Bragard, C., Caffier, D., Dehnen‐Schmutz, K., Gilioli, G., Gregoire, J. C., … Navajas Navarro, M. (2017). Pest categorisation of citrus leprosis viruses. EFSA Journal, 15(12). Retrived from https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2017.5110
- Jirnmci, E. (2013). Efficacy of botanical extracts against termites, macrotermes spp.,(Lsoptera: Termiticlae) under laboratory conditions. Retrived from http://docsdrive.com/pdfs/academicjournals/ijar/0000/57223-57223.pdf
- Jones, R., Gilstrap, F., & Andrews, K. (1988). Biology and life tables for the predaceous earwig, Doru taeniatum [Derm.: Forficulidae]. Entomophaga, 33(1), 43–54. doi:10.1007/BF02372312
- Kumela, T., Simiyu, J., Sisay, B., Likhayo, P., Mendesil, E., Gohole, L., & Tefera, T. (2018). Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. International Journal of Pest Management, 65(1), 1–9.
- Kumela, T., Simiyu, J., Sisay, B., Likhayo, P., Mendesil, E., Gohole, L., & Tefera, T. (2019). Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. International Journal of Pest Management, 65(1), 1–9. doi:10.1080/09670874.2017.1423129
- Luginbill, P. (1928a). The fall armyworm. United States departament of agriculture, Washington, DC, USA. Technical Bulletin, 34. Retrived from https://www.jstor.org/stable/3495378?seq=1#metadata_info_tab_contents
- Luginbill, P. (1928b). The fall armyworm. United States Department of Agriculture (USDA). Technical Bulletin, 34. Retrived from https://www.jstor.org/stable/3495378?seq=1#metadata_info_tab_contents
- Mahmoud, M. (2016). Biology and use of entomopathogenic nematodes in insect pests biocontrol, a generic view. Cercetari Agronomice in Moldova, 49(4), 85–105. doi:10.1515/cerce-2016-0039
- Marenco, R., Foster, R., & Sanchez, C. (1992). Sweet corn response to fall armyworm (Lepidoptera: Noctuidae) damage during vegetative growth. Journal of Economic Entomology, 85(4), 1285–1292. doi:10.1093/jee/85.4.1285
- Martínez, A. M., Aguado-Pedraza, A. J., Viñuela, E., Rodríguez-Enríquez, C. L., Lobit, P., Gómez, B., & Pineda, S. (2017). Effects of ethanolic extracts of Argemone ochroleuca (Papaveraceae) on the food consumption and development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Florida Entomologist, 100(2), 339–345. doi:10.1653/024.100.0232
- Matošević, D., & Melika, G. (2013). Recruitment of native parasitoids to a new invasive host: First results of Dryocosmus kuriphilus parasitoid assemblage in Croatia. Bulletin of Insectology, 66(2), 231–238.
- Metcalf, C. L., Flint, W. P., & Metcalf, R. L. (1965). Insectos destructivos e insectos útiles: Sus costumbres y su control. Compañía Editorial Continental.
- Midega, C. A., Bruce, T. J., Pickett, J. A., & Khan, Z. R. (2015). Ecological management of cereal stemborers in A frican smallholder agriculture through behavioural manipulation. Ecological Entomology, 40, 70–81. doi:10.1111/een.12216
- Miętkiewicz, R., Dzięgielewska, M., & Janowicz, K. (1998). Entomopathogenic fungi isolated in the vicinity of Szczecin. Acta Mycologica, 33(1), 123–130. doi:10.5586/am.1998.011
- Ministry of Agriculture and Natural Resources. (2017). Farmers fear loss of maize to fall army worm. Retrived from https://www.thereporterethiopia.com/article/farmers-fear-loss-maize-fall-armyworm
- Molina Ochoa, J., Hamm, J., Lezama Gutierrez, R., Bojalil Jaber, L., Arenas Vargas, M., & Gonzalez Ramirez, M. (1996). Virulence of six entomophatogenic nematodes (Steinernematidae and Heterorhabditidae) on immature stages of Spodoptera frugiperda (Lepidoptera: Noctuidae). Vedalia Revista Internacional De Control Biologico (mexico). Retrived from http://agris.fao.org/agris-search/search.do?recordID=MX1997002847
- Molina-Ochoa, J., Lezama-Gutierrez, R., Gonzalez-Ramirez, M., Lopez-Edwards, M., Rodriguez-Vega, M. A., & Arceo-Palacios, F. (2003). Pathogens and parasitic nematodes associated with populations of fall armyworm (Lepidoptera: Noctuidae) larvae in Mexico. Florida Entomologist, 86, 244–253. doi:10.1653/0015-4040(2003)086[0244:PAPNAW]2.0.CO;2
- Nagoshi, R. N., Meagher, R. L., & Hay‐Roe, M. (2012). Inferring the annual migration patterns of fall armyworm (Lepidoptera: Noctuidae) in the United States from mitochondrial haplotypes. Ecology and Evolution, 2(7), 1458–1467. doi:10.1002/ece3.268
- Nagoshi, R. N., Meagher, R. L., & Jenkins, D. A. (2010). Puerto Rico fall armyworm has only limited interactions with those from Brazil or Texas but could have substantial exchanges with Florida populations. Journal of Economic Entomology, 103(2), 360–367. doi:10.1603/ec09253
- Nigussie, M., Tanner, D., & Twumasi-Afriyie, S. (2002). In Enhancing the contribution of maize to food security in Ethiopia. Proceedings of the Second National Maize Workshop of Ethiopia, Addis Ababa, Ethiopia, 12–16 November 2001. Ethiopian Agricultural Research Organization
- Pair, S., & Gross, H., Jr. (1984). Field mortality of pupae of the fall armyworm, Spodoptera frugiperda (JE Smith), by predators and a newly discovered parasitoid, Diapetimorpha introita. Journal of the Georgia Entomological Society. Retrived from http://agris.fao.org/agris-search/search.do?recordID=US19850013898
- Pannuti, L., Baldin, E., Hunt, T. E., & Paula-Moraes, S. (2015). On-plant larval movement and feeding behavior of fall armyworm (Lepidoptera: Noctuidae) on reproductive corn stages. Environmental Entomology, 45(1), 192–200. doi:10.1093/ee/nvv159
- Pannuti, L., Paula-Moraes, S., Hunt, T., Baldin, E., Dana, L., & Malaquias, J. (2016). Plant-to-plant movement of Striacosta albicosta (Lepidoptera: Noctuidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize (Zea mays). Journal of Economic Entomology, 109(3), 1125–1131. doi:10.1093/jee/tow042
- Pilkington, L. J., Messelink, G., van Lenteren, J. C., & Le Mottee, K. (2010). “Protected biological control”–Biological pest management in the greenhouse industry. Biological Control, 52(3), 216–220. doi:10.1016/j.biocontrol.2009.05.022
- Pogue, M. G. (2002). A world revision of the genus Spodoptera Guenée:(Lepidoptera: Noctuidae). American Entomological Society Philadelphia. Retrived from https://www.ars.usda.gov/research/publications/publication/?seqNo115=110657
- Portillo, H., Meckenstock, H., & Gómez, F. (1994). Improved chemical protection of sorghum seed and seedlings from insect pests in Honduras. Turrialba (IICA) V, 44(1), 50–55.
- Prasanna, B., Huesing, J., Eddy, R., & Peschke, V. (2018). Fall armyworm in Africa: A guide for integrated pest management. CDMX: CIMMYT.
- Richter, A., & Fuxa, J. (1990). Effect of Steinernema feltiae on Spodoptera frugiperda and Heliothis zea (Lepidoptera: Noctuidae) in corn. Journal of Economic Entomology, 83(4), 1286–1291. doi:10.1093/jee/83.4.1286
- Rojas, J. C., Kolomiets, M. V., & Bernal, J. S. (2018). Nonsensical choices? Fall armyworm moths choose seemingly best or worst hosts for their larvae, but neonate larvae make their own choices. PloS One, 13(5), e0197628. doi:10.1371/journal.pone.0197628
- Romero Sueldo, G. M., Dode, M., & Virla, E. G. (2014). Depredación de Doru luteipes y D. lineare (Dermaptera: Forficulidae) sobre Rhopalosiphum maidis (Hemiptera: Aphididae) en condiciones de laboratorio. Retrived from https://ri.conicet.gov.ar/handle/11336/17140
- Rose, A., Silversides, R., & Lindquist, O. (1975). Migration flight by an aphid, Rhopalosiphum maidis (Hemiptera: Aphididae), and a noctuid, Spodoptera frugiperda (Lepidoptera: Noctuidae). The Canadian Entomologist, 107(6), 567–576. doi:10.4039/Ent107567-6
- Rose, D., Dewhurst, C., & Page, W. (2000). The African armyworm handbook: The status, biology, ecology, epidemiology and management of Spodoptera exempta (Lepidoptera: Noctuidae). Natural Resources Institute. Retrived from https://www.amazon.com/African-Armyworm-Handbook-Epidemiology-Lepidoptera/dp/0859545237
- Schmutterer, H. (1985). Which insect pests can be controlled by application of neem seed kernel extracts under field conditions? Zeitschrift für angewandte Entomologie, 100(1‐5), 468–475. doi:10.1111/j.1439-0418.1985.tb02808.x
- Silva, D. M. D., Bueno, A. D. F., Andrade, K., Stecca, C. D. S., Neves, P. M. O. J., & Oliveira, M. C. N. D. (2017). Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Scientia Agricola, 74(1), 18–31. doi:10.1590/1678-992x-2015-0160
- Silva, G. A., Picanço, M. C., Ferreira, L. R., Ferreira, D. O., Farias, E. S., Souza, T. C., … Pereira, E. J. G. (2018). Yield losses in transgenic Cry1Ab and non-Bt corn as assessed using a crop-life-table approach. Journal of Economic Entomology, 111(1), 218–226. doi:10.1093/jee/tox346
- Silva, M. S., Broglio, S. M. F., Trindade, R. C. P., Ferrreira, E. S., Gomes, I. B., & Micheletti, L. B. (2015). Toxicity and application of neem in fall armyworm. Comunicata Scientiae, 6(3), 359–364. doi:10.14295/cs.v6i3.808
- Simmons, A. M. (1993). Effects of constant and fluctuating temperatures and humidities on the survival of Spodoptera frugiperda pupae (Lepidoptera: Noctuidae). The Florida Entomologist, 76, 333–340. doi:10.2307/3495733
- Sosa-Gomez, D., & Moscardi, F. (1994). Effect of till and no-till soybean cultivation on dynamics of entomopathogenic fungi in the soil. Florida Entomologist, 77(2), 284. doi:10.2307/3495515
- Sparks, A. (1979). A review of the biology of the fall armyworm. The Florida Entomologist, 62, 82–87. doi:10.2307/3494083
- Sparks, A. (1986). Fall armyworm (Lepidoptera: Noctuidae): Potential for area-wide management. The Florida Entomologist, 69, 603–614. doi:10.2307/3495397
- Stokstad, E. (2017). New crop pest takes Africa at lightning speed. American Association for the Advancement of Science. Retrived from https://science.sciencemag.org/content/356/6337/473.summary
- Thrash, B., Adamczyk, J., Jr, Lorenz, G., Scott, A., Armstrong, J., Pfannenstiel, R., & Taillon, N. (2013). Laboratory evaluations of lepidopteran-active soybean seed treatments on survivorship of fall armyworm (Lepidoptera: Noctuidae) larvae. Florida Entomologist, 96(3), 724–728. doi:10.1653/024.096.0304
- Togola, A., Meseka, S., Menkir, A., Badu-Apraku, B., Boukar, O., Tamò, M., & Djouaka, R. (2018). Measurement of pesticide residues from chemical control of the invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a maize experimental field in Mokwa, Nigeria. International Journal of Environmental Research and Public Health, 15(5), 849. doi:10.3390/ijerph15061188
- Tufa, B., & Ketema, H. (2016). Effects of different termite management practices on maize production in Assosa district, Benishangul Gumuz Region, Western Ethiopia. Journal of Biology, Agriculture and Healthcare, 6(26), 27–33.
- van Huis, A. (1981). Integrated pest management in the small farmer’s maize crop in Nicaragua. van Huis. Retrived from https://library.wur.nl/WebQuery/wurpubs/74659
- Vänninen, I. (1996). Distribution and occurrence of four entomopathogenic fungi in Finland: Effect of geographical location, habitat type and soil type. Mycological Research, 100(1), 93–101. doi:10.1016/S0953-7562(96)80106-7
- Vercher, R., Costa-Comelles, J., Marzal, C., & García-Marí, F. (2005). Recruitment of native parasitoid species by the invading leafminer Phyllocnistis citrella (Lepidoptera: Gracillariidae) on citrus in Spain. Environmental Entomology, 34(5), 1129–1138. doi:10.1603/0046-225X(2005)034[1129:RONPSB]2.0.CO;2
- Viana, P. A., & Prates, H. T. (2003). Desenvolvimento e mortalidade larval de Spodoptera frugiperda em folhas de milho tratadas com extrato aquoso de folhas de Azadirachta indica. Bragantia, 62(1), 69–74. doi:10.1590/S0006-87052003000100009
- Vickery, R. A. (1929). Studies on the fall army worm in the gulf coast district of Texas. US Government Printing Office. Retrived from https://tind-customer-agecon.s3.amazonaws.com/d507190e-ff3f-4793-a22e-2d33b868992a?response-content-disposition=inline%3B%20filename%2A%3DUTF-8%27%27tb138.pdf&response-content type=application%2Fpdf&AWSAccessKeyId=AKIAXL7W7Q3XHXDVDQYS&Expires=1563436881&Signature=jdmQeq6XnWIxNZI%2FP7UihC0oTdk%3D
- Westbrook, J., Nagoshi, R., Meagher, R., Fleischer, S., & Jairam, S. (2016). Modeling seasonal migration of fall armyworm moths. International Journal of Biometeorology, 60(2), 255–267. doi:10.1007/s00484-015-1022-x
