?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Scientific evidence suggests that honey is a novel antioxidant and it can reduce the risk of chronic diseases, including cancer and heart disease. It is particularly recommended for children and sportsmen as it can help to support the overall well-being of the elderly as well as the invalids. The objective of this paper is to review on honey with emphasis on its antioxidant activity, the major components responsible for the antioxidant activity, and the factors leading to the variation in the antioxidant activity of honeys. In addition, the advantages and disadvantages of basic analytical methods used for the determination of the antioxidant activities (AOA) of honey are reviewed. It is hoped that this summarized review on the various methods available for the determination of the antioxidant activity of honey can provide the scientific community with reliable information to confirm the benefits of the antioxidant effects of honey and help to provide some basic information before a more expensive and time-consuming effort of identification and characterization of the antioxidant components is embarked. From this review, we can determine that environmental and climatic conditions, the botanical origin/the plant sources, the amount of phenolic compounds in honey or total phenolic content (TPC) and color are among the factors which can influence the antioxidant activity of honey. The review may also help to know that although many analytical methods are available to determine the antioxidant activity of honey, each method has its own limitations; so, it is important to employ a consistent and rapid method and in most cases it is necessary to use several tests to get good reliability.
PUBLIC INTEREST STATEMENT
Antioxidants can be either natural or synthetic; but the demand for natural antioxidants is increasing day to day to a healthy nutrition, both due to the possible negative effects of synthetic food additives on human health and the increased consumer perception of this problem. Epidemiological studies have indicated that frequent consumption of natural antioxidants is associated with a lower risk of cardiovascular disease and cancer. Hence, the applications of synthetic antioxidants have been restricted and there is a trend to substitute them with naturally occurring antioxidants. Honey is a naturally occurring food substance known to be rich in both enzymatic and non-enzymatic antioxidants. Therefore, assessing antioxidant activities and the major components responsible for the antioxidant activity of honey, and evaluating the analytical methods used for the measurement of the antioxidant activity of honey is very important. Thus, this review can give information to researchers in the area and helps honey consumers to know more about the medicinal values of honey.
Competing Interests
The authors declare no competing interests.
1. Introduction
Oxidation reactions can produce free radicals such as superoxide anion (O2•), hydroxyl (•OH), peroxyl (ROO•), and alkoxyl radicals (RO•). These radicals can start chain reactions; when the chain reactions occur in a cell, they can cause damage or death to the cell (Halliwell & Gutteridge, Citation1989). (Pandey, Mehdi, Maurya, & Rizvi, Citation2010) stated that degradation of biological macromolecules, protein, lipid, and DNA damage, cell aging, oxidative stress-originated diseases and cancer is caused by oxidation reactions occurred as a result of free radicals.
Damages related to oxidation reactions can be reduced by antioxidants. Antioxidants are molecules that scavenge or quench reactive oxygen species (ROS) and reactive nitrogen species (RNS) products of respiration including free radicals, and inhibit the oxidation of other molecules (Ames, Shigenag, & Hagen, Citation1993). They are agents which counteract deterioration caused by oxidants such as O2, OH−, superoxide, and/or lipid peroxyl radicals. Cancer, synthesis of mutagens, aging, atherosclerosis, and many chronic and degenerative lingering diseases are susceptible to oxidative stress (Hallowell & Gutteridge, Citation1989).
Antioxidants terminate the chain reactions by removing free radical intermediates and inhibit other oxidative reactions (Shenoy & Shirwaiker, Citation2002). They do so by being oxidized themselves. Antioxidants are often reducing agents such as thiols, ascorbic acid or polyphenols (Sies, Citation1997). The terms “antioxidant activity” and “antioxidant capacity” have different meanings: antioxidant activity deals with the kinetics of a reaction between an antioxidant and the prooxidant or radical it reduces or scavenges, whereas antioxidant capacity measures the thermodynamic conversion efficiency of an oxidant probe upon reaction with an antioxidant. Measuring the antioxidant activity/capacity levels of food and biological fluids (e.g., human serum) is carried out for the meaningful comparison of the antioxidant content of foodstuffs and for the diagnosis and treatment of oxidative stress-associated diseases in clinical biochemistry (Gutteridge, Gregory, & Timothy, Citation1994).
According to Huang, Ou, and Prior (Citation2005), based on their application, antioxidants have been traditionally divided into two classes: primary or chain-breaking antioxidants, and secondary or preventative antioxidants. In chain-breaking mechanisms, radical initiation (by reacting with a lipid radical) or propagation (by reacting with alkoxyl or peroxyl radicals) steps are inhibited.
On the other hand, secondary (preventative) antioxidants are molecules that retard the rate of oxidation, e.g., transition-metal ion chelators may inhibit Fenton-type reactions that produce hydroxyl radicals:
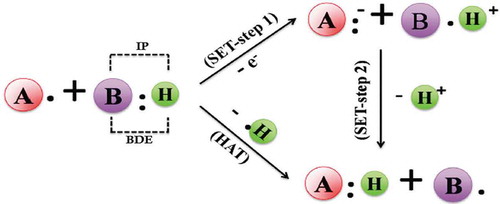
Another classification of antioxidants is based on the types of chemical reactions they can undergo. Wright, Johnson, and DiLabio (Citation2001) stated that based on the type of chemical reactions antioxidants undergo, anti-oxidant assays can be categorized as electron transfer (ET) and hydrogen atom transfer (HAT)-based assays. In the first case, the capacity of the possible antioxidants to transfer an electron and reduce certain compounds, including carbonyls, metals, and radicals is evaluated. In the second case (HAT), the capacity of an antioxidant to scavenge free radicals by proton donation is measured ().
HAT-based assays measure the capability of an antioxidant to quench free radicals. In this case, the hydrogen atom (H) of a phenol (Ar–OH) is transferred to a ROO• radical:
The AH and ArOH species denote the protected biomolecules and phenolic antioxidants, respectively. The aryloxyl radical (ArO•) formed from the reaction is stabilized by resonance. Effective phenolic antioxidants need to react faster than biomolecules with free radicals to protect the latter from oxidation. Prior, Wu, and Schaech (Citation2005) stated that in HAT-based antioxidant assays both the fluorescent probe and antioxidants react with ROO•, and the antioxidant activity can be determined from competition kinetics by measuring the fluorescence decay curve of the probe in the absence and presence of antioxidants, integrating the area under these curves, and finding the difference between them.
The HAT-based assays include oxygen-radical absorbance capacity (ORAC) assay, TRAP assay using R-phycoerythrin as the fluorescent probe, crocin bleaching assay using 2,2ʹ-azobis(2-amidinopropane) hydrochloride (AAPH) as the radical generator, and β-carotene bleaching assay, although the latter bleaches not only by peroxyl radical attack but by multiple pathways (Prior et al., Citation2005).
In ET-based assays, the antioxidant action is simulated with a suitable redox-potential probe, namely, the antioxidants react with a fluorescent or coloured probe (oxidizing agent) instead of peroxyl radicals. Spectrophotometric ET-based assays measure the capacity of an antioxidant in the reduction of an oxidant, which changes colour when reduced. The degree of colour change (either an increase or decrease of absorbance of the probe at a given wavelength) is correlated to the concentration of antioxidants in the sample. The 2, 2ʹAzinobis (3ethylbenzothiazoline 6 sulfonic acid) (ABTS)/Trolox equivalent antioxidant capacity (TEAC) and DPPH were decolorization assays (Brand-Williams, Cuvelier, & Berset, Citation1995) while ferric reducing antioxidant power (FRAP) and cupric reducing antioxidant capacity (CUPRAC) were mentioned as colorization assays where there is an increase in absorbance at a prespecified wavelength as the antioxidant reacts with the reagent (Apak, Guclu, Ozyurek, & Karademir, Citation2004).
Many authors: Gheldof, Wang, and Engeseth (Citation2002), Proteggente et al. (Citation2002), and Beretta, Granata, Ferrero, Orioli, and Facino (Citation2005) mentioned that whole grains, fruits (oranges, lemons, apples) cereals (oat, rice) and vegetables, olive oils, are effective in prevention of some diseases including coronary atherosclerosis. This is due to the presence of antioxidant components such as phenolic and flavonoid groups. As a result, they are considered as primary sources of naturally occurring antioxidants.
Honey is another natural product, rich in antioxidant components, which is made by honeybees (Apis mellifera), mainly composed of a complex mixture of carbohydrates (Blasa et al., Citation2006). Similarly, Cantarelli, Pellerano, Marchevsky, and Camiña (Citation2008) stated that honey is a natural food produced by bees from nectar or secretion of flowers composed of 80–85% carbohydrates, 15–17% water, 0.3% protein, 0.2% ash, and minor quantities of amino-acids and vitamins as well as other components in low levels of concentration. Barganska, Slebioda, and Namiesnik (Citation2013), (Citation2014) explained that honey produced by honey bees from pollen, plant nectars, or honeydew is composed of over 300 chemical substances which belong to different chemical compound groups, mainly of which are carbohydrates and water with minor components comprising of fatty acids, proteins, minerals, dyes, fragrances, enzymes, essential oils, sterols, phospholipids, and organic acids.
Scientific evidence suggests that honey has an antioxidant property and it can reduce the risk for chronic diseases, including cancer and heart disease (Bjelakovic, Nikolova, Simonetti, & Gluud, Citation2004; Khalil &Sulaiman, Citation2010; Paolini, Sapone, Canistro, Chieco, & Valgimigli, Citation2003; Vivekanathan, Penn, Sapp, Hsu, & Topol, Citation2003). Due to its potential as an antioxidant and positive medicinal properties, honey is particularly recommended for children and sportsmen as it can help to support the overall well-being of the elderly as well as the invalids (Blasa et al., Citation2006). Due to this fact, it is crucial that the antioxidant potential of honey is determined, which can also help in the identification of different types of honey. The study conducted by (Bertoncelj, Dobersek, Jamnik, & Golob, Citation2007) has investigated different honey types and reported significant differences in their antioxidant properties. Measuring the total antioxidant capacity of honey will provide an understanding of the functional properties of honey. Although many methods are available to determine the antioxidant activity, it is important to employ a consistent and rapid method and in most cases it is necessary to use several tests to get good reliability (Fukumoto & Mazza, Citation2000). The aim of this paper is to review on the properties of honey mainly: the antioxidant activity, the major components responsible for the antioxidant activity of honey, the factors leading to the differences in the antioxidant activities of honeys, and to evaluate the different assays used by various researchers for the determination of the antioxidant properties of honey. This may be useful for future researchers to select an appropriate analytical method before a more expensive and time-consuming effort of identification and characterization of the methods is embarked. This review can also give information to researchers in the area and helps honey consumers to know more about the medicinal values of honey.
2. Antioxidant activity of honey
Although free radicals of oxygen are a natural byproduct of metabolism within the organism, they cause cellular damage and breakdown the structure of DNA. Free radicals may oxidize nucleic acids, proteins, lipids or DNA and have the potential to initiate degenerative disease. There are also preliminary reports pointing to the role of oxidative stress in ageing (Gilca, Stoian, Atanasiu, & Virgolici, Citation2007; Muller, Lustgarten, Jang, Richardson, & Van Remmen, Citation2007). On the other hand, Nunes et al. (Citation2012) stated that even though ageing can contribute to the occurrence of oxidative stress, the main cause is an imbalance between ROS and the antioxidative defense systems and is considered to be the major reason for many diseases such as cancer, cardiovascular diseases, neurodegenerative diseases. Cells exhibit defense system against oxidative damage. This defense system consists of free radicals and other oxidative protective agents such as catalase, superoxide dismutase, peroxidase, ascorbic acid, tocopherol, and polyphenols (Nagai, Sakai, Inoue, Inoue, & Suzuki, Citation2001). These antioxidant agents stimulate biomolecules such as carbohydrates, proteins, lipids, and nucleic acids. Cells are altered by this stimulation and ultimately provoking antioxidant response (Diplock, Rice-Evans, & Burdon, Citation1994). Antioxidants bind the dangerous molecules (free radicals), preventing their harmful effects (Jaganathan & Mandal, Citation2009). Antioxidants for the treatment of cellular degenerations are beginning to be considered because they inhibit or delay the oxidative process by blocking both the initiation and propagation of oxidizing chain reactions. Gorelik, Ligumsky, Kohen, and Kanner (Citation2008) stated that food antioxidants protect our body against the oxidative damage induced by free radicals and reactive oxygen species generated as byproducts of metabolism or inflammatory processes. Therefore, foods containing significant levels of antioxidants, which can inhibit or delay the oxidation of a substrate, represent a healthy and logical diet choice (Gheldof & Engeseth, Citation2002a).
Due to the possible negative effects of synthetic food additives on human health and to the increased consumer perception of this problem, the demand for natural antioxidants is increasing day to day to a healthy nutrition (Baltrusaityte, Venskutonis, & Ceksteryte, Citation2007). According to the reports of Kamra and Bhatt (Citation2012), currently available synthetic antioxidants, including butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), gallic acid, etc., have been found to cause negative health effects. Hence, their application has been restricted and there is a trend to substitute them with naturally occurring antioxidants. Renaud, Gueguen, Schenker, and Houtaud (Citation1998) and Temple (Citation2000) also stated that natural antioxidants, particularly in fruits and vegetables, have gained increasing interest among consumers and the scientific community because epidemiological studies have indicated that frequent consumption of natural antioxidants is associated with a lower risk of cardiovascular disease and cancer.
Honey exhibits strong antioxidant activity (Ahmed & Othman, Citation2013). Published research denotes it as a novel antioxidant agent (Al-mamary, Al-meeri, & Al-habori, Citation2002; Erejuwa, Sulaiman, & Ab Wahab, Citation2012. Unlike synthetic compounds, honey represents a natural product that does not carry side effects which can be harmful to health. Lachman, Orsak, Hejtmankova, and Kovarova (Citation2010) suggested that the antioxidant power of honey is an eligible quality parameter to accept honey as a natural antioxidant. Thus, finding the antioxidant and therapeutic advantages of honey have been reviewed (Erejuwa et al., Citation2012; Mirunalini, Dhamodharan, & Deepalakshmi, Citation2012). Antioxidant properties of honey act as an antidepressant during high emotional, physical and intellectual stress (Jaganathan & Mandal, Citation2009). This antioxidant capacity of honey contributes to the prevention of several acute and chronic disorders such as inflammatory, allergic, thrombotic, diabetic, cardiovascular, cancer, and others. Honey is known to be rich in both enzymatic and non-enzymatic antioxidants (Aljadi & Kamaruddin, Citation2004; Gheldof et al., Citation2002). Among the compounds found in honey; vitamin C, phenol compounds, catalase, peroxides, glucose oxidase enzymes have antioxidant properties. Honey also contains flavonoids and carotenoids. High levels of these indicators ensure a high level of antioxidants in honey. Researchers also showed that honey (1.2 g/kg) elevated the amount and activity of antioxidant agents such as beta-carotene, vitamin C, glutathione reductase, and uric acid in healthy human subjects (Al-Waili, Citation2003). Uric acid levels can vary based on sex. According to the American College of Rheumatology (ACR, 2019), normal values for women are 2.5 to 7.5 milligrams/deciliter (mg/dL) and for men 4.0 to 8.5 mg/dL. However, the values may vary based on the lab doing the testing. High levels of uric acid (Hyperuricemia) in our blood typically indicate that our body is making too much uric acid or that our kidneys are not removing enough uric acid from our body. Having cancer or undergoing cancer treatment can also raise our uric acid levels. Low levels of uric acid are less common than high levels and are less of a health concern.
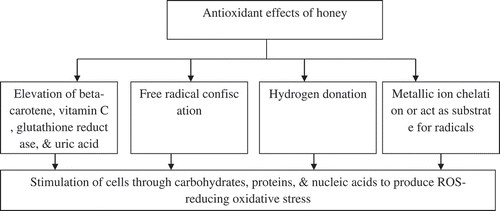
The antioxidant effect of honey is well established, but urges to explore the exact mechanisms involved and extrapolation to clinical trials. The exact antioxidant mechanism is unknown, but the proposed mechanisms include free radical sequestration, hydrogen donation, metallic ion chelation, flavonoids substrate action for hydroxyl, and superoxide radical actions (Al-mamary et al., Citation2002). Figure is presenting all the possible mechanisms involved in the antioxidant effects of honey.
Figure 1. Mechanisms of antioxidant effects of honey (Al-mamary et al., Citation2002).
The antioxidant properties of honey can be measured in the form of antiradical activity using, oxygen-radical absorbance capacity (ORAC) assay, 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay, and ferric reducing antioxidant power (FRAP) assay (Erejuwa et al., Citation2012). The free radical scavenging activity of antioxidants in honey has been substantially investigated and reported in the literature (Taormina, Niemira, & Beuchat, Citation2001). A number of clinical studies (Garcia & Castillo, Citation2008; Kandaswani & Middleton, Citation1994; Middleton, Kandaswami, & Theoharides, Citation2000) have suggested that the antioxidant contents in honey are the main factors for its observed therapeutic action in reducing the incidence of chronic diseases including heart disease and some cancers. In addition, honey can prevent deteriorating oxidation reactions in foods such as enzymatic browning of fruit and vegetables (Chen, Mehta, Berenbaum, Zangerl, & Engeseth, Citation2000), lipid oxidation in meat (Gheldof & Engeseth, Citation2002a; Nagai, Inoue, Kanamori, Suzuki, & Nagashima, Citation2006), and inhibit the growth of foodborne pathogens and food spoilage organisms (Mundo, Padilla-Zakour, & Worobo, Citation2004). The components responsible for the redox properties of honey are likely to be phenolic acids, flavonoids, vitamins, and enzymes, as well as a small amount of mineral content, particularly copper and iron (Erlund, Citation2004; Meda, Lamien, et al., Citation2005b). Similarly, Beretta et al. (Citation2005) and Soares, Dinis, Cunha, and Almeida (Citation1997) stated that antioxidant and antiradical properties of honey extract have mainly been attributed to the presence of phenolic compounds and the effectiveness of a honey fraction is proportional to its phenolic concentrations. Moreover, the study conducted by Khalil, Sulaiman, and Boukraa (Citation2010) reported that honey is considered as a significant source of antioxidants since it is rich in phenolic acids, flavonoids, ascorbic acid and carotenoids. Polyphenols, including flavonoids and phenolic acids, are found in honey and act as free radical scavengers, peroxy-radical scavengers and as metal chelators (Chimi, Cillard, Cillard, & Rahmani, Citation1991; Shahidi & Wanasundara, Citation1992).
3. Factors influencing the antioxidant activity of honey
According to Nigussie, Subramanian, and Gebrekidan (Citation2012), the variation in sensorial and physicochemical characteristics of honey is due to the variations in geographical, climatic, environmental conditions, as well as the botanical origin of plants from which it is harvested. Baltrusaityte et al. (Citation2007) reported that honey properties from different locations should be different because the composition of active components in plants depends on various factors, particularly plant bio and chemo type and climatic conditions. Similarly, Guler, Bakan, Nisbet, and Yavuz (Citation2007) stated that the composition of honey depends on the plant species visited by the honey bees and the environmental processing and storage conditions. Although the composition of honey can be variable and is primarily dependent on its floral, geographical, and entomological source, certain external factors, such as seasonal and environmental factors and processing, handling and storage of honey may also influence its composition and they play important roles in determining honeys properties (Gheldof et al., Citation2002; Khalil et al., Citation2010; Turkmen, Sari, Poyrazoglu, & Velioglu, Citation2006).
Kaskoniene and Venskutonis (Citation2010) suggested that the properties and composition of honey are affected greatly by various factors including its nectar source, collection season, mode of storage, and harvest technology and conditions. The differences in the antioxidant activities of honey depend on the floral or the botanical origin (Al-mamary et al., Citation2002; Jantakee & Tragoolpua, Citation2015; Kaskoniene et al., Citation2009; Kesic et al., Citation2009), seasonal and environmental factors (Estevinho, Pereira, Moreira, Dias, & Pereira, Citation2008), processing, handling, and storage of honey (Al-mamary et al., Citation2002). Different scholars, Lamien et al. (Citation2005b) and Socha, Juszczak, Pietrzyk, and Fortuna (Citation2009) reported that the antioxidant activity of natural honeys depends largely on their chemical composition, such as phenolics, flavonoids, enzymes, organic acids, amino acids, reaction products, ascorbic acid, carotenoids, as well as their origins.
Phytochemicals are chemical substances naturally occurring in plants and many of them are now recognized to have health-promoting activity (Apostolidis, Kwon, & Shetty, Citation2006; Liu, Citation2003, Citation2004; Sun, Chu, Wu, & Liu, Citation2002; Vattem, Ghaedian, & Shetty, Citation2005). Phenolic substances are the largest group of phytochemicals (King & Young, Citation1999). The plants containing phytochemicals might be used as a supply of the bees; thereby bioactive compounds can be transferred to honey. Studies have shown that honey contains great variation in contents of different phytochemicals according to floral sources and climatic conditions, which contribute to different characteristic colors, flavors, aromas, and bioactivities (Abu-Tarboush, Al-Kahtani, & El-Sarrage, Citation1993; Molan, Citation1996). As herbal medicines are derived from different plants, which can produce different therapeutic properties (Villegas et al., Citation1997), some honey derived from these specific plants may provide added value for health promotion. Honey produced by bees fed herbal extracts has shown greater antioxidant activity than normal honey (Rosenblat, Angonet, Goroshit, Tabak, & Neeman, Citation1997).
Polyphenols are groups of phytochemicals currently of particular interest to medical and food nutrition research, mainly because of their functional properties. Besides being radical scavenger, polyphenols could be an effective immune modulator and hormone action inhibitor (Havsteen, Citation2002). Polyphenols are believed to be potent scavengers of peroxyl radicals, mainly because of the presence of high mobility of hydrogen in their molecular structures (Al-mamary et al., Citation2002). They have also been reported to affect the flavor (Steeg & Montag, Citation1988) and physical appearance of honey, particularly honey colour (Alvarez-Suarez et al., Citation2010; Isla et al., Citation2011). Interestingly, they have been given considerable attention to be an eligible parameter for honey quality assessment (Al-mamary et al., Citation2002), as well as for honey marker identification, with the help of the advancement of liquid chromatography and mass spectrometry technology nowadays. Savatović et al. (Citation2011) explained that honey colour depends on its botanical origin, minerals, and other minor components, and darker honey has better radical scavenging activity due mainly to its phenolic content. Similarly, Aldina, Nadira, and Almir (Citation2015) who conducted a research on the phytochemical profile of honey stated that the chemical composition of honey largely depends on botanical origin as an important factor and the botanical origin significantly affects the polyphenol content and the total antioxidant activity of honey samples (Figure ).
Figure 2. Total antioxidant activity of honey samples of different botanical origin (Aldina et al., Citation2015).
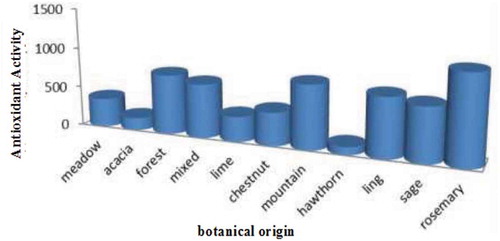
Depending on the plant sources on which the bees forage, honeys may have dark amber to clear white color. One of the factors that lead to this color difference is the difference in the amount of phytochemicals contained in the plant which in turn affects the antioxidant activity of honeys. The study made Mekuanint and Meareg (Citation2019) stated that honey samples with dark colors were found to have higher total phenolic content and higher antioxidant activity as compared to honey samples with relative clear white colors. Similar studies were reported by Dubero, Minaleshawa, Mesfin, and Tewabech (Citation2015) who did on total phenols and antioxidant activities of natural honeys and propolis collected from different geographical regions of Ethiopia and showed that darker honeys had higher total phenolic content and higher antioxidant activities than clear white honeys. Jasicka-Misiak, Poliwoda, Derea, and Kafarski (Citation2011) also reported that the total phenolic content and the antioxidant activity of amber to dark amber Algerian honey samples were higher (459.83 ± 1.92 mg/kg) than light color Borneo (206.33 ± 1.05 mg/kg) and Tualang (352 ± 0.81 mg/kg) Malaysian honey samples and one of the well-investigated Manuka honey (435 mg GAE/kg) of the New Zealand. Saxena, Gautama, & Sharma (Citation2010) reported that color intensity has positive correlations of 0.72–0.83 with the total phenolic content and antioxidant activities. Likewise, Moniruzzaman, Sulaiman, Khalil, and Gan (Citation2013b) reported that Color intensity of honey is a reliable parameter that indicates the presence of pigments which has antioxidant activities such as carotenoids and flavonoids.
4. Correlations between major antioxidant components and antioxidant activities in honey
Studies have found a direct correlation between honey color, its phenolic content and antioxidant activities (Dezmirean et al., Citation2012). A strong correlation between honey phenolic content and antioxidant activity has been documented (Beretta et al., Citation2005; Blasa et al., Citation2006; Bertoncelj et al., Citation2007; Frankel, Robinson, & Berenbaum, Citation1998; Gheldof et al., Citation2002; Gheldof & Engeseth, Citation2002a; Vela, de Lorenzo, & Perez, Citation2007). Siti et al. (Citation2012, Citation2013) stated that the correlations between AOAM obtained from all assays depend upon the nature of the sample. They conducted an experiment on physicochemical and antioxidant properties of Tualang and Malaysian honeys. The mean of total phenolic content (TPC) and flavonoid contents with the corresponding DPPH and FRAP value of Tualang honey samples is summarized in Table . The correlation and correlation matrix showing the interrelation among phenolics, flavonoids, DPPH scavenging and FRAP assays are displayed in Tables and .
Table 1. Phenolic and Flavonoid content, and antioxidant activity of eleven Tualang honey samples (Siti et al., Citation2012)
Table 2. Correlation established between phenolic compounds and the antioxidant activity of Tualang honey samples (Siti et al., Citation2012)
Table 3. Correlation matrix showing the interrelation among phenolics, flavonoids, DPPH scavenging and FRAP assay for Malaysian honeys (Siti et al., 2013)
A significant correlation (P < 0.001) was observed between the total phenolic content and antioxidant activity of the Tualang honey samples. The results of the FRAP assay, however, were not significantly correlated with the levels of phenolics (R2 = 0.093 and P = 0.084). The higher the content of total phenolics in the honey samples was, the higher was the percentage of DPPH radicals that were quenched. A positive correlation was also observed between the flavonoid content and DPPH scavenging activity (R2 = 0.335 and P < 0.001). The correlations between the flavonoid content and the FRAP values were also significant (P = 0.006) (Table ).
These results indicated that the phenolic and flavonoid contents could be good indicators of antioxidant activity.
The correlation matrix (Table ) suggested that there was a strong positive correlation of TPC and Flavonoids with both AOAM (DPPH and FRAP). In such cases, it could be feasible to use TPC and Flavonoids for prediction of AOAM. Other scholars (Lee, Norul, Nur, & Ti Tjih, Citation2013) also tested the correlation between biochemical components and antioxidant activity of honey and confirmed the presence of positive correlations (Table ).
Natacha, Liberato, and Everaldo (Citation2014) reported the correlation of color intensity with total phenolic content and antioxidant activity of honey samples (Table ). Their finding confirmed with the traditional perception that darker honey types are beneficial for treating coughs and colds, which takes advantage of the greater antioxidant activity of darker honey.
In the study by Aldina et al. (Citation2015), 95.7% of the analyzed honey samples with higher polyphenol content were found to have a stronger antioxidant activity. The correlation curve of total phenolic content and antioxidant activity is shown in Figure .
Figure 3. Correlation between polyphenol content and antioxidant activity of honey (Aldina et al., Citation2015).
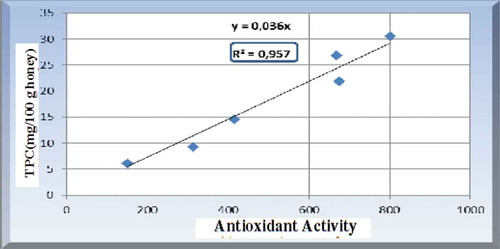
Dubero et al. (Citation2015) studied the total phenols and antioxidant activities of natural honeys from different geographical regions of Ethiopia. They stated that, in general, honey extracts with a high radical scavenging activity showed a high phenolic and flavonoids content. Similarly, Mekuanint and Meareg (Citation2019) reported that there was a strong correlation between the total phenolic content and the radical scavenging activity (RSA) of the honey samples of Amhara and Tigray regions, Ethiopia. The correlation between total phenolic content and antioxidant activity parameters (AEAC, RSA, & IC50) of the analyzed honey samples is depicted in Table with the corresponding correlation coefficients. The correlation results indicated that the radical scavenging activities of the honey samples were strongly correlated with their total phenolic contents with the correlation coefficient (r) of 0.94 (Table ). This implied that honeys with higher phenolic content showed higher radical scavenging activity. The correlation analysis also indicated that there was a strong negative correlation between the total phenolic content and the IC50 values of the investigated honey samples. IC50 is the amount of antioxidant required to reduce 50% of the free radical. Since the lower the IC50 value implies the higher the antioxidant activity, the correlation result revealed that honeys with higher total phenolic content were found to show higher antioxidant activity.
Table 4. Correlation matrix between antioxidant activity and biochemical components of honey (Lee et al., Citation2013)
Table 5. Pearson correlation matrixes for AAE-DPPH, TP, Color and TF (Natacha et al., Citation2014)
Table 6. Correlations between total phenolic contents and RSA of honey samples (Mekuanint & Meareg, Citation2019)
Table 7. Comparison of the analytical methods used for assessing the antioxidant activity (Ronald et al., Citation2005)
However, samples with similar total phenolic content do not necessarily correspond to the same antioxidant responses. Such types of contradictions were explained by Meda, Lamien, et al. (Citation2005b). Atoui, Missouri, Boskou, and Kefalas (Citation2005) stated that Folin–Ciocalteu assay (used for estimating the TPC) results depend on the chemical structure of phenolics. Oumer, Bilge, Osman, and Fatih (Citation2014) also reported that although phenolic compounds are the main components responsible for the antioxidant effects of honey, non-phenolic antioxidants are also involved. Therefore, the higher total phenolic content does not always correlate strongly with the capacity to inhibit oxidation. Because of their structures, some phenolic compounds may be inert to participate in the scavenging of the DPPH radical. Rice-Evans, Miller, and Paganga (Citation1996) stated that only phenolic compounds with a certain structure and particular hydroxyl position in the molecule can act as a proton donor and show radical scavenging activity. The study by Mekuanint and Meareg (Citation2019) showed that two honey samples with the TPC of 1565.266 ±7.70 and 1595.796 ±7.79 mg/Kg were found to have an antioxidant activity of 29.37 ± 0.26 and 26.20 ± 0.31 AEAC/100 g honey, respectively. This confirmed that honey samples with a higher total phenolic content will not always have higher antioxidant activities. So, the radical scavenging activity of a sample cannot be predicted merely from its total phenolic content (Gheldof & Engeseth, Citation2002a). This probability of occurrence of lack of relationships is supported by other literatures (Alvarez-Suarez, Giampieri, & Battino, Citation2013; Teixeira, Message, Negri, Salatino, & Stringheta, Citation2008).
5. Analytical methods for the determination of antioxidant activity
In the modern world, many scientists around the globe (Halliwell & Gutteridge, Citation2015; Willets, Citation2011) attribute the origin of many diseases to oxidative stress. For this reason, many nutritionists recommend the consumption of at least a minimum of foods such as fruits, vegetables, some drinks like grape wine, and spices and also food supplements from natural and synthetic origin containing antioxidants to help keep an individual healthy (Kaur & Kapoor, Citation2001).
The antioxidant molecules present in these foods, drinks, and supplements, among which phenols are included, have been characterized as antioxidants by means of several methods and under different experimental conditions. Sometimes the results from the same molecule may vary when different methods are used (Prior et al., Citation2005). This can be understood in two ways: on the one hand, inside living systems there are multiple radicals and reactive chemical species, as well as the mechanisms involved in oxidative stress; on the other hand, it is important to take into account the chemical nature of other molecules being tested to employ the most adequate assay in order to get results that are closest to reality. For these reasons, there is no single and universal method to characterize the antioxidant chemical abilities of all molecules (Wright et al., Citation2001).
For determination of the antioxidant potential of honey, many analytical methods have been developed (Moniruzzaman, Khalil, Sulaiman, & Gan, Citation2012). The most commonly used assays include DPPH (free radical scavenging activity), FRAP (ferric reducing/antioxidant power), ORAC (oxygen radical absorbance capacity), AEAC (ascorbic acid content), and TEAC (Trolox equivalent antioxidant activity) (Chua, Lee, & Chan, Citation2013). Each proposed method will always have advantages and disadvantages, which need to be taken into account in terms of complexity, required facilities and equipment, the chemical mechanism that it tests, the quantification method, and its relevance in biological systems. There are several methods reported in the literatures to determine the antioxidant activity of polyphenols, flavonoids and other antioxidant components. This review focused mainly on the most common analytical methods used to test the antioxidant activity of honey.
5.1. DPPH free radical scavenging activity (the DPPH method)
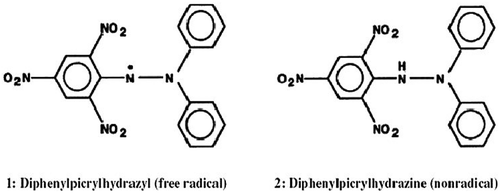
presents the radical and non-radical forms of DPPH (2, 2-diphenyl-1-picryhydrazyl). The DPPH method, developed by Brand-Williams et al. (Citation1995), and modified by Diaz et al. (Citation2011), is based on the absorbance reduction of the radical anion DPPH• by antioxidants. Huang et al. (Citation2005) stated that DPPH is one of the few stable and commercially available organic nitrogen radicals. It is one of the most widely reported methods (Heleno et al., Citation2012; Mau, Huang, Huang, & Chen, Citation2004; Mau, Lin, & Chen, Citation2002; Mau, Tsai, Tseng, & Huang, Citation2005; Merinal & Boi, Citation2012; Sreeramulu & Raghunath, Citation2010) for the determination of antioxidant activity of honey.
The DPPH test is based on the ability of the stable 2, 2-diphenyl-1-picrylhydrazyl free radical to react with hydrogen donors (Fahey & Stephenson, Citation2002; Inoue et al., Citation2005). The DPPH radical displays an intense ultraviolet, visible (UV-vis) absorption spectrum. The reaction of DPPH with an antioxidant or reducing compound produces the corresponding hydrazine DPPH2, followed by a color change from purple (absorbance at 515–528 nm) to yellow. In this test, a solution of radical is decolorized after reduction with an antioxidant (AH) or a radical (R) in accordance with the following equation (Parejo, Codina, Petrakis, & Kefalas, Citation2000):
The resulting decolonization is stoichiometric with respect to the number of electrons captured. The antioxidant activity of honey for DPPH has been assessed by several authors following the methodology described by other authors (Meda, Lamien, et al., Citation2005b; Baltrusaityte et al., Citation2007). The DPPH method with the stable nitrogen-centered organic radical DPPH is used for the determination of free radical scavenging activity, usually expressed as SC50 (concentration of the substrate) (Molyneux, Citation2004; Saxena et al.,Citation2010), the amount of antioxidant necessary to decrease the initial concentration of DPPH by 50%. This means that the lower is the SC50 value of the sample, the higher is its antioxidant activity because it requires a lesser amount of radical scavenger from the antioxidant to reduce DPPH (Molyneux, Citation2004). The percentage of scavenging activity of each extract on DPPH radical can also be calculated as % inhibition of DPPH (I %) using the following equation: I% = [(Ao-As)/Ao] × 100
Where Ao is the absorption of control and As is the absorption of the tested extract solution. In this case, if the antioxidant activity of the sample is higher so does its DPPH scavenging activity (Saxena et al., Citation2010).
5.1.1. Advantages and disadvantages of the DPPH method
DPPH test is reported to be unaffected by certain side reactions such as metal ion chelation and enzyme inhibition (Amarowicz, Pegg, Rahimi-Moghaddam, Barl, & Weil, Citation2004). Isla et al. (Citation2011) stated that the DPPH method is a quick and simple test; it guarantees reliable results and needs only a UV-vis spectrophotometer, which likely explains its widespread use in antioxidant screening. DPPH is also stable, commercially available, and does not have to be generated before assay unlike ABTS•+ as the 2, 2ʹ-azino-bi’s (3-ethylbenzothiazoline-6-sulphonic acid) is known. It has been used to quantify antioxidants in complex biological systems in recent years. The DPPH method can be used for solid or liquid samples and is not specific to any particular antioxidant component, but applies to the overall antioxidant capacity of the sample. A measure of total antioxidant capacity helps to understand the functional properties of foods. For these reasons, it is considered as an easy and useful spectrophotometric method with regard to screening/measuring antioxidant capacity (Alvarez-Suarez, Tulipani, Romandini, Vidal, & Battino, Citation2009). Pooja and Modi (Citation2015) stated that DPPH is not a very tedious assay in terms of preparation of chemicals and also in terms of performing the assay and hence can be used for its operational simplicity. They conducted an experiment to test the reproducibility of the method and they reported that DPPH showed high reproducibility. According to Magalhaes, Segundo, Reis, and Lima (Citation2008), the only disadvantage of this method is that it is not very cost effective and is not suitable for measuring the antioxidant capacity of plasma, because proteins are precipitated in the alcoholic reaction medium.
On the contrary, Noruma, Kikuchi, and Kawakami (Citation1997) stated that use of DPPH to measure the antioxidant capacity (AOC) is plagued by many drawbacks: Interpretation is complicated when the test compounds like carotenoids have spectra that overlap DPPH at 515 nm; the method is not a competitive reaction because DPPH is both radical probe and oxidant; DPPH color can be lost via a radical reaction (HAT) or reduction (SET) as well as unrelated reactions, and steric inaccessibility is a major determinant of the reaction. Thus, small molecules that have better access to the radical site have higher apparent AOC with this test. In addition, DPPH has a relatively small linear reaction range of only 2–3-fold. He also stated that DPPH is stable nitrogen radical that bears no similarity to the highly reactive and transient peroxyl radicals involved in lipid peroxidation. Many antioxidants that react quickly with peroxyl radicals may react slowly or may even be inert to DPPH due to steric inaccessibility. DPPH is also decolorized by reducing agents as well as H transfer, which also contributes to inaccurate interpretations of AOC. Thus, AOC is not fairly rated by the ability of antioxidants to react with DPPH.
5.2. Ferric reducing antioxidant power (FRAP)
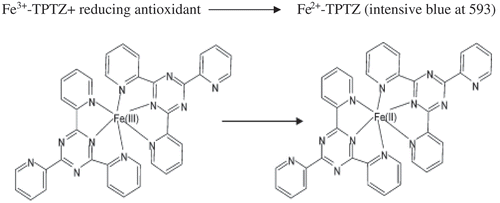
FRAP method was developed by Benzie and Strain (Benzie & Strain, Citation1996) to measure reducing power in plasma. It is considered as a direct method for measuring the total antioxidant power of biological fluids (Aljadi & Kamaruddin, Citation2004). The principle of this method is based on the reduction of a ferric 2, 4, 6-tripyridyl-s-triazine complex (Fe3+-TPTZ) to its ferrous colored form (Fe2+-TPTZ) in the presence of antioxidants (Alvarez-Suarez et al., Citation2010) ().
Fe3+-TPTZ+ reducing antioxidant → Fe2+-TPTZ (intensive blue at 593)
This reduction is monitored by measuring the change in absorption at 593 nm, using a diode-array spectrophotometer (Antolovich et al., Citation2002). The reaction detects compounds with redox potentials of <0.7 V (the redox potential of Fe3+ TPTZ). So, FRAP is a reasonable screen for the ability to maintain redox status in cells or tissues. Reducing power appears to be related to the degree of hydroxylation and extent of conjugation in polyphenols (Pulido, Bravo, & Saura-Calixto, Citation2000). However, FRAP cannot detect compounds that act by radical quenching (HAT), particularly thiols and proteins. This causes a serious underestimation in serum (Ou et al., Citation2002).
5.2.1. Advantages and disadvantages of the FRAP method
Benzie and Strain (Citation1996) stated that even though the FRAP assay is more tedious and time-consuming in terms of preparing the chemicals of the working solution, it is inexpensive method and does not require the use of any exclusive chemicals. It is reproducible and hence, FRAP is a suitable method for the determination of antioxidant activity of honey. Likewise, Ghiselli, Nardini, Baldi, and Scaccini (Citation1998) reported that even though FRAP is primarily used for determining the antioxidant activity of plasma, which is impossible in the DPPH method, it has also been successfully applied to measure antioxidant activity of a number of biological samples and pure substances. According to Thaipong, Boonprakob, Crosby, Cisneros-Zevallos, and Byrne (Citation2006), the FRAP assay is basically used for estimating the antioxidant activity of fruit extracts. They concluded that the FRAP assay showed high reproducibility, was simple, rapidly performed and showed the highest correlations with both ascorbic acid and total phenolics, therefore, it would be an appropriate technique for determining antioxidants of fruit extracts. Pulido et al. (Citation2000) performed an experiment to test the FRAP assay of dietary polyphenols in water and methanol and they suggested that even though FRAP results can vary tremendously depending on the time scale of analysis; it is simple, speedy, inexpensive, and robust and does not require specialized equipment in contrast to other techniques. The study conducted by Ferreira, Aires, Barreira, and Estevinho (Citation2009) and Kesic et al. (Citation2009) showed another advantage of the FRAP method that it can be used to correlate the antioxidant activity of honey with the amount of L-ascorbic acid. They concluded that the total antioxidant activity of the analyzed honey samples was higher in samples with higher content of L-ascorbic acid.
Opposingly, the study done by Katalinic, Milos, Kulisic, and Jukic (Citation2006) suggested that from the methodological point of view, the DPPH method is easy and accurate with regard to measuring the antioxidant activity of the extracts and also the results are highly reproducible and comparable to other antioxidant methods such as ABTS. Jerkovic and Marijanovic (Citation2010) explained that FRAP method has its own limitations, especially for measurements under non-physiological pH values, i.e. at pH 3.6. Likewise, Ou et al. (Citation2002) stated that the FRAP method is unable to detect slowly-reacting polyphenolic compounds. The study by Alvarez-Suarez et al. (Citation2009) reported that any compound, even without antioxidant properties with a redox potential lower than that of the redox pair Fe (3+)/Fe (2+), can theoretically reduce Fe (3+) to Fe (2+) contributing to an increase in the FRAP value and thus inducing false-positive results. Blasa et al. (Citation2006) suggested that this method has limitations; when FRAP is used to determine the antioxidant potential of polyphenols in water and methanol (the solvent typically used for extraction of antioxidants), the change in absorbance continued after 4 min. Therefore, the FRAP values for these compounds cannot be accurately determined at 4 min; for this reason, the ideal reaction time should be at least 10 min.
5.3. Oxygen-radical absorbance capacity (ORAC)
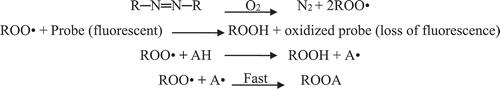
The ORAC method is based upon the early work of Glazer (Citation1990) and Ghiselli, Serafini, Maiani, Azzini, and Ferro-Luzzi (Citation1995), as developed further by Cao, Alessio, and Cutler (Citation1993). ORAC measures antioxidant inhibition of peroxyl radical induced oxidations and thus reflects classical radical chain-breaking antioxidant activity by H atom transfer (HAT) (Ou, Hampsch-Woodill, & Prior, Citation2001).
When antioxidants are present, they mop up the free radicals being produced and therefore inhibit the loss of fluorescence. The stronger the antioxidant property of a substance, the higher is the degree of inhibition of the loss of fluorescence. The measurement is standardized Trolox which has a known ORAC value and is reported in terms of Trolox equivalents (µ mol TE) per gram of sample. This method serves as an excellent way to quantify the ability of various compounds to quench free radicals (Cao et al., Citation1993; Rasmussen et al., Citation2008).
In the basic assay, the peroxyl radical reacts with a fluorescent probe to form a non-fluorescent product, which can be quantified easily by fluorescence. Antioxidant capacity is determined by a decreased rate and amount of product formed over time (Niki, Citation1990):
The most recent version of the ORAC method relies on the ability of peroxyl radicals to quench the fluorescence of fluorescein dye and measures the ability of antioxidants in food samples or sera to protect the dye from the radical damage (http://brunswicklabs.com/oracmeasure.shtml). The peroxyl radicals used in ORAC measurement are generated in aqueous solution from the hydrochloride of 2, 2′-azobis-2-methyl-propanimidamide. The compound quickly produces two mols of peroxyl radical. In the absence of an inhibitor, these radicals will rapidly destroy the fluorescence of the fluorescein dye.
By following the time course of the fluorescence decay, with and without added test substances, a measure of the radical trapping ability of the test substance can be estimated. Figure shows plots of fluorescent intensity versus time for blank and sample runs. The difference between the areas under the curve (AUC) for each parameter is the raw datum for the measurement. ORAC values are reported in comparison to the very efficient quenching of a water-soluble analogue of α-tocopherol called trolox (6–hydroxy–2, 5, 7, 8–tetramethylchroman–2–carboxylic acid). The number of µmol equivalents of trolox (TE) that produces the same AUC as 1 g of the test substance (or mL in the case of liquid samples such as fruit juices) is the ORAC value (µmol TE/g (mL)). Sometimes the value is called the “ORAC unit,” which is defined as the net protection produced by 1 µmol of Trolox (www.uoguelph.ca/~ckay/phytoblue/orac info.html).
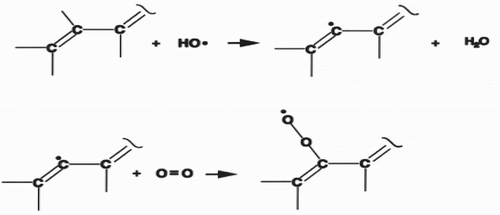
The ORAC procedure is mostly used for evaluating the antioxidant activity of dietary supplements because it involves peroxyl radicals, which are the most abundant radicals in biological systems.
Scheme 5. Formation of peroxyl radical (Joseph, Citation2004).
In the ORAC method, an antioxidant curve (fluorescence versus time) is first normalized to the curve of the blank corresponding to the same assay by multiplying original data by the factor:
fluorescenceblank, t = 0/fluorescencesample, t = 0. From the normalized curves, the area under the fluorescence decay curve (AUC) is calculated as
Where f0 is the initial fluorescence reading at 0 min and fi is the fluorescence reading at time i.
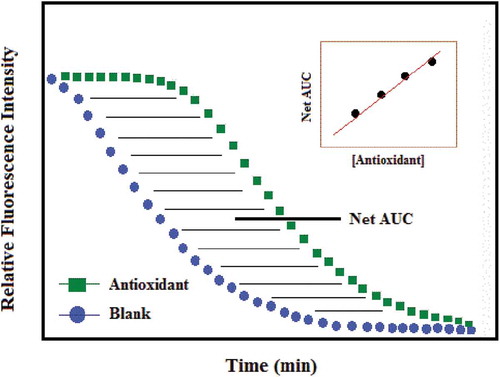
The protective effect of an antioxidant (AOX) is calculated from the net integrated areas under the fluorescence decay curves (Figure ). The net AUC = AUCsample—AUCblank. A standard curve is generated using the AUC for standard concentrations of Trolox, and the Trolox equivalents of the sample are calculated using the following linear or quadratic relationships (Y) = a + bX or Y) = a + bX + cX2 between Trolox concentration (Y)(μM) and the net area under the FL decay curve (X) = (AUCsample—AUCblank).
Figure 4. ORAC antioxidant activity expressed as the net area under the curve (Alberto, Carmen, & Begoña, Citation2004).
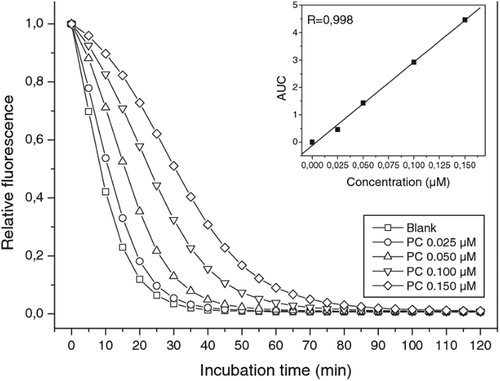
Serena, Francesca, Stefano, and Franco (Citation2010) conducted a research by the ORAC method to determine the antioxidant activity of Phycocyanin (PC) and Phycocyanobilin (PCB) using fluorescein (FL) as a source of fluorescence. The study indicated that in the absence of PC and PCB, most of the fluorescence declined rapidly as indicated for the blank solution (Blk). But when different concentrations of PC and PCB were added as an antioxidant, the loss in the fluorescence probe decreases and different kinetic curve trends were observed for different concentrations of PC and PCB. The fluorescent intensity versus time for the antioxidant PCB using fluorescein (FL) is indicated in Figure .
Figure 5. Kinetics of fluorescein (FL) quenching with different concentrations of Phycocyanobilin (PCB) versus blank. The reaction mixture contains 0.05 µM FL and 4 µM AAPH, with PCB ranging from 0.025 to 0.150 µM. (Inset) Linear plot of the net AUC versus PCB concentrations (Serena et al., Citation2010).
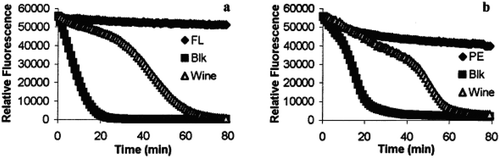
The loss of the fluorescence depends not only on the use of the antioxidants but also on the nature of the fluorescence sources. For example, the study conducted by Huang, Boxin, and Prior (Citation2002) demonstrated how the fluorescence was lost differently when fluorescein (FL) and β-phycoerythrin (PE) were used as a source of fluorescence. The study revealed that PE was found to lose almost 23% of its fluorescence intensity in the first 50 min at 37°C in the absence of AAPH (source of the free radical) (Figure )), but the lose fluorescence intensity in FL was slower as compared to PE (Figure )). When adding wine as an antioxidant, different kinetic curve trends were observed for ORAC-PE and ORAC-FL assays.
Figure 6. Time course of the reaction of fluorescein (FL) and β-phycoerythrin in the presence and in the absence of AAPH with and without antioxidant (wine) (Huang et al., Citation2002).
5.3.1. Advantages and disadvantages of the ORAC method
The ORAC method provides a controllable source of peroxyl radicals that model reactions of antioxidants with lipids in both food and physiological systems, and it can be adapted to detect both hydrophilic and hydrophobic antioxidants by altering the radical source and solvent (Ou et al., Citation2002; Prior et al., Citation2003). Cao, Verdon, Wu, Wang, and Prior (Citation1995) stated that the method is advantageous since it is readily automated, and more recently has undergone additional improvements in instrumentation and fluorescent probe.
The principal drawback of this method is the PE itself; since it varies a lot and is not photo stable, and can be photo bleached after an exposure to excitation light. In addition, PE interactions with polyphenols could cause erroneous ORAC values (Ou et al., Citation2001). To solve such problems, Ou and co-workers developed and validated an improved oxygen-radical absorbance capacity method using fluorescein (FL) as the fluorescent probe (ORAC-FL). Because FL as compared to PE does not interact with antioxidants, shows excellent photostability and reduces the cost of experiments (Ou et al., Citation2001). Frankel and Meyer (Citation2000) have criticized ORAC in that the antioxidant mechanism and protection of the fluorescent probe, β-phycoerythrin (β-PE) by antioxidants mimics critical biological substrates. It is also stated that the ORAC procedure is limited for evaluating the antioxidant activity of dietary supplements because it involves peroxyl radicals, which are the most abundant radicals in biological systems. However, the adaptation for lipophilic antioxidants and other recent variations of the method may extend its sensitivity to other radicals and to the analysis of more specific antioxidants (Ou et al., Citation2002). Above all, there is no single analytical method used for measuring antioxidant activity. All methods have their own strength and weakness. So, the choice of the methods used for assessing the antioxidant activity depends upon: simplicity of the method, instrumentation required biological relevance, mechanism, and whether the method is adaptable to measure lipophilic and hydrophilic antioxidants (Ronald, Boxin & Dejian, Citation2005) (Table ).
6. Conclusion
Honey can be considered as a potential natural antioxidant medicine. The total antioxidant activity of honey is the result of its complex chemical composition and the complex interactions between different substances. From this review, we determine that phenols, flavonoids, phenolic acids, proteins, amino acids and vitamins are the components responsible for the antioxidant activity of honey. There is a high degree of correlation between the total antioxidant activity and polyphenol content of honey. Environmental and climatic conditions, the plant sources, total phenolic content and color differences are among the factors which can influence the antioxidant activity of honey. Due to the presence of multiple radicals, and the variation in the nature of antioxidants, there is no single and universal method to characterize the antioxidant abilities of all molecules. Analytical methods differ from one another in terms of reaction mechanisms, complexity, required facilities and equipment, oxidant species, the quantification method, and their relevance in biological systems, and the way the final results can be expressed. Therefore, the use of the
DPPH assay coupled with other useful methods such as FRAP and ORAC is preferred because they are able to reflect the antioxidant properties more accurately in terms of their time consumption, cost, simplicity and reproducibility.
Additional information
Funding
Notes on contributors
Mekuanint Lewoyehu
Mekuanint Lewoyehu is a full time Lecturer in Analytical Chemistry in the Department of Natural Resource Management, Bahir Dar University, Ethiopia. He obtained BSc. degree in Chemistry in 2014 from Adama Science and Technology University, MSc degree in Analytical Chemistry in 2018 from Bahir Dar University. Currently, he is working as a lecturer and doing research in Agricultural College of Bahir Dar University, Ethiopia. Mekuanint Lewoyehu’s research area is more focused on assessment of the quality of food and agricultural products. He works on comparative assessment of physicochemical parameters, antioxidant and antibacterial activities of honey samples from different regions in Ethiopia. He also works on assessment of the level of heavy metals and other environmental pollutants in the effluent discharges of different industries.
References
- Abu-Tarboush, H. M., Al-Kahtani, H. A., & El-Sarrage, M. S. (1993). Floral-type identification and quality evaluation of some honey types. Food Chemistry, 46(1), 13–24. doi:10.1016/0308-8146(93)90068-Q
- Ahmed, S., & Othman, N. H. (2013). Honey as a potential natural anticancer agent: A review of its mechanisms. Evidence-based Complementary and Alternative Medicine, Article ID 829070, 2013, 7. doi:10.1155/2013/829070
- Alberto, D., Carmen, G., & Begoña, B. (2004). Extending the application of the oxygen radical absorbance capacity (ORAC-fluorescence) assay. Journal of Agriculture and Food Chemistry, 52(1), 48–54. doi:10.1021/jf0305231
- Aldina, K., Nadira, I. M., & Almir, Š. (2015). Phytochemical profile of honey. 105. doi:10.5772/60087
- Aljadi, A. M., & Kamaruddin, M. Y. (2004). Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chemistry, 85(4), 513–518. doi:10.1016/S0308-8146(02)00596-4
- Al-mamary, M., Al-meeri, A., & Al-habori, M. (2002). Antioxidant activities and total phenolic of different types of honey. Nutritional Research, 22(9), 1041–1047. doi:10.1016/S0271-5317(02)00406-2
- Alvarez-Suarez, J. M., Giampieri, F., & Battino, M. (2013). Antioxidant and antimicrobial capacity of honeys. Current Medical Chemistry, 20, 621. doi:10.2174/092986713804999358
- Alvarez-Suarez, J. M., Tulipani, S., Díaz, D., Estevez, Y., Romandini, S., Giampieri, F., … Battino, M. (2010). Antioxidant and antimicrobial capacity of several monofloral cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food and Chemical Toxicology, 48(8–9), 2490–2499. doi:10.1016/j.fct.2010.06.021
- Alvarez-Suarez, J. M., Tulipani, S., Romandini, S., Vidal, A., & Battino, M. (2009). Methodological aspects about determination of phenolic compounds and in vitro evaluation of antioxidant capacity in the honey: A review. Current Analytical Chemistry, 5, 293–302. doi:10.2174/157341109789077768
- Al-Waili, N. S. (2003). Effects of daily consumption of honey solution on hematological indices and blood levels of minerals and enzymes in normal individuals. Journal of Medicinal Food, 6(2), 135–140. doi:10.1089/109662003322233549
- Amarowicz, R., Pegg, R. B., Rahimi-Moghaddam, P., Barl, B., & Weil, J. A. (2004). Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chemistry, 84, 551–562. doi:10.1016/S0308-8146(03)00278-4
- Ames, B. N., Shigenag, M. K., & Hagen, T. M. (1993). Oxidants, antioxidant and the degenerative diseases of aging. Processing of the National Academic Science, 90, 7915–7922. doi:10.1073/pnas.90.17.7915
- Antolovich, M., Prenzler, P.D., & Patsalides, E., McDonald, S., & Robards, K. (2002). Methods for Testing Antioxidant Activity. The Analyst, 127, 183–198. http://dx.doi.org/10.1039/b009171p
- Apak, R., Guclu, K., Ozyurek, M., & Karademir, S. E. (2004). Comparative evaluation of various total antioxidant capacity assays. Journal of Agriculture and Food Chemistry, 52, 7970–7981. doi:10.1021/jf048741x
- Apostolidis, E., Kwon, Y., & Shetty, K. (2006). Potential of cranberry-based herbal synergies for diabetes and hypertension management. Asia Pacific Journal of Clinical Nutrition, 15(3), 433–441.
- Atoui, A. K., Missouri, A., Boskou, G., & Kefalas, P. (2005). Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chemistry, 89(1), 27–36. doi:10.1016/j.foodchem.2004.01.075
- Baltrusaityte, V., Venskutonis, P. R., & Ceksteryte, V. (2007). Radical scavenging activity of different floral origin honey and bee bread phenolic extracts. Food Chemistry, 101(2), 502–514. doi:10.1016/j.foodchem.2006.02.007
- Barganska, Z., Olkowska, E., Dymerski, T., & Namiesnik, J. (2014). Determination of pesticide residues in honey using the GC×GC-TOFMS technique. Journal of Bioprocessing and Biotechniques, 16(4), 1–5.
- Barganska, Z., Slebioda, M., & Namiesnik, J. (2013). Pesticide residues levels in honey from apiaries located of Northern Poland. Journal of Food Control, 31, 196–201. doi:10.1016/j.foodcont.2012.09.049
- Benavente-Garcia, O., & Castillo, J. (2008). Updates on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-Inflammatory Activity. Journal of Agriculture and Food Chemistry, 56, 6185–6205. doi: 10.1021/jf8006568
- Benzie, I. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Journal of Analytical Chemistry, 239, 70–76.
- Beretta, G., Granata, P., Ferrero, M., Orioli, M., & Facino, R. M. (2005). Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Analytical Chemistry, 533, 185–191.
- Bertoncelj, J., Dobersek, U., Jamnik, M., & Golob, T. (2007). Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chemistry, 105, 822–828. doi:10.1016/j.foodchem.2007.01.060
- Bjelakovic, G., Nikolova, D., Simonetti, R. G., & Gluud, C. (2004). Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis. Lancet, 364, 1219–1228. doi:10.1016/S0140-6736(04)17138-9
- Blasa, M., Candiracci, M., Accorsi, A., Piacentini, M. P., Albertini, M. C., & Piatti, E. (2006). Raw millefiori honey is packed full of antioxidants. Food Chemistry, 97, 217–222. doi:10.1016/j.foodchem.2005.03.039
- Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of free radical method to evaluate antioxidant activity. Lebensmittel Wissenschaft Und Technology, 28, 25–30. doi:10.1016/S0023-6438(95)80008-5
- Cantarelli, M. A., Pellerano, R. G., Marchevsky, E. J., & Camiña, J. M. (2008). Quality of honey from Argentina: Study of chemical composition and trace elements. Journal of the Argentine Chemical Society, 96, 33–41.
- Cao, G., Alessio, H. M., & Cutler, R. G. (1993). Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biological Medicines, 14, 303–311. doi:10.1016/0891-5849(93)90027-R
- Cao, G., Verdon, C. P., Wu, A. H., Wang, H., & Prior, R. L. (1995). Automated assay of oxygen radical absorbance capacity. Clinical Chemistry, 41, 1738–1744.
- Chen, L., Mehta, A., Berenbaum, M., Zangerl, A. R., & Engeseth, N. J. (2000). Honeys from different floral sources as inhibitors of enzymatic browning in fruit and vegetable homogenates. Journal of Agricultural and Food Chemistry, 48, 4997–5000. doi:10.1021/jf000373j
- Chimi, H., Cillard, J., Cillard, P., & Rahmani, M. (1991). Peroxyl and hydroxyl radical scavenging activity of some natural phenolic antioxidants. Journal of the American Oil Chemists’ Society, 68, 307–312. doi:10.1007/BF02657682
- Chua, L. S., Lee, J. Y., & Chan, G. F. (2013). Honey protein extraction and determination by mass spectrometry. Analytical and Bioanalytical Chemistry, 405, 3063–3074. doi:10.1007/s00216-012-6630-2
- Dezmirean, G. I., Marghhitas, L. A., Bobies, O., Dezmirean, D. S., Bonta, V., & Erler, S. (2012). Botanical origin causes changes in nutritional profile and antioxidant activity of fermented products obtained from honey. Journal of Agriculture and Food Chemistry, 60, 8025–8035. doi:10.1021/jf3022282
- Diaz, L., De Montijo, S., Medina, A. L., Melendez, P., Laurence, V., & Marti-Mestres, G. (2011). Activity of ethanolic extracts leaves of machaerium floribundum against acne-inducing bacteria, and their cytoprotective and antioxidant effects on fibroblast. Revista Peruana de Biologia, 18, 153–158.
- Diplock, A. T., Rice-Evans, C. A., & Burdon, R. H. (1994). Is there a significant role for lipid peroxidation in the causation of malignancy and for antioxidants in cancer prevention? Cancer Research, 54, 1952–1956.
- Dubero, S., Minaleshawa, A., Mesfin, R. A., & Tewabech, Z. (2015). Total phenols and antioxidant activities of natural honeys and propolis collected from different geographical regions of Ethiopia. Bulletin of the Chemical Society of Ethiopia, 29(2), 163–172. doi:10.4314/bcse.v29i2.1
- Erejuwa, O. O., Sulaiman, S. A., & Ab Wahab, M. S. (2012). Honey: A novel antioxidant. Molecules, 17, 4400–4423. doi:10.3390/molecules17044400
- Erlund, I. (2004). Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutrition Research, 24(10), 851–874. doi:10.1016/j.nutres.2004.07.005
- Estevinho, L., Pereira, A. P., Moreira, L., Dias, L. G., & Pereira, E. (2008). Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chemistry Toxicol, 46, 3774–3779. doi:10.1016/j.fct.2008.09.062
- Fahey, J. W., & Stephenson, K. (2002). Pinostrobin from honey and Thai ginger (Boesenbergia pandurata): A potent flavonoid inducer of mammalian phase 2 chemo protective and antioxidant enzymes. Journal of Agricultural and Food Chemistry, 50, 7472–7476. doi:10.1021/jf025692k
- Ferreira, I. C., Aires, E., Barreira, J. C., & Estevinho, L. M. (2009). Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chemistry, 114, 1438–1443. doi:10.1016/j.foodchem.2008.11.028
- Frankel, E. N., & Meyer, A. S. (2000). The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. Journal of the Science Food and Agriculture, 80, 1925–1941. doi:10.1002/1097-0010(200010)80:13<1925::AID-JSFA714>3.0.CO;2-4
- Frankel, S., Robinson, G. E., & Berenbaum, M. R. (1998). Antioxidant content and correlated characteristics of 14 monofloral honeys. Journal of Apiculture Research, 37, 27–31. doi:10.1080/00218839.1998.11100951
- Fukumoto, L. R., & Mazza, G. (2000). Assessing antioxidant and provident activities of phenolic compounds. Journal of Agricultural & Food Chemistry, 48, 3597–3604. doi:10.1021/jf000220w
- Gheldof, N., & Engeseth, N. J. (2002a). Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitr lipoprotein oxidation in human serum samples. Journal of Agriculture and Food Chemistry, 50(10), 3050–3055. doi:10.1021/jf0114637
- Gheldof, N., Wang, X. H., & Engeseth, N. J. (2002). Identification and quantification of antioxidant components of honeys from various floral sources. Journal of Agriculture and Food Chemistry, 50(21), 5870–5877. doi:10.1021/jf0256135
- Ghiselli, A., Nardini, M., Baldi, A., & Scaccini, C. (1998). Antioxidant activity of different phenolic fractions separated from Italian red wine. Journal of Agriculture and Food Chemistry, 46, 361–367. doi:10.1021/jf970486b
- Ghiselli, A., Serafini, M., Maiani, G., Azzini, E., & Ferro-Luzzi, A. (1995). A fluorescence-based method for measuring total plasma antioxidant capability. Biological Medicines, 18, 29–36.
- Gilca, M., Stoian, I., Atanasiu, V., & Virgolici, B. (2007). The oxidative hypothesis of senescence. Journal of Postgraduates, Medicines, 53, 207–213. doi:10.4103/0022-3859.33869
- Glazer, A. N. (1990). Phycoerythrin fluorescence-based assay for reactive oxygen species. Methods in Enzymology, 186, 161–168.
- Gorelik, S., Ligumsky, M., Kohen, R., & Kanner, J. (2008). The stomach as a “bioreactor”: When red meat meets red wine. Journal of Agriculture and Food Chemistry, 56(13), 5002–5007. doi:10.1021/jf703700d
- Guler, A., Bakan, A., Nisbet, C., & Yavuz, O. (2007). Determination of important biochemical properties of honey to discriminate pure and adulterated honey with sucrose (Saccharum officinarumL.) syrup. Food Chemistry, 105, 1119–1125. doi:10.1016/j.foodchem.2007.02.024
- Gutteridge, J. M. C., Gregory, J. Q., & Timothy, W. E. (1994). Oxidative damage to plasma proteins in adult respiratory distress syndrome. Free Radical Research, 20(5), 289–298. doi:10.3109/10715769409145628
- Halliwell, B., & Gutteridge, J. M. (2015). Free radicals in biology and medicine (5th ed., pp. 905). Clarendon: Oxford University Press.
- Halliwell, B., & Gutteridge, J. M. (1989). Lipid peroxidation: A radical chain reaction. Free Radical Biology and Medicine, 2, 188–218.
- Havsteen, B. H. (2002). The biochemistry and medical significance of the flavonoids. Pharmacology and Therapeutics, 96(2–3), 67–202. doi:10.1016/S0163-7258(02)00298-X
- Heleno, S. A., Barros, L., Martins, A., Quiroz, M. J., Santos-Buelga, & Ferreira, C. I. (2012). Fruiting body spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Research International, 46, 135–140. doi:10.1016/j.foodres.2011.12.009
- Huang, D., Boxin, O. U., & Prior, P. L. (2002). The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry, 55, 1841–1856.
- Huang, D., Ou, B., & Prior, R. L. (2005). The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry, 53, 1841–1856. doi:10.1021/jf030723c
- Inoue, K., Murayarna, S., Seshimo, F., Takeba, K., Yoshimura, Y., & Nakazawa, H. (2005). Identification of phenolic compound in manuka honey as specific superoxide anion radical scavenger using electron spin resonance (ESR) and liquid chromatography with coulometric array detection. Journal of the Science Food Agriculture, 85, 872–878. doi:10.1002/jsfa.1952
- Isla, M. I., Craig, A., Ordoñez, R., Zampini, C., Sayago, J., Bedascarrasbure, E., … Maldonado, L. (2011). Physico chemical and bioactive properties of honeys from Northwestern Argentina. Lebensmittel-Wissenschaft & Technologie, 44(9), 1922–1930. doi:10.1016/j.lwt.2011.04.003
- Jaganathan, S. K., & Mandal, M. (2009). Antiproliferative effects of honey and of its polyphenols: A review. Journal of Biomedicine and Biotechnology, 2009, 306. doi:10.1155/2009/830616
- Jantakee, K., & Tragoolpua, Y. (2015). Activities of different types of Thai honey on pathogenic bacteria causing skin diseases, tyrosinase enzyme and generating free radicals. Biological Research, 48(1), 4. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4417269
- Jasicka-Misiak, I., Poliwoda, A., Derea, M., & Kafarski, P. (2011). Phenolic compounds and abscisic acid as potential markers for the floral origin of two polish unifloral honeys. Food Chemistry, 10, 1016.
- Jerkovic, I., & Marijanovic, Z. (2010). Honeydew honey-approach to screening of volatile organic composition and antioxidant capacity (DPPH and FRAP assay). Molecules, 15, 3744–3756.
- Joseph, R. C. (2004). Alternative and complementary therapies. The Biochemistry of Alternative Medicines, 10(3), 170.
- Kamra, A., & Bhatt, A. B. (2012). Evaluation of antimicrobial and antioxidant activity of ganoderma lucidum extracts against human pathogenic bacteria. International Journal of Pharmacy and Pharmaceutical Sciences, 4(2), 359–362.
- Kandaswani, C., & Middleton, E. (1994). Free radical scavenging and antioxidant activity of plant flavonoids. Advanced Experiments, Medical. Biology, 336, 351–376.
- Kaskoniene, V., Maruska, A., Kornysova, O., Charczun, N., Ligor, M., & Buszewski, B. (2009). Quantitative and qualitative determination of phenolic compounds in honey. Chemine Technologija, 3(52), 1–7.
- Kaskoniene, V., & Venskutonis, P. R. (2010). Floral markers in honey of various botanical and geographical origins: A Review. Comprehensive Reviews in Food Science and Food Safety, 9, 620–634. doi:10.1111/j.1541-4337.2010.00130.x
- Katalinic, V., Milos, M., Kulisic, T., & Jukic, M. (2006). Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chemistry, 94, 550–557. doi:10.1016/j.foodchem.2004.12.004
- Kaur, C., & Kapoor, H. C. (2001). Antioxidants in fruits and vegetables the millennium’s health. International Journal of Food Science and Technology, 36, 703–725. doi:10.1046/j.1365-2621.2001.00513.x
- Kesic, A., Mazalovic, M., Crnkic, A., Catovic, B., Hadzidedic, S., & Dragosevic, G. (2009). The influence of L–ascorbic acid content total antioxidant activity of bee honey. ESJ, 32, 95–101.
- Khalil, M. I., & Sulaiman, S. A. (2010). The potential role of honey and its polyphenols in preventing heart diseases: A review. African Journal of Tradit Complement and Alternative Medicine, 7, 315–321.
- Khalil, M. I., Sulaiman, S. A., & Boukraa, L. (2010). Antioxidant properties of honey and its role in preventing health disorder. The Open Nutraceuticals Journal, 3, 6–16. doi:10.2174/18763960010030100006
- King, A., & Young, G. (1999). Characteristics and occurrence of phenolic phytochemicals. Journal of the American Dietetic Association, 99, 213–218. doi:10.1016/S0002-8223(99)00051-6
- Lachman, J., Orsak, M., Hejtmankova, A., & Kovarova, E. (2010). Evaluation of antioxidant activity and total phenolics of selected Czech honeys. Journal of Food Science and Technology,43, 52–58.
- Lee, S. C., Norul, L. A., Nur, A. A., & Ti Tjih, E. T. (2013). Antioxidant activity of three honey samples in relation with their biochemical components. Journal of Analytical Methods in Chemistry, 2013, 8. Article ID 313798 doi:10.1155/2013/313798
- Liu, R. (2003). Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. The American Journal of Clinical Nutrition, 78, 517–520. doi:10.1093/ajcn/78.3.517S
- Liu, R. (2004). Potential synergy of phytochemicals in cancer prevention: Mechanism of action. The Journal of Nutrition, 134, 3479–3485. doi:10.1093/jn/134.12.3479S
- Magalhaes, L. M., Segundo, M. A., Reis, S., & Lima, J. L. (2008). Methodological aspects about in vitro evaluation of antioxidant properties. Analytical Chemistry, 613, 1–19.
- Mau, J. L., Huang, P. N., Huang, S. J., & Chen, C. C. (2004). Antioxidant properties of methanolic extracts from two kinds of Antrodia camphorata mycelia. Food Chemistry, 86, 25–31. doi:10.1016/j.foodchem.2003.08.025
- Mau, J. L., Lin, H. C., & Chen, C. C. (2002). Antioxidant properties of several medicinal mushrooms. Journal of Agricultural and Food Chemistry, 50, 6072–6077. doi:10.1021/jf0201273
- Mau, J. L., Tsai, S. Y., Tseng, Y. H., & Huang, S. J. (2005). Antioxidant properties of methanolic extracts from ganoderma tsugae. Food Chemistry, 93, 641–649. doi:10.1016/j.foodchem.2004.10.043
- Meda, A., Lamien, C. E., Romito, M., Millogo, J., & Nacoulma, O. G. (2005a). Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry, 91(3), 571–577. doi:10.1016/j.foodchem.2004.10.006
- Meda, A., Lamien, C. E., Romito, M., Millogo, J., & Nacoulma, O. G. (2005b). Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry, 91, 571–577. doi:10.1016/j.foodchem.2004.10.006
- Mekuanint, L., & Meareg, A. (2019). Comparative assessment on selected physicochemical parameters, antioxidant and antimicrobial activities of honey samples from selected districts of the Amhara and Tigray regions, Ethiopia. International Journal of Food Science ID 4101695, 2019. doi:10.1155/2019/4101695
- Merinal, S., & Boi, V. S. (2012). In vitro antioxidant activity and total phenolic content of leaf extracts of Li monia crenulata (Roxb.).G. Journal of Natural Product and Plant Resource, 2(1), 209–214.
- Middleton, E., Kandaswami, C., & Theoharides, T. C. (2000). The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacol Review, 52, 673–751.
- Mirunalini, S., Dhamodharan, G., & Deepalakshmi, K. (2012). Antioxidant potential and current cultivation aspects of an edible milky mushroom-calocybe indica. International Journal of Pharmacy and Pharmaceutical Sciences, 4, 137–143.
- Molan, P. C. (1996). Authenticity of honey. In P. R. Ashurst & M. J. Dennis (Eds.), Food authentication (pp. 259–303). London: Blackie Academic and Professional.
- Molyneux, P. (2004). The use of the stable free radical diphenyl picrylhydrazyl (DPPH) for estimating antioxidant activity. Journal of Science and Technology, 26, 211–219.
- Moniruzzaman, M., Khalil, M. I., Sulaiman, S. A., & Gan, S. H. (2012). Advances in the analytical methods for determining the antioxidant properties of honey: A review. African Journal of Traditional Complement Alternative Medicines, 9, 6–42.
- Moniruzzaman, M., Sulaiman, S. A., Khalil, M. I., & Gan, S. H. (2013b). Evaluation of physicochemical and antioxidant properties of sourwood and other Malaysian honeys: A comparison with Manuka honey. Chemistry Central Journal, 7, 138. doi:10.1186/1752-153X-7-138
- Muller, F. L., Lustgarten, M. S., Jang, Y., Richardson, A., & Van Remmen, H. (2007). Trends in oxidative ageing theories. Free Radicals, Biological Medicines, 43, 477–503. doi:10.1016/j.freeradbiomed.2007.03.034
- Mundo, M. A., Padilla-Zakour, O. I., & Worobo, R. W. (2004). Growth inhibition of food borne pathogens and food spoilage organisms by select raw honeys. International Journal of Food Microbial, 97(1), 1–8. doi:10.1016/j.ijfoodmicro.2004.03.025
- Nagai, T., Inoue, R., Kanamori, N., Suzuki, N., & Nagashima, T. (2006). Characterization of honey from different floral sources. Its functional properties and effects of honey species on storage of meat. Food Chemistry, 97(2), 256–262. doi:10.1016/j.foodchem.2005.03.045
- Nagai, T., Sakai, M., Inoue, R., Inoue, H., & Suzuki, N. (2001). Antioxidative activities of some commercially honeys, royal jelly, and propolis. Food Chemistry, 75(2), 237–240. doi:10.1016/S0308-8146(01)00193-5
- Natacha, M., Liberato, C., & Everaldo, A. (2014). The Antioxidant activity of maltese honey. Journal of Apicultural Science, 58(1), 60.
- Nigussie, K. M., Subramanian, P. A., & Gebrekidan, M. (2012). Physicochemical analysis of Tigray honey: An attempt to determine major quality markers of honey. Bulletin of the Chemical Society of Ethiopia, 26(1), 127–133. doi:10.4314/bcse.v26i1.14
- Niki, E. (1990). Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods in Enzymology, 186, 100–108.
- Noruma, T., Kikuchi, M., & Kawakami, Y. (1997). Proton-donative antioxidant activity of fucoxanthin with 1, 1-diphenyl-2-picrylhydrazyl (DPPH). Biochemistry and Molecular Biology, International, 42, 361–370.
- Nunes, X. P., Silva, F. S., DaS Almeida, J. R., deA Ribeiro, L. A., Junior, L. J., de Lima, J. T., & Filho, J. M. (2012). “Biological oxidations and antioxidant activity of natural products”,in Venketeshwer, phytochemicals as nutraceuticals global approaches to their role in nutrition and health. Molecules, 15, 3744–3756.
- Ou, B., Hampsch-Woodill, M., Flanagan, J., Deemer, E. K., Prior, R. L., & Huang, D. (2002). Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. Journal of Agriculture and Food Chemistry, 50, 2772–2777. doi:10.1021/jf011480w
- Ou, B., Hampsch-Woodill, M., & Prior, R. L. (2001). Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. Journal of Agriculture and Food Chemistry, 49, 4619–4926. doi:10.1021/jf010586o
- Oumer, S. T., Bilge, T., Osman, S., & Fatih, T. (2014). Quality characterization of artisanal and retail Turkish blossom honeys: Determination of physicochemical, microbiological, bioactive properties and aroma profile. Industrial Crops and Products, 46, 124–131.
- Pandey, K. B., Mehdi, M. M., Maurya, P. K., & Rizvi, S. I. (2010). Antioxidant and free radical activity of honey. Disease Markers, 29, 31. doi:10.1155/2010/964630
- Paolini, M., Sapone, A., Canistro, D., Chieco, P., & Valgimigli, L. (2003). Antioxidant vitamins for prevention of cardiovascular disease. Lancet, 362, 920. doi:10.1016/S0140-6736(03)14318-8
- Parejo, L., Codina, C., Petrakis, C., & Kefalas, P. (2000). Evaluation of scavenging activity assessed by Co (II)/EDTA-induced luminol chemiluminescence and DPPH center dot (2, 2-diphenyl-1-picrylhydrazyl) free radical assay. Journal of Pharmacol Toxicol Methods, 44, 507–551. doi:10.1016/S1056-8719(01)00110-1
- Pooja, S., & Modi, H. A. (2015). Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. International Journal for Research in Applied Science & Engineering Technology (IJRASET), 3(6), 636–641.
- Prior, R. L., Hoang, H., Gu, L., Wu, X., Bacchiocca, M., Howard, L., … Jacob, R. (2003). Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. Journal of Agriculture and Food Chemistry, 51, 3273–3279. doi:10.1021/jf0262256
- Prior, R. L., Wu, X., & Schaech, K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agriculture and Food Chemistry, 53, 4290–4302. doi:10.1021/jf0502698
- Proteggente, A. R., Pannala, A. S., Paganga, G., Van Buren, L., Wagner, E., Wiseman, S., … Rice-Evans, C. A. (2002). Free radical scavenging studies of honey. Free Radicals, 36, 217. doi:10.1080/10715760290006484
- Pulido, R., Bravo, L., & Saura-Calixto, F. (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. Journal of Agriculture and Food Chemistry, 48, 3396–3402. doi:10.1021/jf9913458
- Rasmussen, C. N., Wang, X. H., Leung, S., Andrae-Nightingale, L. M., Schmidt, S. J., & Engeseth, N. J. (2008). Selection and use of honey as an antioxidant in a French salad dressing system. Journal of Agriculture and Food Chemistry, 56, 8650–8657. doi:10.1021/jf800635d
- Renaud, S. C., Gueguen, R., Schenker, J., & Houtaud, A. (1998). Alcohol and mortality in middle-aged men from eastern France. Epidemiology, 9, 184–188. doi:10.1097/00001648-199803000-00014
- Retrieved from http://brunswicklabs.com/oracmeasure.shtml
- Retrieved from www.uoguelph.ca/~ckay/phytoblue/orac info.html
- Rice-Evans, C. A., Miller, N. J., & Paganga, G. (1996). Free radicals. Biological Medicines, 20(7), 933–956.
- Ronald, L., & Boxin, O. U., & Dejian. (2005). The chemistry behind Antioxidant Capacity Assays. Journal of Agriculture & Food Chemistry, 53(6), 1841–1856. doi: 10.1021/if030723c
- Rosenblat, G., Angonet, S., Goroshit, A., Tabak, M., & Neeman, I. (1997). Antioxidant properties of honey produced by bees fed with medical plant extracts. Proceedings of the International Conference on Bee Products: Properties, Applications and Apitherapy (pp. 49–55). New York, NY: Plenum Press.
- Savatović, S. M., Dimitrijević, D. J., Dilas, S. M., Čanadanović-Brunet, J. M., Ćetković, G. S., Tumbas, V. T., & Štajner, D. I. (2011). Antioxidant activity of three different Serbian floral honeys. Acta Periodica Technologica, 4242, 145–155. doi:10.2298/APT1142145S
- Saxena, S., Gautama, S., & Sharma, A. (2010). Physical, biochemical and antioxidant properties of some Indian honeys. Journal of Food Chemistry, 118, 391–397. doi:10.1016/j.foodchem.2009.05.001
- Serena, B., Francesca, B., Stefano, S., & Franco, C. (2010). Oxygen radical absorbance capacity of phycocyanin and phycocyanobilin from the food supplement aphanizomenon flos-aquae. Journal of Medicinal Food, 13(1), 223–227. doi:10.1089/jmf.2008.0257
- Shahidi, F., & Wanasundara, J. P. (1992). Phenolic antioxidants. Critical review. Food Science and Nutrition, 32, 67–103. doi:10.1080/10408399209527581
- Shenoy, K., & Shirwaiker, A. (2002). Anti-inflammatory and free radical scavenging studies of Hyptis snravolens. Indian Drug, 39, 574–577.
- Sies, H. (1997). Oxidative stress, oxidants and antioxidants. Experimental Physiology, 82, 291–295. doi:10.1113/expphysiol.1997.sp004024
- Siti, A., Ibrahim, K., Nadia, A., Mahaneem, M., Saringat, B., & Gan, S. (2012). Content and antioxidant properties of processed tualang honey collected from different regions in Malaysia. International Journal of Pharmacy and Pharmaceutical Sciences, 4, 2014–2019.
- Siti, A. S., Moniruzzaman, M. I., Khalil, M., Sulaiman, S. A., & Siew, H. G. (2013). Physicochemical and antioxidant properties of malaysian honeys produced by apis cerana, apis dorsata apis mellifera. BMC Complementary and Alternative Medicine, 13, 43. doi: 10.1186/1472-6882-13-43
- Soares, J. R., Dinis, T. C., Cunha, A. P., & Almeida, L. M. (1997). Antioxidant activities of some extracts of Thymus zygis. Free Radical Research, 26, 469–478. doi:10.3109/10715769709084484
- Socha, R., Juszczak, L., Pietrzyk, S., & Fortuna, T. (2009). Antioxidant activity and phenolic composition of herb honeys. Food Chemistry, 113, 568–574. doi:10.1016/j.foodchem.2008.08.029
- Sreeramulu, D., & Raghunath, M. (2010). Antioxidant activity and phenolic contents of roots, tubers and vegetables commonly consumed in India. Food Research International, 43, 1017–1020. doi:10.1016/j.foodres.2010.01.009
- Steeg, E., & Montag, A. (1988). Quantitative determination of aromatic carboxylic acids in honey. Journal of Food Examination and Research,187(2), 115–120. doi: 10.1007/BF01042621
- Sun, J., Chu, Y., Wu, X., & Liu, R. (2002). Antioxidant and antiproliferative activities of common fruits. Journal of Agricultural and Food Chemistry, 50(25), 7449–7454. doi:10.1021/jf0207530
- Taormina, P. J., Niemira, B. A., & Beuchat, L. R. (2001). Inhibitory activity of honey against food borne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. International Journal of Food Microbial, 69, 217–225. doi:10.1016/S0168-1605(01)00505-0
- Teixeira, E. W., Message, D., Negri, G., Salatino, A., & Stringheta, P. C. (2008). Evidence based complement. Alternative Medicines, 7, 307.
- Temple, N. J. (2000). Antioxidants and disease: More questions than answers. Nutrition Research, 20, 449–459. doi:10.1016/S0271-5317(00)00138-X
- Thaipong, K., Boonprakob, U., Crosby, K., Cisneros-Zevallos, L., & Byrne, D. H. (2006). Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis, 19, 669–675. doi:10.1016/j.jfca.2006.01.003
- Turkmen, N., Sari, F., Poyrazoglu, E. S., & Velioglu, Y. S. (2006). Effects of prolonged heating on antioxidant activity and color of honey. Food Chemistry, 95, 653–657. doi:10.1016/j.foodchem.2005.02.004
- Vattem, D., Ghaedian, R., & Shetty, K. (2005). Enhancing health benefits of berries through phenolic antioxidant enrichment: Focus on cranberry. Asia Pacific Journal of Clinical Nutrition, 14(2), 120–130.
- Vela, L., de Lorenzo, C., & Perez, R. A. (2007). Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. Journal of Food Science and Agriculture, 87, 1069–1075. doi:10.1002/jsfa.2813
- Villegas, L., Fernández, I., Maldonado, H., Torres, R., Zavaleta, A., Vaisberg, A., & Hammond, G. B. (1997). Evaluation of the wound-healing activity of selected traditional medicinal plants from Peru. Journal of Ethnopharmacology, 55(3), 193–200. doi:10.1016/S0378-8741(96)01500-0
- Vivekanathan, D. P., Penn, M. S., Sapp, S. K., Hsu, A., & Topol, E. J. (2003). Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomized trials. Lancet, 361, 2017–2023. doi:10.1016/S0140-6736(03)13637-9
- Willets, W. C. (2011). Eat, drink and be healthy-the Harvard medical school guide to healthy eating. New York: Simon and Schuster.
- Wright, J. S., Johnson, E. R., & DiLabio, G. A. (2001). Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. Journal of the American Chemistry Society, 123, 1173–1183. doi:10.1021/ja002455u