Abstract
Fungi grow everywhere in agricultural produce, food and surface of indoor and outdoor environment. The aim of this paper is to expand the growth and synthesis contributing factors and prevention mechanisms of fungi and its metabolites. Fungi, grouped as hydrophilic, mesophilic and xerophilic, grow under a wider range of water activity, temperature, pH, gases and substrate. Besides the beneficial properties, the harmful fungi species are gaining attention due to their toxicity effect on consumer and economic losses. Taking into consideration their prevalence, food group, daily intake, sampling, analytical techniques and consumer type’s regulatory limit have been established and promising prevention mechanisms discovered. Prevention of growth and production of toxic metabolites includes good practices, use of plant extracts/probiotics, oxygen-reactive scavenging substances and molecular silencing technology for a wide range of commodities. Nevertheless, application and commercialization of those techniques are limited.
Graphic Abstract

PUBLIC INTEREST STATEMENT
Farmers, growers, food producers at different scales of production, food retailers and consumers are facing problems with the growth of pathogenic microorganisms such as fungi and biosynthesis of the fungi toxic metabolites. Fungi and fungi toxic metabolites' mitigation technologies have been investigated and continuing on investigation and promising finding are coming out. In this review paper, the possible mitigation mechanisms of mycotoxins and mycotoxin inducing fungi to minimize the risks on the economic and environmental damage, public health problems and sustain food and nutrition security have been presented.
1. Introduction
Spore-forming fungi are a microbial hazard (Gacem & Hadj-khelil, Citation2016) that produce toxic metabolites known as mycotoxins which cause economic and public health problems in human and livestock (Bhat, Rai, & Karim, Citation2010; Fountain et al., Citation2015; Pitt, Citation2000). The spore-forming fungi grow everywhere in the indoor and outdoor environment where humidity, temperature, hygienic condition and product composition are conducive (Kumar, Mahato, Kamle, Mohanta, & Kang, Citation2017). Studies show that fungi and mycotoxins exist at high dose levels in soils and walls (Diba, Rezaie, & Mahmoudi, Citation2007; Visagie et al., Citation2014a, Citation2014b), mixed mycotoxicosis in water, damaged buildings (Gray et al., Citation2003), in agricultural commodities (Fountain et al., Citation2015; Lai, Zhang, Liu, & Liu, Citation2015; Thathana, Murage, Luther, & Abia, Citation2017), fresh and stored sorghum (Taye, Ayalew, Chala, & Dejene, Citation2016), livestock (EFSA, Citation2013) and food stuffs such as sun-dried potato chips (Amri & Lenoi, Citation2016), edible oils (Yang et al., Citation2011), sesame oil (Idris, Hassan, & Mariod, Citation2013; Kollia, Tsourouflis, & Markaki, Citation2016); groundnut, sesame and cottonseed oils (Idris, Mariod, Elnour, & Mohamed, Citation2010), groundnut and sunflower oils (Mariod & Idris, Citation2015) dried vine fruits (Kollia, Kanapitsas, & Markaki, Citation2014), wheat grain (Sifuentes et al., Citation2013), wheat flour (Li et al., Citation2016) and melon seeds when consumed raw (Somorin, Akinyemi, Bertuzzi, & Pietri, Citation2016).
Fungi are categorized into three major groups based on their stage of occurrence and moisture content for their growth (Egmond Van & Jonker, Citation2003; Hassane et al., Citation2017; Iram et al., Citation2016; Mannaa & Kim, Citation2017; Nayak, Agarwal, Pandey, Sudini, & Jayale, Citation2017; Perczak, Goliński, Bryła, & Waśkiewicz, Citation2018). First, fungi are grown in the field in the early stage of farm at high moisture content (aw = 1) referred to as hydrophilic or also called pre-harvest fungi such as Alternaria, epicaccum and fusarium species. Second category are the mesophilic or intermediate fungi which occur at the optimal maturation for harvesting at water activity (aw = 0.95–1.0) such as aureobasidium species, cladosporium species and verticillium species. Third, xerophilic post-harvest fungi occur during handling and processing at water activity level (aw = 0.6–0.95) such as aspergillus species, eurotium species and penicillium species. However, not all fungi species are harmful.
Visagie et al. (Citation2014a) identified species of fungi of aspergillus (1160 species), penicillium (1459 species) and talaromyces (98 species) isolates in house dust from around the world using extraction-2-dilution method (e2d), and Visagie et al. (Citation2014b) identified 339 aspergillus isolates using different agar medias in the indoor environments. Diba et al. (Citation2007) identified more than 205 isolates of aspergillus species from the surface (wall, floor, beds and trolleys), environment (air) and other surfaces of teaching hospitals in Iran with higher fungi dose in the indoor environment. Thathana et al. (Citation2017) identified 43 aspergillus flavus isolates in maize and soils in Jomo Kenyatta University of Agriculture and Technology in Kenya. Those fungi isolates and strains significantly impact biotechnology, food production, environment and human health. More than 37% of rice was contaminated by aflatoxin B1 (175–30,797 µg/kg) and B2 (70–10,329 µg/kg) (Lai et al., Citation2015) and heavily contaminated maize supplied to local mills by aflatoxin B1 (≤155.6 µg/kg), fumonisin (≤9,638 µg/kg) and deoxynivalenol (≤7,394 µg/kg) in China due to prolonged and inappropriate storage conditions, lack of restrict receiving and inspection, and poor processing practices (Liu et al., Citation2016).
In another study, it was found that 11 of the fungi species (aspergillus flavus, niger, fumigatus, nidulans, tereus, parasiticus, penicilliod, tamarii, ochraceus, sojae and niveus) were found to be more toxigenic to humans, livestock, caused economic loss and affected the food market (Alberts, Van Zyl, & Gelderblom, Citation2016). The growth of fungi reduces the enzymatic activity, disorder of mitotic divisions of meristematic cells and restrained biosynthesis of proteins, and produces secondary metabolites (Podolska et al., Citation2017). The responses include oxygen-reactive stress, environmental stress and interaction of fungi species in the agricultural commodities and foods in the value chain (Fountain et al., Citation2015). The physical features of fungi are important for its identification and characterization, and vary depending on the type of media used to cultivate. The aspergillus flavus cultivated in potato dextrose agar (PDA) is wrinkled, olive to dark-green colored concentrated at the center with a colony diameter of 65–75 mm and have exudates; in Czapeck Dox Agar (SDA) is floccose isolated, yellowish-green and olive candida, with no exudates and 55–75 mm in diameter; in Rose-Bengal Chloramphenicol Agar (RBCA) is yellowish conidia turned to olive and dark green, tiny uncolored exudates with 50–70 mm diameter; in malt extract agar (MEA) is olive and dark conidia with variable shape and uncolored exudates visible at the center (Diba et al., Citation2007; Thathana et al., Citation2017). Isolates of A. flavus (28%) have the ability to produce aflatoxins B1, B2 and G1. Physical features of Aspergillus parasiticus are 250–500 µm in size, colorless, rough surface and spherical in shape. They are inhibited by the application of a metabolite of streptomyces dioctatin A (Dot A) (Diba et al., Citation2007; Yoshinari et al., Citation2007).
Mycotoxins are acute (single exposure) and chronic (repeated exposure) toxic metabolites (Darwish, Ikenaka, Nakayama, & Ishizuka, Citation2014; Krska et al., Citation2008; Tola & Kebede, Citation2016), which play an important source of energy for the growth of other microorganisms. The toxicity of mycotoxin to human beings precipitates allergic responses to immunosuppression, potent carcinogens and results in different types of cancers and even mortality (Pitt, Citation2000; Zain, Citation2011). The occurrence of mycotoxins in food and feedstuffs are influenced by external and internal factors in the food value chain (Darwish et al., Citation2014). Their recoveries and level of detection (LOD) significantly influenced based on the extraction method, separation technique and type of food (Andrade, Da Silva, & Caldas, Citation2013; Frenich, Vidal, Romero-González, & Del Aguilera-Luiz, Citation2009). Several mycotoxins are known to have various physicochemical, morphological and physiological properties associated with food (Berthiller, Sulyok, Krska, & Schuhmacher, Citation2007; Guchi, Citation2015; Krska et al., Citation2016), prompt potential health risks and food insecurity to human. However, mycotoxins are gaining attention in the food value chain due to their toxicity, frequent occurrence and adverse health effect. Examples include aflatoxins, ochratoxins, fumonisins, trichothecenes and Zearalenone, patulin and ergot alkaloids (Krska et al., Citation2008; Pitt, Citation2000).
Aflatoxins-inducing fungi species are mostly grown in tropical and subtropical regions where optimal conditions such as temperature and humidity are conducive for their growth and toxin production (Krska et al., 2017; Chowdhury, Hossain, & Ahmed, Citation2015). The tolerance limit of aflatoxins is less than 2 µg/kg in food; if it exceeds that, then it is potentially carcinogenic (Kollia et al., Citation2016). Aflatoxins B1, B2, G1 and G2 might be found in food and feed staffs, and M1 and M2 are the metabolites of B1 and B2 in dairy products and can cause mutagenic, teratogenic and carcinogenic problems (Kollia et al., Citation2016; Somorin et al., Citation2016; Taye et al., Citation2016; Mi, Citation2014; Idris et al., Citation2013; Torlak, Sert, & Serin, Citation2013; Yang et al., Citation2011; Idris et al., Citation2010; Chen et al., Citation2002). In a study by Garrido, Iha, Ortolani, and Favaro (Citation2003), it was found that the incidence of aflatoxins M1 and M2 in dairy products exceeds the tolerable limit. Most studies conducted and ongoing, in particular, in developing countries are focusing on assessing their prevalence (Taye, Ayalew, Dejene, & Chala, Citation2018). Likewise, the conditions are conducive for the growth of fungi and production of toxin metabolites where the conditions of storage facilities are poor for its prevention.
2. Factors influencing fungi growth and mycotoxin production
Most important influencing factors for the growth of fungi andthe production of fungi metabolites are moisture, gas, temperature, time and composition (Hassane et al., Citation2017; Iram et al., Citation2016; Lahouar, Marin, Crespo-sempere, Saïd, & Sanchis, Citation2016; Leggieri, Decontardi, Bertuzzi, Pietri, & Battilani, Citation2017; Nayak et al., Citation2017). The optimal growth temperature varies by species, strain types and growth medium. Most fungi grow in the range of 5–35°C, optimal at 25°C (Hassane et al., Citation2017; Leggieri et al., Citation2017), influenced by the post-harvest drying temperature (Hawkins & Windham, Citation2005). Higher temperature inhibits the growth of fungi and the synthesis of mycotoxins by attacking the transcription genes (Gacem & Hadj-khelil, Citation2016).
Mycotoxins are synthesized at a wide range of temperature (5–40°C; 25°C) (Hassane et al., Citation2017; Leggieri et al., Citation2017), and even continue during cooking up to 110°C for 20 min (Sandoval-contreras, Villarruel-lópez, & Sierra-beltrán, Citation2017; Zhou, Chen, Kong, Ma, & Liu, Citation2017). The optimal temperatures of AFB and AFG synthesis in wheat induced by aspergillus flavus are 25°C and 28°C, respectively (Hassane et al., Citation2017). According to Hawkins and Windham (Citation2005), aflatoxin synthesis in maize hybrids during post-harvest drying is high at a wide range of temperature (40–70°C), but no significant effect on aflatoxin concentration up to 100°C explains aflatoxins are resistant to a wide range of temperature. In conclusion, post-harvest drying of grains should not exceed 43°C to prevent the growth of fungi and synthesis of mycotoxins, to avoid loss of viability, minimize breakage susceptibility and quality reduction. Nevertheless, once the mycotoxin-inducing fungi are secreted in the food, it is hard to remove at moderate temperature treatment without affecting the physical and nutritional quality. The rate of AFB1 reduction was 0.71–7.8% during thermal treatment (25–60°C) and rate of AFB1 reduction with prolonged holding time of 6–72 h was 0.71–2.86% which is not practical from the economic, processing and nutritional value perspectives (Hassane et al., Citation2017; Hawkins & Windham, Citation2005). The basic condition, however, inhibits the growth of fungi and synthesis of the mycotoxins whereby the pH can be altered by mutation of the acid-tolerant enzymes or amino acid (Moreno-pedraza et al., Citation2015). Mutation of Xylanase in Aspergillus kawachii alters the pH condition, and hence the biosynthesis of mycotoxin and growth of fungi (Qiu et al., Citation2016).
Increased moisture content (5–25%) in the agri-value chain is susceptible to the growth of fungi and increased synthesis of mycotoxins (Hassane et al., Citation2017). Several species of fungi grow at water activities of 0.87–0.99, but no mycotoxins are detected at <0.93 water activity (Leggieri et al., Citation2017). The longer the incubation/storage time resulted, the higher the fungi colonies with bigger diameter at increased water activity. A combined significant Ochratoxin A accumulation with increased incubation time at high water activity and relatively elevated temperature due to Aspergillus carbonarius was observed (Lappa, Kizis, & Panagou, Citation2017). Mycotoxins are, in general, synthesized under acidic condition (Sandoval-contreras et al., Citation2017; Sulyok, Krska, & Schuhmacher, Citation2007), optimally active at 4.0–4.5 pH and reduction was observed at the pH of 5.5–8.0 (Brzonkalik, Hümmer, Syldatk, & Neumann, Citation2012; Iram et al., Citation2016), which explains they are acid tolerant. High gas (CO2) concentration increases in fungi biomass without affecting the growth rate because fungi are facultatively anerobic (Brzonkalik et al., Citation2012; Gacem & Hadj-khelil, Citation2016). Similarly, the composition of the growth medium affects the growth of fungi and mycotoxin synthesis. Amino acids of tryptophan, alkaline condition and secondary plant metabolites such as octanal reduce the growth of fungi, hydrolyzable tannin; antioxidants such as phenolic compounds, ascorbic acid and caffeic acid inhibit or decreases the growth of fungi and toxin production. However, amino acids of tyrosine, lipids, organic C and N, simple sugars of glucose and fructose, acidity, increased water activity, increased CO2, secondary plant metabolites such as octanal cause super enhancement of toxin production by encouraging the growth and synthesis of mycotoxins. Simple sugars of sorbose and lactose have no effect on either of the fungi growth or mycotoxin synthesis (Gacem & Hadj-khelil, Citation2016). In general, the combined effect of several parameters affects the fungi colonies and mycotoxin synthesis. The Aspergillus carbonarius strain type, optimal water activity, optimal incubation time and temperature show a significant increase in Ochratoxin synthesis (Lappa et al., Citation2017).
3. Health impact
Mycotoxin is a noiseless threat (Allen, Citation2017; Bhat et al., Citation2010; Karlovsky et al., Citation2016) where food and feed handling and storage condition are uncontrolled with prolonged storage (Pitt, Citation2000). Mycotoxins are chronic (Bryden, Citation2007; Knutsen et al., Citation2018), respiratory signs and neurological dysfunction due to mycotoxin exposure from building (Rea et al., Citation2003). More than half death and children malnutrition such as stunting, wasting and growth restriction are prevalent due to mycotoxins (Mitchell et al., Citation2017; Mupunga, Mngqawa, & Katerere, Citation2017; Tariku, Biks, Derso, Wassie, & Abebe, Citation2017). Children stunting in developing country (Wirth et al., Citation2017) is associated with frequent exposure to aflatoxin, fumonisin and deoxynivalenol in their staple foods through environmental enteric dysfunction (EED) and disturbance of the insulin-like growth factor (Smith et al., Citation2015). Deoxynivalenol (DON, vomitoxin) in wheat and wheat-based products is causing vomiting, diarrhea, gastrointestinal inflammation and immunomodulation (Kushiro, Citation2008) with frequent consumption and/or exceeding the tolerable limit (8 µg per kg body weight per day) (EFSA, Citation2013).
Aflatoxin is the most toxic, mutagenic and carcinogenic type affecting liver function, exacerbating malnutrition (Knipstein et al., Citation2015; Verheecke, Liboz, Anson, Diaz, & Mathieu, Citation2015) and increasing the susceptibility to hepatitis and liver cancer (Abrar et al., Citation2013; Šarkanj et al., Citation2018), immune system deficiency, reducing child growth and increasing incidences of stillbirth or newborn jaundice (Chen et al., Citation2018; Verheecke et al., Citation2015), and interfere with protein metabolism and multiple micronutrients leads to hepatocellular carcinoma (HCC) which may lead to death (Marchese et al., Citation2018). Aflatoxin is hepatocarcinogenic (Abebe et al., Citation2017; Egmond Van & Jonker, Citation2003) that binds nucleic acids and nucleoproteins which are essential to cellular viability and buildup of hepatic lipids, causes enlargement of liver, proliferation of bile epithelium and necrosis (Abrar et al., Citation2013). Aflatoxin remains a global burden (Kumar et al., Citation2017). Aflatoxin exposure in sub-Saharan Africa (Mupunga et al., Citation2017), Latin America and Asia causes hepatocellular carcinoma, chronic growth impairment in childhood. Dietary aflatoxin and fumonisin exposure have an impact on children’s growth measured as stunting and underweight in Tanzania which is believed to be associated with breastfeeding and weaning practices (Chen et al., Citation2018). The AFB1-lysine concentrations in diets in Nigeria lead to stunting and acute child malnutrition (Mitchell et al., Citation2017), weight loss and mortality as a result of a significant reduction in packed cell volume, hemoglobin concentration, red blood cell and protein concentration (Hussein, Citation2015).
4. Regulatory limit
According to the codex alimentarius report in 2003, 87% of the world inhabitant have had a forced mycotoxin regulation and the remaining 13% either do not have forced regulation or no information is available (Egmond, Schothorst, & Jonker, Citation2007; Egmond Van & Jonker, Citation2003). The regulations of tolerable limit are settled based on the absence or level of occurrence, toxicological studies considering absorption and excretion, analytical methods, sampling, associated intake, direct consumption with or without treatment and requirements for further processing (Egmond et al., Citation2007; Solerizzo et al., Citation2018; Zhang et al., Citation2018). As a result, countries adopt the Codex standards or develop their own standards based on Codex standards considering the intake level and handing condition in a specified environment. However, in developing world, the existence of the regulation, implementation and monitoring is lagging behind (Alberts et al., Citation2016) where mycotoxin might be contributing to child malnutrition and associated health problems are higher. The set of regulatory limits as shown in table , are according to the maximal tolerable limit in food and feed, used as a guideline to avoid contamination, health risk to humans and animals, and promote food international trade (Egmond et al., Citation2007). However, depending on the daily intake of food or group of foods, consumer type and presences or absence of subsequent treatments, the limits can vary (Egmond Van & Jonker, Citation2003).
5. Mycotoxin detoxification and reduction
Understanding of the location or distribution mechanism of mycotoxin in grains, fluid food and biosynthesis is important to identify the toxic-inducing part and their prevention methods. According to the Yu, Bhatnagar, and Cleveland (Citation2004) investigation, the aspergillus parasiticus-induced AFB1, AFB2 and AFG1 synthesis involves 14–15 pathways and more than 25 enzymes which alter the genome sequences and facilitate the toxin synthesis process. The phytohormone signaling in the food or agricultural commodities is responsible for the activation of the calcium signaling which resulted oxidative stress to damage the tissues or nucleotides within few exposure time, while it can be prevented by calcium signaling blockers (channel blockers) such as LaCl3 (Li et al., Citation2019; Rentel & Knight, Citation2004). Extracellular hydrogen bonds are communication bridges where external fungi contaminates associate themselves to the crop/food (Fountain et al., Citation2015; Gacem & Hadj-khelil, Citation2016). Controlling the stress-inducing factors such as humidity, hotness and mechanical stress, quorum sensor understanding, protein signaling pathways (Gilbert et al., Citation2016) contribute to the prevention of aflatoxigenic and mycotoxin occurrence (Gacem & Hadj-khelil, Citation2016). Studies showed numerous controlling mechanisms of fungi growth and its metabolites in the value chain as shown in Figure and summarized Table
Figure 1. Prevention of potential health hazards and post-harvest losses in the food supply and value chain. GAP is good agricultural practices, MC is moisture content, and HACCP is hazard analysis and critical control point.
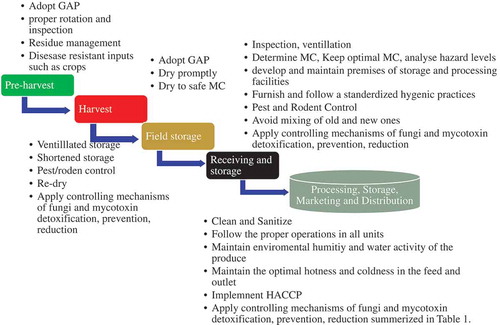
Table 1. The tolerable limit of common mycotoxins in food considered to be consumed without further treatment or processing
Table 2. Mycotoxin detoxification and/or reduction mechanisms in a wide range of agricultural/food produce
6. Conclusion and future perspectives
In conclusion, fungi grow virtually everywhere which affects the quality, safety and productivity of agricultural produce, and produce toxic metabolites.Taking into consideration the consumption frequency, toxicity and bioavailability of mycotoxins, chemical, physical and biological prevention methods have been discovered. The chemical preservatives include high-oxidative power ozone with a wider spectrum, nanoparticles to bind/detoxify and fungicides. Physical prevention includes good practices from farm to fork; biological mitigation techniques include genome transcriptomes, mRNA silencing to advance disease-resistant breeds or monitor toxin biosynthesis, alerting the process condition and product composition by maintaining optimal quality. Commercialization and application of easily accessible, available, affordable and biologically safe bioactive plant extracts, single or mixed probiotics,enzyme extracts is a promising prevention in the food-value chain and promoting healthy consumption. However, the techniques, formulation and stability of the mitigation methods and substrates are not clear, and further investigations are required.
Competing Interest
The authors declare no competing interest.
Acknowledgements
We acknowledge the Dutch organization for internationalization in education (Nuffic), Organization for women in science for the developing world (OWSD) and the World Academy of Sciences (TWAS) for financial support.
Additional information
Funding
Notes on contributors
Abrehet F. Gebremeskel
The author, Abrehet Fisseha Gebremeskel was awarded her first degree (BSc.) in Food Science and Post-harvest Technology at Hawassa University, Ethiopia in 2007, masters of Science (MSc.) in Food Technology at the inter university program of University of Ghent and Catholic university of Leuven, Belgium in 2011. Currently she is an assistant professor of Food Technology since 2017 and PhD student at Egerton University, Kenya with the area of research interest in Food Safety and Quality management.
References
- Abdel-hadi, A. M., Caley, D. P., Carter, D. R. F., Magan, N., Group, A. M., Health, C., & Building, V. (2011). Control of aflatoxin production of aspergillus flavus and aspergillus parasiticus using RNA silencing technology by targeting aflD (nor-1) gene. Toxins, 3(1), 647–14. doi:10.3390/toxins3060647
- Abebe, S. M., Andargie, G., Shimeka, A., Alemu, K., Kebede, Y., Wubeshet, M., … Birku, M. (2017). The prevalence of non-communicable diseases in northwest Ethiopia: Survey of dabat health and demographic surveillance system. BMJ Open, 7(10), 1–9. doi:10.1136/bmjopen-2016-015496
- Abrar, M., Anjum, F. M., Butt, M. S., Randhawa, M. A., Saeed, F., & Waqas, K. (2013). Aflatoxins : Biosynthesis, occurrence, toxicity, and remedies aflatoxins : Biosynthesis, OCCURRENCE, TOXICITY, AND REMEDIES. Critical Reviews in Food Science and Nutrition, 53, 862–874. doi:10.1080/10408398.2011.563154
- Agriopoulou, S., Koliadima, A., Karaiskakis, G., & Kapolos, J. (2016). Kinetic study of aflatoxins’ degradation in the presence of ozone. Food Control, 61(September), 221–226. doi:10.1016/j.foodcont.2015.09.013
- Alberts, J. F., Van Zyl, W. H., & Gelderblom, W. C. A. (2016). Biologically based methods for control of fumonisin-producing fusarium species and reduction of the fumonisins. Frontiers in Microbilogy, 7(April), 1–33. doi:10.3389/fmicb.2016.00548
- Allen, L. N. (2017). Financing national non-communicable disease responses Financing national non-communicable disease responses. Global Health Action, 10(1), 1–6. doi:10.1080/16549716.2017.1326687
- Amri, E., & Lenoi, S. (2016). Aflatoxin and fumonisin contamination of sun-dried sweet potato (Ipomoea batatas L .) Chips in Kahama. Journal of Applied & Environmental Microbiology, 4(3), 55–62. doi:10.12691/jaem-4-3-2
- Andrade, P. D., Da Silva, J. L. G., & Caldas, E. D. (2013). Simultaneous analysis of aflatoxins B1, B2, G1, G2, M1 and ochratoxin A in breast milk by high-performance liquid chromatography/fluorescence after liquid-liquid extraction with low temperature purification (LLE-LTP). Journal of Chromatography A, 1304, 61–68. doi:10.1016/j.chroma.2013.06.049
- Ansari, F., Khodaiyan, F., Rezaei, K., & Rahmani, A. (2015). Enviromental health modelling of aflatoxin G1 reduction by kefir grain using response surface methodology. Journal of Enviromental Healh Science and Enginerning, 13(40), 1–7. doi:10.1186/s40201-015-0190-2
- Audenaert, K., Callewaert, E., Höfte, M., Saeger, S. D., & Haesaert, G. (2010). Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum. BMC Micro, 10(112), 1–14.
- Bedre, R., Rajasekaran, K., & Mangu, V. R. (2015). Genome-wide transcriptome analysis of cotton (gossypium hirsutum L .) identifies candidate gene signatures in response to aflatoxin producing fungus aspergillus flavus. PLoS One, 10(9), 1–23. doi:10.1371/journal.pone.0138025
- Berthiller, F., Sulyok, M., Krska, R., & Schuhmacher, R. (2007). Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. International Journal of Food Microbiology, 119(1–2), 33–37. doi:10.1016/j.ijfoodmicro.2007.07.022
- Bhat, R., Rai, R. V., & Karim, A. A. (2010). Mycotoxins in food and feed : Present status and future concerns. Comprehensive Reviewa in Food Science and Food Safety, 9(1), 57–81.
- Bryden, W. L. (2007). Mycotoxins in the food chain : Human health implications. Asia Pacific Journal of Clinical Nutrition, 16(1), 95–101.
- Brzonkalik, K., Hümmer, D., Syldatk, C., & Neumann, A. (2012). Influence of pH and carbon to nitrogen ratio on mycotoxin production by Alternaria alternata in submerged cultivation. AMB Express, 2(28), 1–8. doi:10.1186/2191-0855-2-28
- Chen, C., Mitchell, N. J., Gratz, J., Houpt, E. R., Gong, Y., Egner, P. A., … Wu, F. (2018). Exposure to aflatoxin and fumonisin in children at risk for growth impairment in rural Tanzania. Environment International, 115(1), 29–37. doi:10.1016/j.envint.2018.03.001
- Chen, R.-S., Tsay, J.-G., Huang, Y.-F., & Chiou, R. Y. Y. (2002). Polymerase chain reaction – mediated characterization of molds belonging to the aspergillus avus group and detection of aspergillus parasiticus in peanut kernels by a multiplex. Journal of Food Protection, 65(5), 840–844.
- Chowdhury, A., Hossain, N., & Ahmed, M. M. (2015). Characterization of aflatoxin producing Aspergillus flavus from food and feed samples. Springer Plus, 4(159), 1–6. doi:10.1186/s40064-015-0947-1
- Darwish, W. S., Ikenaka, Y., Nakayama, S. M. M., & Ishizuka, M. (2014). An overview on mycotoxin contamination of foods in Africa. Toxicology, 1(1), 9. doi:10.1292/jvms.13-0563
- Deepak, M. B., Jhanvi, S. P., & Anuappaiah, K. A. (2015). Original research article aflatoxin binding and detoxification by non-saccharomyces yeast vista for decontamination. International Journal of Current Microbiology and Applied Sciences, 4(5), 310–317.
- Diba, K., Rezaie, S., & Mahmoudi, M. (2007). Identification of aspergillus species using morphological characteristics. Pakistan Journal off Medical Science, 23(6), 867–872.
- EFSA. (2013). Deoxynivalenol in food and feed: Occurrence and exposure. European Food Safety Authority, 11(10), 1–56. doi:10.2903/j.efsa.2013.3379
- Egmond, H. P., Schothorst, R. C., & Jonker, M. A. (2007). Regulations relating to mycotoxins in food perspectives in a global and European context. Analytica Bioanal Chemistry, 389(1), 147–157. doi:10.1007/s00216-007-1317-9
- Egmond Van, H. P., & Jonker, M. A. (2003). Worldwide regulations for mycotoxins in food and feed in 2003. FAO Food and Nutrition, 81, 1–7.
- El-desouky, T. A., & Ammar, H. A. M. (2016). Honey mediated silver nanoparticles and their inhibitory effect on aflatoxins and ochratoxin A. Applied Pharmaceutical Science, 6(06), 83–90. doi:10.7324/JAPS.2016.60615
- Fountain, J. C., Khera, P., Yang, L., Nayak, S. N., Scully, B. T., Lee, R. D., … Guo, B. (2015). ScienceDirect Resistance to Aspergillus fl avus in maize and peanut : Molecular biology, breeding, environmental stress, and future perspectives. The Crop Journal, 3, 229–237. doi:10.1016/j.cj.2015.02.003
- Frenich, A. G., Vidal, J. L. M., Romero-González, R., & Del Aguilera-Luiz, M. M. (2009). Simple and high-throughput method for the multimycotoxin analysis in cereals and related foods by ultra-high performance liquid chromatography/tandem mass spectrometry. Food Chemistry, 117(4), 705–712. doi:10.1016/j.foodchem.2009.04.045
- Gacem, M. A., & Hadj-khelil, E. A. O. (2016). Asian Paci fi c journal of tropical biomedicine. Asian Pacific Journal of Tropical Biomedicine, 6(9), 808–814. doi:10.1016/j.apjtb.2016.07.012
- Garrido, N. S., Iha, M. H., Ortolani, M. R. S., & Favaro, R. M. D. (2003). Occurrence of aflatoxins M 1 and M 2 in milk commercialized in Ribeirão Preto-SP, Brazil Occurrence of aflatoxins M 1 and M 2 in milk ˜ o Preto-SP, Brazil commercialized in Ribeira. Food Additives and Contaminants - Part A, 20(1), 70–73. doi:10.1080/0265203021000035371
- Gemeda, N., Woldeamanuel, Y., Asrat, D., & Debella, A. (2014). Effect of essential oils on Aspergillus spore germination, growth and mycotoxin production: A potential source of botanical food preservative. Asian Pacific Journal of Tropical Biomedicine, 4(Suppl 1), S373–S381. doi:10.12980/APJTB.4.2014C857
- Generotti, S., Cirlini, M., Šarkanj, B., Sulyok, M., Berthiller, F., Dall, C., & Suman, M. (2017). Formulation and processing factors affecting trichothecene mycotoxins within industrial biscuit-making. Fod Chemistry, 229, 597–603. doi:10.1016/j.foodchem.2017.02.115
- Gilbert, M. K., Mack, B. M., Wei, Q., Bland, J. M., Bhatnagar, D., & Cary, J. W. (2016). RNA sequencing of an nsdC mutant reveals global regulation of secondary metabolic gene clusters in Aspergillus flavus. Microbilogical Research, 182, 150–161.
- Gray, M. R., Thrasher, J. D., Crago, R., Madison, R. A., Campbell, A. W., Vojdani, A., … Madison, R. A. (2003). Archives of environmental health : an international mixed mold mycotoxicosis : Immunological changes in humans following exposure in water- damaged buildings mixed mold mycotoxicosis : immunological changes in humans following exposure in water-damaged Bu. Archives of Environmental Health: an International Journal, 58(7), 1–11. doi:10.1080/00039896.2003.11879142
- Guchi, E. (2015). Implication of aflatoxin contamination in agricultural products. American Journal of Food and Nutrition, 3(1), 12–20. doi:10.12691/ajfn-3-1-3
- Hassane, A. M. A., El-Shanawany, A. A., Abo-Dahab, N. F., Abdel-Hadi, A. M., Abdul-Raouf, U. M., & Mwanza, M. (2017). Influence of different moisture contents and temperature on growth and production of aflatoxin B 1 by a toxigenic aspergillus flavus isolate in wheat flour. Journal of Environmental Science and Health, 83(3), 77–83.
- Hawkins, L. K., & Windham, G. L. (2005). Effect of different postharvest drying temperatures on aspergillus flavus survival and aflatoxin content in five maize hybrids. Journal of Food Protection, 68(7), 1521–1524.
- Huang, L., Duan, C., Zhao, Y., Gao, L., Niu, C., Xu, J., & Li, S. (2017). Reduction of aflatoxin B 1 toxicity by lactobacillus plantarum C88 : A potential probiotic strain isolated from Chinese traditional fermented food “Tofu.”. PloS One, 12(1), 1–16. doi:10.1371/journal.pone.0170109
- Huang, W., Chang, J., Wang, P., Liu, C., Yin, Q., & Zhu, Q. (2018). Effect of the combined compound probiotics with mycotoxin – Degradation enzyme on detoxifying a fl atoxin B 1 and zearalenone. The Journal of Toxicological Sciences, 43(6), 377–385.
- Hussein, H. Z. (2015). Plant pathology & microbiology activity of pomegranate peels and clove powders in detoxification of aflatoxin B1 and ochratoxin A from contaminated poultry diet. Plant Pathology and Microbiology, 6(1), 6–9. doi:10.4172/2157-7471.1000249
- Idris, Y. M. A., Hassan, S. A., & Mariod, A. A. (2013). Physicochemical characteristics and aflatoxin levels in two types of sudanese sesame oil. Journal of the American Oil Chemists’ Society, 90(7), 989–998. doi:10.1007/s11746-013-2241-0
- Idris, Y. M. A., Mariod, A. A., Elnour, I. A., & Mohamed, A. A. (2010). Determination of aflatoxin levels in Sudanese edible oils. Food and Chemical Toxicology, 48(8–9), 2539–2541. doi:10.1016/j.fct.2010.05.021
- Iram, W., Anjum, T., Iqbal, M., Ghaffar, A., Abbas, M., & Brown, R. L. (2016). Structural analysis and biological toxicity of aflatoxins B1 and B2 degradation products following detoxification by ocimum basilicum and cassia fistula aqueous extracts. Frontiers in Microbiology, 7(1), 1–18. doi:10.3389/fmicb.2016.01105
- Jouni, F. J., Zafari, J., Abdolmaleki, P., Vazini, H., Ghandi, L., & Satari, M. (2018). Aflatoxin - M 1 detoxification from infected milk using - Fe 3 O 4 nanoparticles attached to specific aptamer. Journal of Nanostructure in Chemistry, 8(1), 13–22. doi:10.1007/s40097-017-0250-5
- Karlovsky, P., Suman, M., Berthiller, F., Meester, J. D. E., Perrin, G. I., & Speijers, G. (2016). Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Research, 32(1), 179–205. doi:10.1007/s12550-016-0257-7
- Knipstein, B. H., Barr, J., Sossenheimer, E., Philip Dietzen, D., Egner, P. A., Groopman, J. D., & David, R. A. (2015). HHS Public Access. European Journal of Vascular and Endovascular Surgery, 78(2), 120–127. doi:10.1038/pr.2015.84.
- Knutsen, H. K., Alexander, J., Barregård, L., Bignami, M., Brüschweiler, B., Ceccatelli, S., … Petersen, A. (2018). Effect on public health of a possible increase of the maximum level for ‘aflatoxin total’ from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs. EFSA Journal, 16(2), 1–32. doi:10.2903/j.efsa.2018.5175
- Kollia, E., Kanapitsas, A., & Markaki, P. (2014). Food additives & contaminants : Part B occurrence of aflatoxin B 1 and ochratoxin A in dried vine fruits from Greek market. Food Additives & Contaminants: Part B, 7(1), 11–16. doi:10.1080/19393210.2013.825647
- Kollia, E., Tsourouflis, K., & Markaki, P. (2016). Aflatoxin B 1 in sesame seeds and sesame products from the Greek market. Food Additives & Contaminants: Part B, 9(3), 217–222. doi:10.1080/19393210.2016.1179349
- Krska, R., De Nijs, M., McNerney, O., Pichler, M., Gilbert, J., Edwards, S., & van Egmod, H. P. (2016). Safe food and feed through an integrated toolbox for mycotoxin management : The mytoolbox approach. World Mycotoxin Journal, 9(4), 487–495. doi: 10.3920/WMJ2016.2136
- Krska, R., Schubert-ullrich, P., Molinelli, A., Macdonald, S., Crews, C., Krska, R., … Crews, C. (2008). Mycotoxin analysis : An update. Food Additives and Contaminants, 25(December), 152–163. doi:10.1080/02652030701765723
- Kumar, P., Mahato, D. K., Kamle, M., Mohanta, T. K., & Kang, S. G. (2017). Aflatoxins: A global concern for food safety, human health and their management. Frontiers in Microbiology, 7(JAN), 1–10. doi:10.3389/fmicb.2016.02170
- Kushiro, M. (2008). Effects of milling and cooking processes on the deoxynivalenol content in wheat. International Journal of Molecular Sciences, 9, 2127–2145. doi:10.3390/ijms9112127
- Lahouar, A., Marin, S., Crespo-sempere, A., Saïd, S., & Sanchis, V. (2016). Effects of temperature, water activity and incubation time on fungal growth and aflatoxin B1 production by toxinogenic Aspergillus flavus isolates on sorghum seeds. Revista Argentina de Microbiología, 48(1), 78–85. doi:10.1016/j.ram.2015.10.001
- Lai, X., Zhang, H., Liu, R., & Liu, C. (2015). Potential for aflatoxin B 1 and B 2 production by Aspergillus flavus strains isolated from rice samples. Saudi Journal of Biological Sciences, 22(2), 176–180. doi:10.1016/j.sjbs.2014.09.013
- Lappa, I. K., Kizis, D., & Panagou, E. (2017). Monitoring the temporal expression of genes involved in ochratoxin a production of aspergillus carbonarius under the influence of temperature and water activity. Toxins, 9(296), 1–15. doi:10.3390/toxins9100296
- Lee, K. R., Yang, S. M., Cho, S. M., Kim, M., Hong, S.-Y., & Chung, S. H. (2017). Aflatoxin B 1 detoxification by aspergillus oryzae from Meju, a traditional Korean fermented soybean starter. Journal of Microbial Biotechnology, 27(1), 57–66.
- Leggieri, M. C., Decontardi, S., Bertuzzi, T., Pietri, A., & Battilani, P. (2017). modeling growth and toxin production of toxigenic fungi signaled in cheese under different temperature and water activity regimes. Toxins, 9(4), 1–17. doi:10.3390/toxins9010004
- Li, F., Jiang, D., Zhou, J., Chen, J., Li, W., & Zheng, F. (2016). Mycotoxins in wheat flour and intake assessment in Shandong province of China. Food Additives and Contaminants: Part B Surveillance, 9(3), 170–175. doi:10.1080/19393210.2016.1154109
- Li, T., Yan, A., Bhatia, N., Altinok, A., Eldad, A., Durand-smet, P., … Meyerowitz, E. M. (2019). Calcium signals are necessary to establish auxin transporter polarity in a plant stem cell niche. Nature Communications, 10(726), 1–9. doi:10.1038/s41467-019-08575-6
- Liu, B., Wu, T., Yu, F., & Su, C. (2007). Induction of oxidative stress response by the mycotoxin patulin in mammalian cells. Toxicological Science, 95(2), 340–347. doi:10.1093/toxsci/kfl156
- Liu, Z., Zhang, G., Zhang, Y., Jin, Q., Zhao, J., Li, J., … Jin, Q. (2016). Factors controlling mycotoxin contamination in maize and food in the Hebei province, China To cite this version : HAL Id : Hal-01532531 Factors controlling mycotoxin contamination in maize and food in the Hebei province, China. Agronomy for Sustainable Development, 36(2), 36–39. doi:10.1007/s13593-016-0374-x
- Mallakian, S., Rezanezhad, R., Jalali, M., & Goobadi, F. (2017). The effect of ozone gas on destruction and detoxification of aflatoxin. Bulletin de La Société Royale Des Sciences de Liège, 86(1), 1–6.
- Mannaa, M., & Kim, K. D. (2017). Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology, 45(4), 240–254. doi:10.5941/MYCO.2017.45.4.240
- Marchese, S., Polo, A., Ariano, A., Velotto, S., Costantini, S., & Severino, L. (2018). Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins, 10(6), 1–19. doi:10.3390/toxins10060214
- Mariod, A. A., & Idris, Y. M. A. (2015). Aflatoxin B1 levels in groundnut and sunflower oils in different Sudanese states. Food Additives and Contaminants: Part B Surveillance, 8(4), 266–270. doi:10.1080/19393210.2015.1082511
- Marrez, D. A., Shahy, E. M., El-sayed, H. S., & Sultan, Y. Y. (2018). Detoxification of aflatoxin B1 in milk using lactic acid bacteria. Journal of Biological Science, 18(3), 144–151. doi:10.3923/jbs.2018.144.151
- Mi, T. (2014). Determination of aflatoxins in sesame, rice, millet and acha from Nigeria using HPLC. Chemical Science Transactions, 3(4), 1516–1524. doi:10.7598/cst2014.749
- Mitchell, N. J., Hsu, H. H., Chandyo, R. K., Shrestha, B., Bodhidatta, L., Tu, Y. K., … Wu, F. (2017). Aflatoxin exposure during the first 36 months of life was not associated with impaired growth in Nepalese children: An extension of the MAL-ED study. PLoS ONE, 12(2), 1–12. doi:10.1371/journal.pone.0172124
- Moreno-pedraza, A., Valdés-santiago, L. C. D., Hernández-valadez, L. J., Higuera, A. R., Winkler, R., & Guzmán-de Peña, C., . D. (2015). Reduction of aflatoxin B 1 during tortilla production and identification of degradation by-products by direct-injection electrospray mass spectrometry. Salud Pública de México, 57(1), 50–57.
- Mupunga, I., Mngqawa, P., & Katerere, D. R. (2017). Peanuts, aflatoxins and undernutrition in children in Sub-Saharan Africa. Nutrients, 9(12), 1–12. doi:10.3390/nu9121287
- Nayak, S. N., Agarwal, G., Pandey, M. K., Sudini, H. K., & Jayale, A. S. (2017). Aspergillus flavus infection triggered immune responses and host-pathogen cross-talks in groundnut during in-vitro seed colonization. Scientific Reports, 7(February), 1–14. doi:10.1038/s41598-017-09260-8
- Perczak, A., Goliński, P., Bryła, M., & Waśkiewicz, A. (2018). The efficiency of lactic acid bacteria against pathogenic fungi and mycotoxins. LAB Efficiency against Pathogenic Fungi and Mycotoxins, 69(1), 32–45. doi:10.2478/aiht-2018-69-3051
- Pitt, J. I. (2000). Toxigenic fungi : Which are important? Medical Mycology, 38(1), 17–22.
- Podolska, G., Bryła, M., Sulek, A., Waskiewicz, A., Szymczyk, K., & Jedrzejczak, R. (2017). Influence of the cultivar and nitrogen fertilisation level on the mycotoxin contamination in winter wheat. Quality Assurance and Safety of Crops and Foods, 9(4), 451–461. doi:10.3920/QAS2016.1064
- Qiu, J., Han, H., Sun, B., Chen, L., Yu, C., & Peng, R. (2016). Residue mutations of xylanase in Aspergillus kawachii alter its optimum pH. Microbiological Research, 182, 1–7.
- Rea, W. J., Didriksen, N., Simon, T. R., Pan, Y., Ervin, J., Griffiths, B., … Simon, T. R. (2003). Archives of environmental health : An international effects of toxic exposure to molds and mycotoxins in building-related illnesses effects of toxic exposure to molds and mycotoxins in building-related illnesses. Archives of Environmental Health: an International Journal, 58(7), 1–7. doi:10.1080/00039896.2003.11879140
- Rentel, M. C., & Knight, M. R. (2004). Oxidative Stress-Induced Calcium Signaling. Plant Physiology, 135(July), 1471–1479. doi:10.1104/pp.104.042663.1
- Sandoval-contreras, T., Villarruel-lópez, A., & Sierra-beltrán, A. P. (2017). Effect of pH and temperature in production of mycotoxins and antibiotics by phytopathogenic moulds for Persian lime (Citrus latifolia T .) in a complex lime pericarp-base medium. Journal of Food and Agriculture, 29(10), 751–759. doi:10.9755/ejfa.2017.v29.i10.1293
- Šarkanj, B., Ezekiel, C. N., Turner, P. C., Abia, W. A., Rychlik, M., Krska, R., … Warth, B. (2018). Ultra-sensitive, stable isotope assisted quantification of multiple urinary mycotoxin exposure biomarkers. Analytica Chimica Acta, 1019, 84–92. doi:10.1016/j.aca.2018.02.036
- Scarpino, V., Reyneri, A., Sulyok, M., Krska, R., & Blandino, M. (2014). Effect of fungicide application to control Fusarium head blight and 20 Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L. World Mycotoxin Journal, 8(4), 1–12. doi:10.3920/wmj2014.1814
- Sifuentes, J., Montagner, T., Yurie, E., Ono, S., Hiromi, E., Carlos, M., … Yoko, E. (2013). Natural occurrence of deoxynivalenol in wheat from Paraná State, Brazil and estimated daily intake by wheat products. Food Chemistry, 138(1), 90–95. doi:10.1016/j.foodchem.2012.09.100
- Smith, L. E., Prendergast, A. J., Turner, P. C., Mbuya, M. N. N., Mutasa, K., Kembo, G., & Stoltzfus, R. J. (2015). The potential role of mycotoxins as a contributor to stunting in the SHINE Trial. Clinical Infectious Diseases, 61(August), S733–S737. doi:10.1093/cid/civ849
- Solerizzo, M., Gambacort, L., Biba, R., Ciriaci, M., Paoloni, A., & Pecorelli, I. (2018). Multimycotoxin analysis by LC-MS/MS in cereal food and feed: Comparison of different approaches for extraction, purification, and calibration. Journal of AOAC International, 101(3), 647–657.
- Somorin, Y., Akinyemi, A., Bertuzzi, T., & Pietri, A. (2016). Co-occurrence of aflatoxins, ochratoxin A and citrinin in “ egusi ” melon (Colocynthis citrullus L .) seeds consumed in Ireland and the United Kingdom. Food Additives & Contaminants: Part B, 9(3), 230–235. doi:10.1080/19393210.2016.1183051
- Sulyok, M., Krska, R., & Schuhmacher, R. (2007). A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Analytical BioAnalytical Chemistry, 389, 1505–1523. doi:10.1007/s00216-007-1542-2
- Taheur, B. F., Fedhila, K., Chaieb, K., Kouidhi, B., Bakhrouf, A., & Abrunhosa, L. (2017). International journal of food microbiology adsorption of a fl atoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Ke fi r grains. International Journal of Food Microbiology, 251(1), 1–7. doi:10.1016/j.ijfoodmicro.2017.03.021
- Tariku, A., Biks, G. A., Derso, T., Wassie, M. M., & Abebe, S. M. (2017). Stunting and its determinant factors among children aged 6 – 59 months in Ethiopia. Italian Journal of Pediatrics, 43(112), 1–9. doi:10.1186/s13052-017-0433-1
- Taye, W., Ayalew, A., Chala, A., & Dejene, M. (2016). Aflatoxin B1and total fumonisin contamination and their producing fungi in fresh and stored sorghum grain in East Hararghe, Ethiopia. Food Additives and Contaminants: Part B Surveillance, 9(4), 237–245. doi:10.1080/19393210.2016.1184190
- Taye, W., Ayalew, A., Dejene, M., & Chala, A. (2018). Fungal invasion and mycotoxin contamination of stored sorghum grain as influenced by threshing methods. International Journal of Pest Management, 0874(May), 11. doi:10.1080/09670874.2017.1327681
- Thathana, M. G., Murage, H., Luther, A., & Abia, K. (2017). Morphological characterization and determination of aflatoxin-production potentials of aspergillus flavus isolated from maize and soil in Kenya. Agriculture, 7(80), 1–14. doi:10.3390/agriculture7100080
- Tola, M., & Kebede, B. (2016). Occurrence, importance and control of mycotoxins : A review. Cogent Food & Agriculture, 21(1), 1–12. doi:10.1080/23311932.2016.1191103
- Torlak, E., Sert, D., & Serin, P. (2013). Fate of Salmonella during sesame seeds roasting and storage of tahini. International Journal of Food Microbiology, 163(2–3), 214–217. doi:10.1016/j.ijfoodmicro.2013.03.010
- Verheecke, C., Liboz, T., Anson, P., Diaz, R., & Mathieu, F. (2015). Reduction of aflatoxin production by Aspergillus flavus and Aspergillus parasiticus in interaction with Streptomyces. Journal of Microbilogy, 161, 967–972. doi:10.1099/mic.0.000070
- Vijayanandraji, S., Brinda, R., Kannan, K., Adhithya, R., Vinothini, S., Senthil, K., … Velazhahan, R. (2014). Detoxification of aflatoxin B1 by an aqueous extract from leaves of Adhatoda vasica Nees ଝ. Microbiological Research, 169(4), 294–300. doi:10.1016/j.micres.2013.07.008
- Visagie, C. M., Hirooka, Y., Tanney, J. B., Mwange, K., Meijer, M., Amend, A. S., & Samson, R. A. (2014a). Aspergillus, 78(1), 63–139. doi: 10.1016/j.simyco.2014.07.002
- Visagie, C. M., Houbraken, J., Frisvad, J. C., Hong, S. B., Klaassen, C. H. W., Perrone, G., … Samson, R. A. (2014b). Identification and nomenclature of the genus Penicillium. Studies in Mycology, 78(1), 343–371. doi:10.1016/j.simyco.2014.09.001
- Wang, L., Shao, H., Luo, X., Wang, R., Li, Y., Li, Y., & Luo, Y. (2016). Effect of ozone treatment on deoxynivalenol and wheat quality. PloS One, 11(1), 1–13. doi:10.1371/journal.pone.0147613
- Wirth, J. P., Rohner, F., Petry, N., Onyango, A. W., Matji, J., Bailes, A., … Woodruff, B. A. (2017). Original Article Assessment of the WHO Stunting Framework using Ethiopia as a case study. Maternal and Child Nutrition, 13(e12310), 1–16. doi:10.1111/mcn.12310
- Yang, L., Liu, Y., Miao, H., Dong, B., Yang, N., Yang, L., & Sun, J. (2011). Food additives and contaminants : Part B determination of aflatoxins in edible oil from markets in Hebei Province of China by liquid chromatography – Tandem mass spectrometry. Food Additives and Contaminants: Part B Surveillance, 4(4), 244–247. doi:10.1080/19393210.2011.632694
- Yoshinari, T., Akiyama, T., Nakamura, K., Kondo, T., Takahashi, Y., Muraoka, Y., … Sakuda, S. (2007). Dioctatin A is a strong inhibitor of aflatoxin production by Aspergillus parasiticus. Microbiology, 153(2007), 2774–2780. doi:10.1099/mic.0.2006/005629-0
- Yu, J., Bhatnagar, D., & Cleveland, T. E. (2004). Completed sequence of aflatoxin pathway gene cluster in Aspergillus parasiticus. FEBS Letters, 564(1–2), 126–130. doi:10.1016/S0014-5793(04)00327-8
- Zain, M. E. (2011). Impact of mycotoxins on humans and animals. Journal of Saudi Chemical Society, 15(2), 129–144. doi:10.1016/j.jscs.2010.06.006
- Zhang, B., Chen, X., Han, S.-Y., Li, M., Ma, T.-Z., Sheng, W.-J., & Zhu, X. (2018). Simultaneous analysis of 20 mycotoxins in grapes and wines from Hexi Corridor Region (China): Based on a QuEChERS – UHPLC – MS/MS Method. Molecules, 23(1), 17. doi:10.3390/molecules23081926
- Zhou, G., Chen, Y., Kong, Q., Ma, Y., & Liu, Y. (2017). Detoxification of aflatoxin B 1 by zygosaccharomyces rouxii with solid state fermentation in peanut meal. Toxins, 9(42), 1–9. doi:10.3390/toxins9010042
