?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The consumption of indigenous leafy vegetables has increased tremendously over the last decade and in Africa, for example, research shows that they account for 30% of all vegetables sold in market outlets. However, there is a need to address problems of low production and improve the quality of seed used by farmers. The objective of this study was to evaluate seed processing methods used by farmers and recommend the most suitable one for the production of clean nightshade seed. One hundred and twenty seed samples were collected from farm fields. Seed quality tests were conducted at University of Nairobi plant pathology laboratory and field evaluation experiments in Muthara; Meru County in Kenya. Four seed processing methods: dry processing, wet processing, dry fermentation and wet fermentation were evaluated. The study revealed that farmers in Upper midland zone two (UM2) and Lower highland zone two (LH2) preferred to use dry seed fermentation while those in Upper midland zone four (UM4) and lower midland zone two (LM2) preferred wet seed processing. Seed processing methods determined the level of seed purity. Evaluation of seed processing methods revealed that the wet seed fermentation method produced seeds with the highest seed purity of 96.3% and yields (913.8kg/ha). This implies that the method of seed processing with the highest seed purity was the most suitable as it yielded more. Therefore, this study recommends wet seed fermentation method for use by farmers as it leads to the production of pure seeds with high germination, vigor index and high yields.
PUBLIC INTEREST STATEMENT
Indigenous leafy vegetables play an important role as income and food security crops in many rural and urban households in Africa. Edible nightshade besides being used as food also provides income to farmers. The leaves and seeds provide vitamins A and C, calcium, iron, protein, carbohydrates and lipids. The health benefits include anti-oxidation, anti-carcinogenic, analgesic and immune-modulatory properties. However, farmers’ capacity to meet the growing demand for these vegetables has been limited by a lack of good quality seed. The informal seed system in Kenya accounts for 90% of the seed used in production which is of poor quality with low germination rates. The success of seed production depends on the seed processing method used as it determines the seed purity. The findings of this study will be useful to seed producers to increase the production of edible nightshades to meet the increasing demand.
1. Introduction
Cultivation and consumption of edible leafy nightshade has continued to increase due to the significant role it plays in nutrition, food security and income generation (Abukutsa-Onyango, Citation2010). Currently, they are traded for higher prices compared to exotic vegetables due to increasing demand (Abukutsa-Onyango, Citation2010). Lack of quality seeds is a major constraint in production of nightshade vegetable. Poor quality is responsible for low yields; for example, in Kenya, nightshade leaves have the potential to yield 30 tonnes, but farmers produce less than 2 tonnes per ha (Elizabeth & Adeniji, Citation2015). The seed processing method is a major determinant of the seed quality. According to Louwaars and De Boef (Citation2012), lack of knowledge on the processing of nightshade has led to poor seed stocks. Solanaceous crops where fruits are borne on succulent berries are better processed by wet fermentation method. Ekhuya, Wesonga, and Abukutsa (Citation2018) reported that wet seed fermentation ensures clean seed production that is free from physical impurities due to washing and removal of sugars that inhibit seed germination.
Proper seed processing enhances seed quality. Seed quality is measured using the method proposed by ISTA (International Seed Testing Association rules). The germination test is the most widely accepted physiological method for testing the quality of seed (Odeyemi, Ajayi, & Olakojo, Citation2010). In addition, seed vigor tests help to predict the performance of seed in the field (Milošević, Citation2010). Growth tests and seedling evaluation are also important because high-quality seeds produce plants that are normal, vigorously growing with low sensitivity to external factors (Milošević, Citation2010). Farmers’ process own seeds in Kenya without using any conventional protocol and thus lack a uniform, standard and optimal method for processing seeds.
The objective of this study was to evaluate seed processing methods used by farmers, their effects on the seed quality and recommend the most suitable method for clean African nightshade seed production. Seed quality tests helped to determine the status of seed produced by farmers in Kenya. Sthapit, Ram, Pashupati, and Pratap (Citation2008) reported that the absence of a good seed processing method leads to significant production losses affecting household incomes and food security. The informal seed acquisition in Kenya accounts for 90% of the African nightshade seed used by farmers (Ghosh, Singh, Magsumbol, Kamboj, & Goldberg, Citation2016). Few farmers in Kenya use certified seed due to limited supply, high prices and lack of knowledge on its importance (Sthapit et al., Citation2008).
2. Materials and methods
2.1. Agroecological sites and seed collection
A total of 120 seed samples were collected from farms to be used in this study. During seed collected the method of processing the seed was recorded. These sites were Suneka and Ogembo in Kisii; Lurambi and Amalemba in Kakamega, Kenya. Global positioning system (GPS) was used to locate the sites. Suneka is located at a latitude of 0° 40ʹ 43.5” S and longitude of 34° 42ʹ 27.7”E in upper midland zones AEZ UM 2. Ogembo is located at a latitude of 0° 50ʹ 18.8” S and longitude of 34° 43ʹ47.6”E in lower highlands zones AEZ LH 2. Amalemba is located at latitude 0° 16ʹ 14.4” N and longitude 34° 45ʹ 14.6” E in upper midlands zone AEZ UM 4. Lurambi is located at latitude 0° 17ʹ 42.5” N and longitude 34° 4ʹ 47.9” E in the lower midlands AEZ LM 2 (FAO/UNESCO, Citation2000; Jaetzold, Schmidt, Hornet, & Shisanya, Citation2006). The following methods were used by farmers to process farm-saved seeds: wet seed fermentation, dry seed fermentation, wet and dry seed processing. The methods were not standard and farmers slightly modified them but the most critical aspects like fermentation and use of water were common. The following were the seed processing methods as described by farmers. Wet seed processing involved seed extraction and fermentation of seeds by placing them in water for 2 to 4 days. This was followed by seed cleaning and drying. Dry seed fermentation involved seed extraction and fermentation without the use of water by placing them in woven sacks or polythene bags for periods ranging from 5 to 14 days. This was followed by seed dying and removal of chaff. Wet seed processing is done by extracting the seeds in water to remove fruit pulp followed by seed cleaning and drying. Dry seed processing was done by extracting the seeds and drying them together with the chaff. In addition, some farmers considered seed processing tedious and time-consuming and did not process their seeds. Instead, they uprooted old nightshade plants with berries and placed them in the garden to germinate.
2.2. Seed quality tests
From each seed sample, 10 g replicated thrice was used in the determination of seed purity. Seed samples were separated into pure seeds, other crop seeds, inert matter, discolored, shriveled and insect-damaged seeds. Each component was weighed separately and the percentage fraction calculated as:
Seed moisture content was determined by removing water from seed by heating in an oven, and the lost weight determined as follows. Two empty containers were weighed; seeds placed into containers and weighed subtracting the weight of the container; seeds were crushed into small pieces and then dried in the oven at ± 103°C for ± 17 h. The samples were allowed to cool in an incubator for 15 min, and then the dry sample was weighed and moisture content calculated (Taylor, Citation2014). The percentage moisture content was calculated by subtracting the weight of seed materials before drying (initial weight) and weight of seed material after drying (final weight) divided by initial weight of seed material and multiplied by 100. Three replications of 50 seeds from each source were distributed over blotting paper sheets, moisturized with an amount of water equivalent to 2.5 times weight of paper, inside plastic boxes (11.0 x 11.0 x 3.5 cm) and exposed to 20–30°C with 8 h of light and 16 h of darkness. The evaluations on first and final germination percentages, seedling emergence, and seedling vigor index and seedling length were performed at 7 and 14 days in germination chamber after sowing in compliance with the rules for seed testing (International Seed Testing Association [ISTA], Citation2014). Seedling vigor index was calculated as seedling length (cm) x germination percentage (Dezfuli, Sharif-Zadeh, & Janmohammadi, Citation2008).
Germination index (GI) was calculated as described by International rules for seed testing (ISTA, Citation2014) by the following formula.
Time for 50% of germination (t ½/T50) is the point of the distribution in which the mean, medium or mode (point at which the highest frequency of germinated seeds) is observed. It represents the peak of germination. The time to 50% germination (T50) was calculated according to the formula modified by Farooq, Basra, Hafeez, and Ahmad (Citation2005).
where N is the final number of germination and ni, nj are cumulative number of seeds germinated by adjacent counts at times ti and tj when ni < N/2 < nj.
2.3. Field experiment
The field experiment was conducted during short rains (SR) of October–November, 2018 in Muthara location Meru County (Figure ).
Field sowing was done on 18th October using farm-saved seeds obtained from four Agroecological zones (UM2, LH2, UM4 and LM2). Seeds were processed following different methods including wet seed processing, dry seed processing, dry fermentation and wet fermentation were sown in different plots. A randomized complete block design (RCBD) with four treatments (seed processing method) and four replications, separately for each experimental site, was adopted. The treatments were arranged in split-plot. The Agroecological sites were the main plots (UM2, LH2, UM4 and LM2) and the processing methods represented the subplot (Table ).
Table 1. Field experiment plots' layout
The plot size was 3x3m2 with a spacing of 60 cm by 30 cm and two seeds per hill. Two manual cultivations were done to control weeds and supplementary irrigation done when needed. No pesticides were applied and all standard agronomic practices were carried out for all plots for uniformity.
2.4. Field data collection
Data were collected on stand count, days to 1st flower, plant height, number of fruits per plant and seed yields. The stand count was recorded by counting the number of plants that emerged in each plot and the data converted to plants/hectare. Plant height was determined by measuring the height of 10 randomly selected plants per plot. Ten plants from each plot were tagged and then fruits of each harvest were cut to evaluate seed processing methods. For seed yield data, the fruits were harvested separately in each plot, processed and weighed with an electronic balance to record seed yield. The seed weight was evaluated with values corrected for 10% moisture content. All seeds were air-dried to a constant moisture content of 10% and then kept in temporary storage at 20°C during initial testing. A determination of moisture content (fresh weight basis) was done using seed moisture meter (GMK-310 RT, G-Won Hitech co.ltd).
2.5. Evaluation of processing methods under controlled environment
To evaluate the seed processing using the standard methods, seeds were planted in the field following the experimental design and agronomic practices described in section 6.5. Ten plants were tagged in each plot using different color tags for each processing method. The plants on reaching maturity were harvested; fruits of each harvest were cut and processed following standard methods. The following procedures were modified and adopted using indigenous knowledge obtained from farmers during the survey on seed processing and the standard procedures as described by (Colley, Alex, & Linda, Citation2015; Desai, Kotecha, & Salunkhe, Citation1997; ISTA, Citation2014).
2.5.1. Wet seed processing
This method involved removing the seed from the overripe fruit, crushing them by hand in a basin or inside a bag. A jar of water was used to separate seeds from debris. Seeds usually sink and debris floats. This was followed by removing debris through repeated washing, and then drying.
2.5.2. Dry seed processing
was done by leaving the seeds to ripen on the plant, after which the fruits were harvested and then dried. The fruits were crushed together with the pulp and seeds were manually separated from the fruits chaff through sieving and winnowing.
2.5.3. Dry seed fermentation
involved harvesting of ripe nightshade fruits, they were then crushed and the mixture was placed in woven sacks without adding any water for a period of 5 days. The mixture was then dried. The mixture was manually separated to remove the chaff from seeds through winnowing.
2.5.4. Wet seed fermentation processing
Steps used for this method are as follows: Fully ripe fruits were opened by hand or gently squeezed while inside a woven bag. A mixture of seeds, pulp and juice obtained was cleaned by hand washing. The dirt and debris were rinsed from the mixture. The remaining mash of seeds, pulp and juice were poured in a basin or bucket. The mixture in a basin or bucket was fermented up to 3 days at an ambient temperature of 25°C. The mixture was stirred three times a day for aeration and even fermentation. During fermentation, the scum that appeared on the top of the mixture indicated that the process was successfully taking place. The top layer of the scum and pulp was poured off after 3 days of fermentation. Water was poured into the remaining mixture so that the volume was doubled. The mixture was stirred and allowed to settle again, and the top layer of pulp and debris poured off. Some lighter, less viable, seeds were poured off with top layer. The process was repeated severally until the water was fairly clean. The remaining contents (seed) were poured through a strainer retaining the seed and draining off the remaining water. The seeds were spread on woven or sack mats to dry. They were turned severally for even drying under a shade. Processed seed samples were put in brown paper (khaki) bags and kept at 5°C cool storage cabinet at the University of Nairobi awaiting seed quality and germination analysis.
2.6. Seed purity
Seed samples were separated into pure seeds, other crop seeds, inert matter, discolored, shriveled and insect-damaged seeds following the described in 2.3.
2.7. Seed quality tests
Seed quality tests were done on the seeds processed using different methods as described in 2.3. Pearson’s correlation coefficients were used to relate seed processing methods with seed quality parameters. Data obtained was used to determine the most optimal seed processing method for use by farmers for clean African nightshade seed production.
2.8. Data analysis
Data were presented using tables and graphs. The mean values for seed quality and germination tests were used for statistical analysis using statistical software version 9.2 (SAS; Citation2002). Principal component analysis (PCA) and Pearson’s correlation coefficient were used to infer relationships to determine the most suitable seed processing method. Means separation was done using the least significant difference (LSD) at (p ≤ 0.05) probability level.
3. Results
3.1. Survey on seed processing
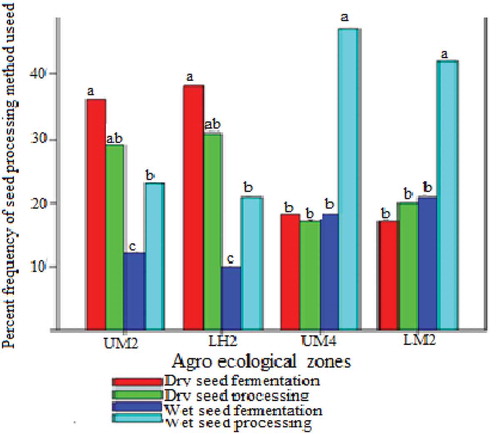
To obtain African nightshade seeds, farmers process berries using different methods. These methods generally follow a similar trend of seed extraction, cleaning and drying. The processing methods used include dry seed processing (Plate ), wet seed processing (Plate ), dry seed fermentation (Plate ) and wet seed fermentation (Plate ). The farmers in each Agroecological zone prefer a particular method of processing and there were significant (p ≤ 0.05) differences comparing the method of processing and the Agroecological zones. In UM2, 36% of farmers prefer dry seed fermentation with the highest and the least frequently used method being wet seed processing at 12%. In LH2, the method with the highest frequency was dry seed fermentation at 38%, while the lowest was wet seed processing at 10%. However, in UM4 and LM2, wet seed processing was the most preferred with 47% and 42%, respectively, and the least preferred in both zones was dry seed fermentation at 17% for each (Figure ).
3.2. Seed growth tests
Moisture content (MC) level differed (p ≤ 0.05) significantly comparing the seed processing methods. The MC was higher in wet-processed seed at 11.1% and lower in dry seed processing method 8.6%. Germination percentage, germination index and seedling emergence were higher in wet seed fermentation: 89.2%, 18.2 and 91.2%, respectively. These germination parameters were lower under dry seed processing recording 80.3%, 11.2 and 82.4%, respectively (Table ). Comparing time to 50% germination seeds under wet fermentation took less than 3 days while seed under dry seed fermentation took more than 4 days. In addition, the energy of germination was high in wet-processed seeds 0.86 and lowest in dry-processed seed at 0.70. Seedling vigor index a key germination parameter was highest in wet seed fermentation at 347.9 and the lowest was recorded for seeds processed by drying at 303.1 (Table ).
Table 2. Farm-saved seed moisture content, germination and growth tests for seeds processed using different seed processing methods under laboratory conditions
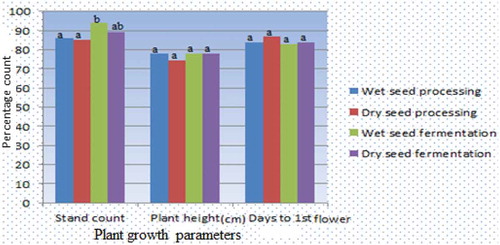
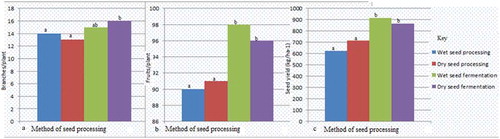
Comparing field parameters, stand count recorded 94% highest in wet fermentation processing method compared to other methods which did not differ significantly (Figure ). Seed processing methods had no significant effect on plant height and the time of the first flowering with plants flowering almost at the same time. In addition, seed processing method had no significant effect on the number of branches bearing fruits per plant (Figure ). However, the processing methods had significant variability in the number of fruits per plant and seed yields (kg/ha). Wet seed fermentation had the highest fruits per plant and seed yields/ha recording 98% and 913.8 kg/ha, respectively, and the lowest was wet seed processing with 90% and 622.5 kg/ha (Figure ).
3.3. Seed purity and growth tests obtained for field experiment
Seeds harvested and processed from the field experiment were subjected to purity tests. Wet fermentation processing method had the highest purity of 96.3% and low in other crop seeds, inert matter, discolored seeds and no insect damage. Dry seed fermentation produced seeds with the lowest purity of 72% and was high in other crop seeds, inert matter, discolored seeds and insect damage (Table ).
Table 3. Seed quality parameters for different seed processing methods
Comparing growth tests for the different processing methods, there were significant differences in the various parameters tested. Wet fermentation processed seeds exhibited a high level of seed quality; the germination percentage (GP) was 97.2% and seedling vigor index (SVI) 408.2 and other parameters followed a similar trend (Table ). Dry seed processing method was the lowest in all growth parameters with germination percentage (GP) and seedling vigor index (SVI) recording 84.1% and 319.6, respectively. In addition, the duration of seed storage had no significant effect of growth parameters due to short storage time (Table ).
Table 4. Seed moisture content, germination and growth tests for seeds processed using different seed processing methods under laboratory conditions
The principal component analysis (PCA) was carried out with four germination parameters to determine their effect on the seed processing method. Seed purity was the most important parameter as it showed an excellent correlation with seedling vigor index, germination percentage and yields per ha (Figure ). Seed processing method contributed to a maximum variance of 93.59% and growth factors contributing to a variance of 6.22% on the Y-axis (Figure ). The first principal components were seed purity and seedling vigor index and strongly correlated with the germination percentage and yields per hectare (Figure ).
Figure 5. Principal component analysis (PCA) of different germination parameters under different treatment methods.
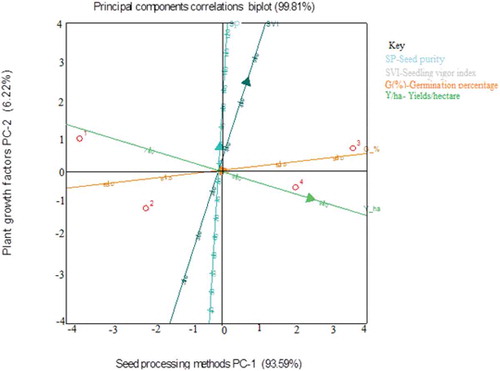
There was a positive and significant correlation between crop growth parameters. Seed purity (SP) had a positive correlation with germination percentage (GP) (r = 0.922), germination index (GI) (r = 0.855), seedling vigor index (SVI) (r = 0.811) and yields per hectare (Y/HA) (r = 0.828). GP was positively correlated with SVI (r = 0.756) and Y/HA (r = 0.766). GI was positively corrected to GP (r = 0.867) and SVI (r = 0.723). SVI was positively correlated with SP (r = 0.9130, GP (r = 0.898) and GI (r = 0.766). In addition, crop yield was positively influenced by SP (r = 0.922), GP (r = 0.913) and SVI (r = 0.782) and no significant correlation with GI (r = 0.388) and fruits/plant (r = 0.234) (Table ).
Table 5. Correlation between different germination parameters with different seed processing methods
4. Discussion
This study revealed that four methods of seed processing are variably preferred by farmers in different agroecological zones to process edible nightshade seed. These methods were wet seed processing, dry seed processing, dry seed fermentation and wet seed fermentation. In addition, some farmers preferred to have plants mature and ripen in the field, uproot and place them in the garden to dry and germinate without any processing. The processing method preference varied in the agroecological zones and this was attributable to the indigenous knowledge passed on from one generation of farmers to the next. Dry seed fermentation was the most preferred by farmers in agroecological zones UM2 and LH2. Farmers preferred to crush the seeds and keep them in sacks to ferment for 2–3 days and dry them under the sun. Due to poor fermentation farmers reported rotting of berries. Rotting of berries due to poor fermentation has also been reported by (Ekhuya et al., Citation2018). It is recommended that fermentation should be done under a controlled environment and seeds washed severally before drying (Amaza, Udoh, Abdoulaye, & Kamara, Citation2010). This is done to remove germination inhibitors (Oiye, Shiundu, & Oniang’o, Citation2009). However, in UM4 and LM2 agroecological zones, majority of farmers preferred wet seed processing. They reported that this method was fast and less tedious and needed not to wait for seeds to ferment. This shows that farmers are not aware of the benefits of proper seed fermentation in quality seed production. Farmers planting low-quality seed risk poor field emergence and low plant vigor as a result of the poor physiological quality of seed (Matthews, Noli, Demir, Khajeh, & Wagner, Citation2012).
Seed washing is a critical step in seed processing and was done by more than half of the farmers’. This allows the washing off of sugars in the nightshade fruit pulp that is a cause of poor germination. Elizabeth and Adeniji (Citation2015) reported low vigor of indigenous vegetable seeds caused by inadequate removal of sugars and germination inhibitors. In addition, many farmers reported difficulties in seed processing, lacked a standard processing procedure and poor extension services leading to poor quality seed. Abukutsa-Onyango (Citation2010) reported that farmers lack knowledge on the processing of indigenous vegetable seeds due to lack of training or poor extension services. Poorly processed seeds constrain yields due to poor germination and disease pressure as reported by Odeyemi et al. (Citation2010)
Wet seed fermentation processing resulted in seeds with high germination and yields per ha. This could be attributed to high seed quality resulting from this method. This method entails frequent washing to remove the pulp, fruit juice and the scum. In addition, it allows for the removal of plant impurities and proper separation of clean seed. Oiye et al. (Citation2009) reported that fermentation helps break germination inhibition by removing the mucilage in the seeds. Proper dying, packaging and storage of seeds are critical in maintaining high seed quality. Odeyemi et al. (Citation2010) reported that rapid sun-drying affects the quality of seeds. In addition, packaging of seeds prevents damp conditions leading to poor quality seeds. Farmers reported that the seeds can remain viable for many months when kept dry, but rapidly lose germination capacity when stored in humid conditions.
The principal component analysis revealed that seed processing method correlated with seed purity, seedling vigor, germination percentage and yields/ha. This implies that the method of seed processing with the highest seed purity and seedling vigor index is the most suitable for use to obtain high crop yields. Seed purity had a strong positive correlation with other germination parameters. Farmers rely on these poorly processed seeds leading to low germination and reduced yields. Most of the farmers produce seeds that are associated with high seedling mortality and reduced yields (FAO, Citation2013). Farmers select and store part of their harvest for future planting, exchange seeds with relatives and other farmers or sell at local markets (International Food Policy Research Institute [IFPRI], Citation2012). This agrees with the finding by Elizabeth and Adeniji (Citation2015) that more than 90% of farmers in Kenya use own saved seeds. Farmers continue with on-farm seed multiplication without seed renewal with poor knowledge on seed processing and handling contributing to poor yields (Amaza et al., Citation2010). The lack of overhead, distribution and seed testing costs enables farmers to offer own produced seeds at relatively lower prices. However, the risks of planting such seeds are high due to poor quality and infections (Louwaars & De Boef, Citation2012). The government and seed companies in Kenya have failed to educate farmers or offer incentives to help in the production of clean edible nightshade seeds. Proper seed production and handling would increase leafy nightshade vegetable yields. The increased production can meet the demand and alleviate the hidden hunger and malnutrition among resource-poor communities.
5. Conclusions
Few farmers grow the vegetable crop for the purpose of seed production despite the increasing demand against limited supply. In addition, a lack of knowledge on production and processing has exacerbated the problem. Knowledge on edible nightshade seed production is indigenous and the sector is neglected by government agricultural extension. Farmers prefer to process their seeds quickly by employing methods like dry and wet processing which led to low seed quality. The focus should be on production of high-quality seed as a critical component for realizing high nightshade vegetable yields.
6. Recommendations
This study recommends wet seed fermentation processing method that farmers can adapt to process own seeds. The method ensures proper fermentation, washing and drying which guarantees quality seeds. The study also recommends standard quality tests that can be used to test for quality. This is an important step towards formalization of edible African nightshade seed in Kenya. The information from this study can be utilized by seed companies and extension workers to increase seed production in Kenya to meet the growing demand.
Competing interests
The author declares no competing interests.
Acknowledgements
The National Research Fund-Kenya and the University of Nairobi are acknowledged for providing facilities and partially funding this study.
Additional information
Funding
Notes on contributors
Kimaru S. Linguya
Kimaru S. Linguya is a researcher in the Department of Plant Sciences, University of Nairobi-Kenya in the final year of his PhD studies. His key research area is to improve production of indigenous vegetables to boast food security in African countries. This research is a collaborative effort by him together with Prof. Kimenju J.W, Dr Maina W.M and Dr. Kilalo D.C all of the University of Nairobi. The title of his research work is “Detection and characterization of plant pathogens associated with African nightshade seed and methods of seed processing to reduce infection”. The author main focus is to develop an optimal seed processing method for quality seed production to address the hidden hunger and malnutrition in African countries. This study recommends a suitable seed processing method for use by all seed producers to upscale the production of indigenous leafy nightshade seed to meet the increasing demand by consumers.
References
- Abukutsa-Onyango, M. O. (2010). Strategic repositioning of African indigenous vegetables in the horticulture sector Résumé.4, 1413–16.
- Amaza, P., Udoh, E., Abdoulaye, T., & Kamara, A. (2010). Analysis of technical efficiency among community-based seed producers in the savannas of Borno State, Nigeria. Journal of Food, Agriculture & Environment, 8, 73–79.
- Colley, M., Alex, S., & Linda, B. (2015). Organic seed processing: Threshing, cleaning and storage: Organic agriculture handbook 354.Ohio state University.http://www.oardc.ohio-state.educ/seedid.
- Desai, B. B., Kotecha, P. M., & Salunkhe, D. K. (1997). Seeds handbook: Biology, production, processing and storage. New York, NY: Marcel Dekker Inc.
- Dezfuli, P. M., Sharif-Zadeh, F., & Janmohammadi, M. (2008). Influence of priming techniques on seed germination behavior of maize inbred lines (Zea mays L). ARPN Journal of Agricultural and Biological Science, 3, 22–25.
- Ekhuya, N. A., Wesonga, J. M., & Abukutsa, M. O. (2018). Production, processing and storage techniques of African nightshade (solanum spp.) seeds and their correlations with farmers’ characteristics in western Kenya. African Journal of Food, Agriculture, Nutrition and Development, 18(2), 13338–133351. doi:10.18697/ajfand.82.17445
- Elizabeth, S., & Adeniji, O. T. (2015). Contract production of indigenous vegetable seeds; a case of East and Southern Africa. African J. Agric. Res, 3, 303–309.
- FAO. (2013). The state of food and agriculture: Food systems for better nutrition (plant production and protection paper 202).Rome, Italy: Food and Agriculture Organization of the United Nations Rome.
- FAO/UNESCO. (2000). Revised legend for the soil map of Kenya (World soil resources report. 68). Rome
- Farooq, M., Basra, S. M. A., Hafeez, K., & Ahmad, N. (2005). Thermal hardening: a new seed vigor enhancement tool in rice. Acta Botanica Sinica, 47, 187–193.
- Ghosh, J. S., Singh, A., Magsumbol, M. S., Kamboj, P., & Goldberg, G. (2016). Exploring the potential of indigenous foods to address hidden hunger: nutritive value of indigenous foods of santhal tribal community of jharkhand, India. Journal of Hunger and Environmental Nutrition, 11(4), 548–568. doi:10.1080/19320248.2016.1157545
- IFPRI. (2012). Global food policy report (pp. 114). Washington DC: International Food Policy Research Institute.
- ISTA. (2014). International Rules for Seed Testing. Seed Sci. Technol., 24, 39–42.
- Jaetzold, R., Schmidt, H., Hornet, B., & Shisanya, C. (2006). Farm management handbook of Kenya (Vol. II/C1). Ministry of Agriculture, Kenya and German Agency Technical Cooperation team (CTZ).
- Louwaars, N. P., & De Boef, W. S. (2012). Integrated seed sector development in Africa: A conceptual framework for creating coherence between practices, programs, and policies. Journal of Crop Improvement, 26, 39–59. doi:10.1080/15427528.2011.611277
- Matthews, S., Noli, E., Demir, I., Khajeh, H. M., & Wagner, M. H. (2012). Evaluation of seed quality: from physiology to international standardization. Seed Science Research, 22, 69–73. doi:10.1017/S0960258511000365
- Milošević, M. (2010). Vigour tests as indicators of seed viability. Genetika, 42, 103–118. doi:10.2298/GENSR1001103M
- Odeyemi, O. B., Ajayi, S. A., & Olakojo, S. A. (2010). Physiological quality of informal-sector maize seeds in Nigeria. Journal of New Seeds, 11, 190–199. doi:10.1080/1522886X.2010.486226
- Oiye, S., Shiundu, K., & Oniang’o, R. (2009). The contribution of African leafy vegetables (ALVs) to vitamin A intake and the influence of income in Rural Kenya. African J. Food Agric. Nutr. Dev., 4, 19–25.
- SAS. (2002). SAS online doc, Version 9.2. Cary, NC: Author.
- Sthapit, B. R., Ram, C., Pashupati, B., & Pratap, S. (2008). Informal seed systems and on-farm conservation of local varieties. In M. H. Thijssen, Z. Bishaw, A. Bashir, & W. S. de Boef (Eds.), Farmers, seeds and varieties: supporting informal seed supply in Ethiopia (Vol. 4, pp. 56–60). Wageningen: Wageningen International.
- Taylor, J. (2014). The international rules seed testing: 2014 edition ISTA ordinary general meeting Antalya Turkey.