?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study investigated the effects of ratio of ethanol to water (green solvent), extraction temperature and reaction time on properties of carotenoids ultrasonically extracted from pericarp of gac fruit. The solvent ratio and temperature significantly affected the antioxidant activity and carotenoid contents. Ultrasonic-assisted extraction (UAE) with absolute ethanol at 75°C for 15 min produced the extract with the highest antioxidant activity and carotenoid level similar to the extract extracted for 30 min. This extract consisted of lycopene, β-carotene, and lutein, as confirmed by HPLC-DAD and did not degrade during extraction as detected by FTIR analysis. However, extraction with a high temperature in UAE changed carotenoid isomerization from all trans to x-cis lycopene. Additionally, the anti-inflammatory properties of this ethanolic extract on THP-1 cells were investigated. As a result, the extract at the concentration of 0.25–1.00 µg/ml should be considered for its benefit in enhancing IL-1β cytokines and safe for THP 1 cell.
PUBLIC INTEREST STATEMENT
In this research, the extraction of carotenoids from gac fruit pericarp assisted with ultrasonic was study. The extraction with high temperature did not increase carotenoid contents. The optimal condition of ultrasonic-assisted extraction was obtained by using absolute ethanol as an effective solvent and extracting at 75°C for 15 min. This carotenoid extract possessed the highest antioxidant activity and carotenoid level. Moreover, the improvement of extraction yield was received while the structure and properties of carotenoids were not affected as confirmed by FTIR. The extract of gac fruit pericarp did not suppress pro-inflammatory cytokine and nor did it affect the level of anti-inflammatory cytokine.
Competing Interests
The authors declares no competing interests.
1. Introduction
Gac fruit (Momordica cochinchinensis) is a tropical fruit that is considered a super fruit because it contains high levels of bioactive compounds, such as phenolic compounds, flavonoids and, especially, carotenoids. The aril of the gac fruit is particularly rich in lycopene (eight times higher than tomatoes) and is suitable for use as a functional ingredient in food and cosmetic products. However, Kubola and Siriamornpun (Citation2011) found that other parts of gac fruit also contained high levels of bioactive compounds, especially carotenoids. Normally, the pericarps are disposed of as waste. To create a high value for this fruit byproduct, its utilization as a raw material for phytochemical extraction is proposed.
Carotenoids (yellow to red pigments) such as lycopene are lipid-soluble compounds containing 9–11 double bonds in the structure. Moreover, carotenoids structure contains isoprenoids (C5), which is made up of 8 isoprene units and conjugates with the π electron system (Kaulmann & Bohn, Citation2014; Murakami et al., Citation2017). This structure is only synthesized in plants and microorganisms; consequently, humans and animals cannot synthesize carotenoids by themselves and must uptake carotenoids from other sources (Kaulmann & Bohn, Citation2014). However, carotenoids can dissolve easily in nonpolar solvents such as tetrahydrofuran, n-hexane or dichloromethane. These solvents are toxic to consumers and the environment. Currently, a more environmentally friendly alternative to solvent extraction is ultrasonic-assisted extraction (UAE), which has been shown to be a viable technology to increase the mass transfer in the carotenoid extraction process. The growth bubbles within liquids that are generated by ultrasound induce cavitation in the solvent. When the bubbles reach an unstable size, they collapse and release a large amount of energy. This energy can increase mass transfers and breakdown the cell walls at the surface (Mason, Paniwnyk, Chemat, & Vian, Citation2011). Although a weak affinity between ethanol and carotenoids might result in a low carotenoid concentration in the solvent extraction process; however, the report of Sun, Liu, Chen, Ye, and Yu (Citation2011) and Phan-Thi and Wache (Citation2014) revealed that the use of ethanol as a solvent in the UAE process can produce the equivalent level of carotenoids to that obtained from using hexane, dichloromethane, ethyl acetate, and tetrahydrofuran as the solvents for extraction. Thus, not only the affinity and polarity of a solvent that affected the UAE extraction process but also the physical properties of the solvent including the surface tension, viscosity and vapor pressure (Hemwimol, Pavasant, & Shotipruk, Citation2006). An environmentally friendly solvent, such as water and ethanol, was considered for the lipid solvent extraction in this study.
Moreover, the UAE process is known as an alternative method that presents enhanced efficiency of the extracted yielded in a short time. The concentration of carotenoids in an extract isolated under UAE was 3.6 times higher than that extracted without ultrasonic assistance (Schlucker, Szeghalmi, Schmitt, Popp, & Kiefer, Citation2003) with comparing on similar time. However, the energy raised by ultrasound could change the carotenoid quality and resulted in carotenoid degradation or isomerization. The UAE conditions will be investigated on carotenoid structural changes that induced carotenoid enhanced or reduced on antioxidant activity. Carotenoids are known to be strong antioxidant and anti-inflammatory compounds that are correlated with the prevention of chronic diseases such as cancer, cardiovascular diseases and Alzheimer’s disease (Makon-Sebastien et al., Citation2014; Napolitano, Depascale, Wheeler-Jones, Botham, & Bravo, Citation2007). Chung, Leanderson, Lundberg, and Jonasson (Citation2017) found that lutein at 1–25 µM suppressed IL-1β and TNF-α cytokines in PBMC cells by inhibiting NF-kB signaling. They found that lutein could suppress the mRNA expression of IL-6, IL-1β, and TNF-α and inhibit NF-kB signaling because the inflamed cells produced cytokines that recruited macrophage immune cells to the site of damage. This process then induces the transcriptional signal of NF-kB binding to DNA in the nucleus. Moreover, Lycopene can reduce ROS (reactive oxygen species) levels by 60% in human monocyte THP-1 cells stimulated by cigarette smoke (Kaulmann & Bohn, Citation2014). The THP-1 cell line was developed in 1980 for use in immune response studies which were isolated from the peripheral blood of an acute leukemia patient. The in vitro study of the expression of inflammatory genes is one of the reliable methods using these monocyte cells, the secretion of some pro- and anti-cytokines occurs. Cytokines are small-secreted proteins that are released from infected cells to communicate with other cells, and they can be categorized into two groups depending on their function. Pro-inflammatory cytokines (such as TNF-α, IL-1β or IFN-γ) are cytokines that promote inflammation, which aggravates conditions such as fever, inflammatory tissue destruction, and loss of function. Tissue damage is caused by the circulation of inflammatory cytokines or other inflammatory makers, and oxidative stress is associated with an increased incidence of chronic inflammatory disease; however, anti-inflammatory cytokines (such as IL-10) suppress pro-inflammatory cytokines (Zhang & An, Citation2007).
The objective of this study was to determine the ideal parameters of the UAE of carotenoids from gac fruit, including the solvent ratio, temperature and time to extract. The antioxidant activity and carotenoid content of extracts from gac fruit pericarp were studied using different extraction parameters. To the best of our knowledge, this study is the first to use crude gac fruit extract to investigate gene expression in THP-1 cells.
2. Material and method
2.1. Chemicals
Absolute ethanol (pharmaceutical grade) was purchased from the Liquor Distillery Organization, Excise Department, Thailand. All solvents for HPLC analysis (acetonitrile, methyl alcohol, dichloromethane) were of HPLC isocratic liquid chromatography grade and were purchased from Merck (Darmstadt, Germany). The lutein and β-carotene standards were purchased from Sigma Aldrich (St. Louis, MO, USA). All reagents for the antioxidant measurements, including TPTZ (2, 4,6-tris(2-pyridyl)-s-triazine); TPTZ, FRAP- 2,4,6-tris (2-pyridyl)-s-triazine; FRAP, ABTS (2,2′-Azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid) and potassium persulfate, were analytical grade and purchased from Sigma Aldrich (St. Louis, MO, USA).
2.2. Ultrasonic-assisted extraction method
Fully ripe Thai gac fruit (Momordica cochinchinensis) were purchased from the Yardthip orchard in Thailand. The fruit was cleaned, and the pericarp was separated from the other parts of the fruit. The separated pericarp was stored at −20°C for a few days and then dried by spreading to a depth of 0.5 cm on metal trays that were loaded into a tray dryer at 60°C for 7 h. The carotenoids of the pericarp were extracted in an ultrasonic bath (GT sonic-D6, 40 kHz, 150 W, China) (Luengo et al., Citation2014). A dried pericarp (all particles larger than 210 µm) of 5 mg dried pericarp was mixed with 50 ml solvent in a 250 ml Erlenmeyer flask. The water in the ultrasonic bath was kept above the level of the solvent in the Erlenmeyer flask, and all experiments were performed in dim light. The crude extract that contained the carotenoid dissolved in the solvent was filtered through Whatman filter paper No. 4, and the solvent was evaporated at 40°C, leaving the carotenoid residue. The residue was then dissolved in 4 ml ethanol (HPLC grade) (Kha, Nguyen, Roach, & Stathopoulos, Citation2015; Sun et al., Citation2011) and finally stored at −20°C before analysis.
The extraction process was studied to determine the best conditions. Three parameters were adjusted—solvent, time and temperature. In the first experiment, the temperature of the solvent was fixed at 75°C with the extraction time of 30 min, and the solvent (ethanol to water ratio) was varied at 70:30, 80:20, 90:10 and 100:0 (absolute ethanol) v/v. In the second experiment, the time was fixed for 30 min, the solvent was absolute ethanol and the temperature was changed (30°C, 60°C, and 75°C). In the third experiment, the temperature was fixed at 75°C, the solvent was absolute ethanol and the time was varied (0, 5, 10, 15 and 30 min). For all samples, the carotenoid content and the antioxidant value were measured. Three replicate of experiments were performed.
2.3. Carotenoid content measurement method
A sample (100 µl) of each of the 11 samples from the above experimental combinations was dissolved in 2 ml of n-hexane, mixed using a vortex, and stirred for 2 min. Then, the absorbance was measured using a UV-Vis spectrophotometer (BioMate 3S, Thermo Fisher Scientific) at 445 nm wavelength. Finally, the absorbance of each sample was compared with a standard curve (R2 = 0.99) to calculate the concentration of carotenoids in micrograms per gram of crude extract. The standard curve was prepared following Ye, Feng, Xiong, and Xiong (Citation2011)
2.4. Carotenoid analysis using HPLC-DAD
The crude extracts were analyzed to determine their carotenoid content (lutein, lycopene, and β-carotene) using HPLC (Agilent 1100 series) with a G1315B diode array detector and a ChromSep HPLC SS C18 column (250 × 4.6 mm, Varian, Inc.). The crude extracts were filtered through a 0.45 µm membrane filter, and 20 µl was injected under the following conditions: solvent A was acetonitrile: methanol: dichloromethane: water (94: 2: 2: 2 v/v); solvent B was acetonitrile: methanol: dichloromethane (70: 20: 10 v/v/v). The gradient elution started with 100% solvent A for 1–15 min and 100% solvent B for 15.1–30 min, and the final eluent was solvent A for 30.1–60 min at a 0.9 ml/min flow rate. The temperature of a column was 30°C, and the absorbance was read at 475 nm with a scanning spectrum of 201–600 nm. Lutein and β-carotene were confirmed by comparing their retention times with the standard, and lycopene was confirmed (Phan-Thi & Wache, Citation2014).
2.5. Determinations of the quality of crude extract using FTIR spectroscopy
The FTIR spectra of degradation products in the crude extract were obtained by a Thermo Fisher Scientific Nicolet 6700 series. The wavenumber range was 400–4000 cm−1 in the single-bounce-ATR mode. Before measurement, the ethanol solvent was evaporated until dryness, and the residual was used for FTIR analysis.
2.6. Antioxidant property measurement
The two methods to measure antioxidant activity, ferric reducing activity power (FRAP) and 2,2ʹ-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid radical scavenging activity (ABTS), were selected to assess the properties of the pericarp of gac fruit extract.
The ferric reducing activity of the crude extracts under various conditions was determined using ferric reducing antioxidant power assay following the method of Kubola and Siriamornpun (Citation2011). In a reaction tube, the crude extract (100 µl) was reacted with 1.9 ml of FRAP solution at 37°C for 4 min, and the absorbance was read at 593 nm using a spectrophotometer. The results were compared with the FeSO4 standard curve using known concentrations in the units of millimoles FeSO4 per gram of crude extract.
The ABTS radical scavenging activity was measured using an ABTS radical cation solution. The ABTS solution was prepared by mixing 7 mM ABTS and 2.45 mM potassium persulfate in water and incubating for 16 h before use. An ABTS working solution was mixed with methanol until the absorbance of the solution was 0.700 ± 0.01. The reaction of 0.1 µl crude extract with 1.9 ml ABTS solution was incubated for 6 min at 30°C. Then, the absorbance was measured at 734 nm using a UV-Vis spectrophotometer. The absorbance of the sample was calculated using a standard curve of gallic acid in micrograms of gallic acid per gram of crude extract. (Phan-Thi & Wache, Citation2014)
2.7. The inflammation modulation study
The human leukemia monocyte THP-1 cells were cultured at the Department of Food Science and Technology, Kasetsart University, Thailand based on the method of Chanput, Krueyos, and Ritthiruangdej (Citation2016). THP-1 cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum and 1% penicillin/streptomycin in 5% CO2 at 37°C. The density of the cells was 8.2 × 104 cells ml−1.
An MTT (3-[4, 5 dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay was used to determine the cytotoxicity of pericarp gac fruit extract on the cell viability of THP-1 cells. The pericarp gac fruit extract was filtered through 0.2 µm before the filtrate was used in the experiment. Gac fruit extract concentrations of 0.05, 0.10, 0.25, 0.50 and 1.00 µg ml−1 were incubated for 3 and 6 h with THP-1 cells. Subsequently, THP-1 cells were dialyzed in DMSO: ethanol (1:1; v/v). The violet crystal of Formazan was detected at 570 nm absorbance.
The crude extract concentrations of 0.25, 0.50 and 1.00 µg ml−1 were chosen to study inflammation gene expression in THP-1 cells. The cells were stimulated with 100 ng ml−1 LPS (lipopolysaccharide) together with the crude extract for 5 h. Then, the gene JET RNA purification kit (Thermo Fisher Scientific) was used to isolate the total RNA from the THP-1 cells. The quality of isolated DNA (cDNA) was determined by agarose gel electrophoresis under UV detection. The gene expression of inflammatory cytokines (IL-1β, TNF-α, and IL-10) were analyzed using RT-PCR (CFX96 TouchTM, Bio Rad). The results show the relative gene expression, which was calculated by normalizing the cycle threshold values of genes of interest with the cycle threshold values of GADPH using the 2^Ct method (Chanput et al., Citation2016).
2.8. Statistical analysis
All experiments were performed in triplicate, and differences among mean values were analyzed using one-way analysis of variance (ANOVA) with the Duncan post hoc comparison test using the SPSS for Microsoft version 12 software package (SPSS Inc., Chicago, USA). The results were reported as the mean value ± standard deviation and were considered significantly different at p< 0.05.
3. Results and discussion
3.1. Effect of ethanol to water ratio on properties of the extracts
Table shows the values of the antioxidant activity and carotenoid content for the different ethanol to water ratios (70:30, 80:20, 90:10 and absolute ethanol; v/v) used in the UAE process. The ratio of ethanol to water had a significant influence on both the antioxidant activity and carotenoid content. The ideal condition for extraction that provided the highest carotenoid content and antioxidant activity was with absolute ethanol. The results also showed that the carotenoid content increased with absolute ethanol, indicating that the carotenoid content and the antioxidant activity increased with increasing ethanol. Our results were in agreement with Jaeschke et al. in 2017, who extracted carotenoids from Heterochlorella luteoviridis using ethanol as a solvent in the UAE process. Their study explained that at a concentration higher than 75% ethanol, there was a possible disruption of the cell structure due to interactions between the solvent and the cell phospholipid membrane that are associated with ethanol favorably interacting with the carotenoids. In addition, ultrasound can rupture the cell membranes or chloroplasts, leading to the carotenoids being released into the cytoplasm where it can easily interact with ethanol (Jaeschke, Rech, Marczak, & Mercali, Citation2017: John, Kuhn, Braeken, & Van Gerven, Citation2018).
Table 1. Effect of ethanol to water ratio on carotenoid content and antioxidant properties of the extracts at the extraction temperature and time of 75°C and 30 min
Water is a solvent that has the physical properties to enhance cavitation in the UAE process because it has lower viscosity, lower vapor pressure and higher surface tension than ethanol (Hassas-Roudsari, Chang, Pegg, & Tyler, Citation2009). In particular, high vapor pressure is negatively correlated with cavitation intensity in the UAE process (Sun, Ma, Ye, Kakuda, & Meng, Citation2010). However, in our study, increasing the proportion of water did not enhance the level of carotenoids or of other bioactive compounds with active antioxidant properties. When the dry sample was mixed with a solvent that contained a proportion of water, the dried sample swelled, which might have led to the interruption of the cavitation phenomenon during the UAE process.
3.2. Effect of temperature on the properties of the extracts
The effect of temperature is presented in Table . The FRAP ferric reducing activity did not change significantly, which was similar to the carotenoid content results. This result might have occurred because the extraction temperature negatively affected the intensity of cavitation in the UAE process (Xu & Pan, Citation2013). However, the ABTS radical scavenging activity increased significantly with increasing temperature. At a temperature of 75°C, the best of the ABTS radical scavenging activity results were detected. The study of Phan-Thi and Wache (Citation2014) showed the effect of maintaining temperature at 80°C with hexane extraction. The rapid isomerization of the all-trans isomer to the 13-cis isomer was detected. Their results indicated that some cis isomers of the lycopene structure enhanced the antioxidant activity as measured by the ABTS method due to the increased solubility of the cis-form. Their results agreed with those reported by Murakami et al. (Citation2017). They found that at 80°C for 15 min, 23.8% of the all-trans isomer content changed to the cis-form, and the percent change continued to increase with increasing heating time. Furthermore, the cis lycopene has greater solubility in ethanol than the all-trans lycopene and may have induced the increase in ABTS radical scavenging capacity with increasing temperature in our experiment.
Table 2. Effect of temperature on carotenoid contents and antioxidant properties of the extracts extracted with absolute ethanol for 30 min
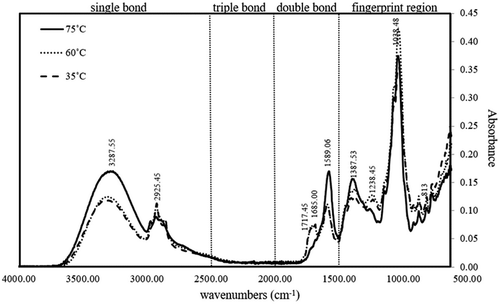
Ultrasound cavitation destroys the double bond and generates a C = O structure or breaks the carbon chain. Additionally, the energy from the bubble collapse might affect the carotenoid structure because the structure of carotenoids contains many conjugated double bonds (-C = C-)n that are sensitive to isomerization and can lead to the degradation of the carotenoid structure (Sun et al., Citation2010; Ye et al., Citation2011). In this study, the effect of temperature on the carotenoid structures was confirmed using the FTIR technique. The IR spectra of the dried crude extract following the variation of temperature at 35°C, 60°C, and 75°C using absolute ethanol as the solvent for 30 min are reported (Figure ). The spectra at 3287.55 cm−1 were assigned as the stretching vibration of—OH of primary aliphatic alcohol, while the IR spectra of aliphatic hydrocarbons were reported at the position of 2925.45 cm−1 (Vahur, Teearu, Peets, Joosu, & Leito, Citation2016). Furthermore, the extracts at different temperatures showed band position shifts in the double bond (2000–1500 cm−1) region. The different bands of 1717.45 and 1658.00 cm−1 were designated the nonconjugated and conjugated ketone functional groups (Dutta, Citation2017; Lorand, Molnar, DELI, & Toth, Citation2002), respectively, that were not present in the IR spectral of 75°C. These spectra represented the functional groups of the other bioactive compounds that might be present in the crude extract, such as flavonoids or some phenolic compounds. Additionally, the height of the IR spectrum at 1589.06 cm−1 increased as the extraction temperature increased from 35°C to 75°C. This result indicated the stretching vibration of the (C = C)cis form of cis carotenoid (Schlucker et al., Citation2003). Additionally, in the fingerprint region, the spectra of all extracts did not shift; furthermore, the spectrum of molecular lycopene containing the vibration spectral of (RH)-C = C-(RH) at nearly 813 cm−1 (Lorand et al., Citation2002; Ye et al., Citation2011). However, there have been no reports on the degradation of carotene at 1187 cm−1 from the construction of (C = O) (Ye et al., Citation2011). As mentioned above, the IR results implied that the increase in temperature (35-75°C) did not degrade carotenoid structure in our crude extract.
3.3. Effect of extraction time
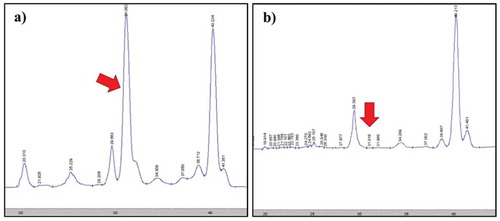
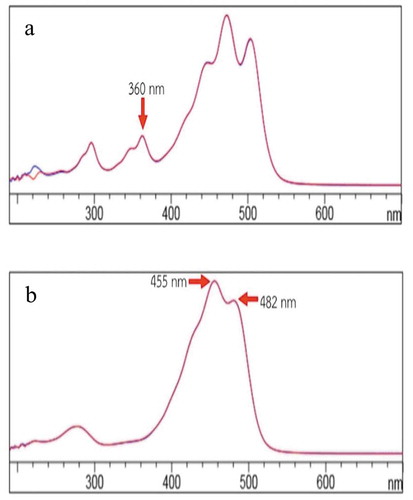
Table shows the results of extraction time on the carotenoid content and antioxidant activity. The results showed that the carotenoid content and antioxidant activity increased with increasing extraction time from 0 to 30 min at 75°C. The highest carotenoid content was produced after extraction for 30 min (p < 0.05). However, the carotenoid content and antioxidant properties of the extracts extracted for 15 and 30 min were not significantly different. This result indicated that after 15 min, time did not predominate in the UAE process. Ye et al. (Citation2011) also found the similar result from corn extracted carotenoids using ethanol as the solvent in an ultrasonic bath at 40°C. Their results showed that the concentration of carotenoids increased with increasing extraction time in the first 1–12 min; however, afterwards, the concentration of carotenoids in the extract was stable until 300 min. Sun et al. (Citation2011) explained that this was due to the turbulence caused by the bubbles imploding and that the energy released by cavitation then facilitates the diffusion of all-trans β carotene extracts, making the osmotic pressure between the inside and the outside of the cell quickly achieve equilibrium (Hemwimol et al., Citation2006). Additionally, the chromatograms of crude extract without UAE (0 min) and with UAE (5 min) were compared to evaluate their carotenoid types with HPLC-DAD (Figure ). The results showed that the chromatogram of all- trans- lycopene at 31.06 min that used UAE (Figure )) was drastically reduced compared with the non-UAE extract (Figure )). Furthermore, the isomer of x-cis lycopene chromatogram was found in the crude extract at the retention time of 29.39 min and was affirmed with the appearance of a peak at approximately 360 nm in the spectra (Figure )) (Konwarh, Pramani, Kalita, Mahanta, & Karak, Citation2012; Phan-Thi & Wache, Citation2014; Poojary & Passamonti, Citation2015). The effect of heat in the UAE process induced some single and double bonds in the structure to change it to x-cis-lycopene (Phan-Thi & Wache, Citation2014). However, all-trans carotenoid isomers are the main configuration in natural fruits and vegetables because they are more stable than cis carotenoids. However, the cis isomers have better bioavailability and are more soluble in bile acids than trans isomers (Calvo et al., Citation2007, Honest et al., Citation2011)
Table 3. Effect of extraction time on carotenoid contents and antioxidant properties of the extracts extracted with absolute ethanol at 75°C
3.4. Structure of carotenoid in the extract
The structure of carotenoids in the crude extract was confirmed using HPLC-DAD. Three types of carotenoids were found in the extract: lycopene, β-carotene, and lutein. The retention times were as follows: lutein at 10.17 min; all-trans lycopene at 31.06 min; and all-trans β-carotene at 40.21 min. The lutein and β-carotene were confirmed using a standard, but the lycopene was confirmed from the absorption spectrum at 447, 471 and 503 nm. (Konwarh et al., Citation2012; Phan-Thi & Wache, Citation2014; Poojary & Passamonti, Citation2015). Furthermore, another isomer of x-cis lycopene was found in the crude extraction at the retention time of 29.39 min, and the structure was confirmed the main peak spectrum as shown in Figure .
3.5. Modulation of the inflammatory system of THP-1 cells
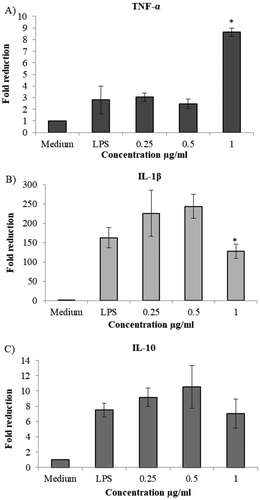
In our study, the crude extract from the pericarp of gac fruit was used to investigate the in vitro modulation of the expression of inflammatory genes on monocyte leukemia THP-1 cells. The percent cell viability of THP-1 cells was studied using the MTT assay. Our results showed that treatment with the crude extract at concentrations of 0.05–1.00 µg/ml had more than 80% viability (data not shown) after 6 h of incubation. These results revealed that the crude extract was safe for THP-1 cells. Then, the cells were activated by incubation with the crude extract and 100 ng/ml lipopolysaccharide (LPS) for 5 h. The effect of the crude extract on dormant THP-1 cells is presented in Figure . Treatment with 1 µg/ml crude extract had a significant effect on the suppression of IL-1β compared to that of the LPS treatment. This treatment concentration might block NF-kB activation in the cells. The inflamed cells also produce cytokines that enhance the signal transduction and transcription of NF-kB, whose activation induces the production and secretion of cytokines. NF-kB has been associated with oxidative stress-induced cell damage (Yeh, Wang, Chen, & Wu, Citation2009) Our results agreed with Makon-Sebastien et al. (Citation2014) regarding the 2 µM lycopene enhancement of IL-1β secreted on LPS active cells; however, when they stimulated the cells with PMA (phorbol myristate acetate), there was significant suppression of IL-1β by lycopene. LPS is produced by gram-negative bacteria such as E. coli and is used to stimulate bacterial activation in inflammatory studies. Alternatively, PMA is used to mimic the intracellular signaling functions of diacylglycerol. Although both LPS and PMA can induce cytokine secretion through the transcription factor NF-kB, they use different signaling pathways that result in different gene expressions. However, at 1 µg/ml, TNF-α expression was significantly enhanced. This result might be influenced by a biphasic effect of the β-carotene compound. From our previous results, the crude extract mainly contained β-carotene. Yeh et al. (Citation2009) found that β-carotene at high concentrations (20 µM) stimulated the enhanced secretion of TNF-α, but at low concentrations (2 µM), the secretion of TNF-α in HL-60 cells and RAW264.7 cells was unchanged. Unfortunately, our results showed only a low correlation with IL-10 expression at all concentrations of crude extract. In addition, these results were different from Chung et al. (Citation2017) in that low concentrations of lutein might not suppress IL-1β and TNF-α cytokines.
Figure 4. Effect of the extract on expression of pro- and anti-inflammatory cytokines in THP 1 cell. The relative fold induction of cytokines as IL-1β, TNF-α and IL-10 was determined on active THP1 cells in various concentrations of crude extract (0.25–1 µg/ml).
*Symbol means there are significant differences in the same cytokine type (P < 0.01) significant of concentrations compared with other concentrations by Duncan’s multiple range test.
4. Conclusions
Our results present that both extraction temperature and time influenced the carotenoid content and antioxidant properties in the UAE process. Moreover, the ratio of ethanol to water was also the critical parameter driven the properties of the carotenoid extracts. The high antioxidant activity of carotenoid extracted with UAE might occur from the isomerization of lycopene which was resulted from high temperature and cavitation on the carotenoid structure as confirmed by HPLC-DAD and FTIR. The gac fruit extract, which contains x-cis carotenoid form, did not suppress the secretion of pro-inflammatory cytokines or affect the level of anti-inflammatory cytokines. Additionally, the antioxidant-rich extract is safe and suited for using in food systems as a functional ingredient.
Acknowledgements
This research was supported by the Graduate Program Scholarship from Graduate School, Kasetsart University.
Additional information
Funding
Notes on contributors
Supassara Natnoi
Supassara Natnoi is doing her Master Degree in Product Development with scholarship obtained from Graduate School, Kasetsart University for studying in Department of Product Development, Faculty of Agro-Industry, Bangkok, Thailand.
Tantawan Pirak
Dr.Tantawan Pirak is an Assistant Professor at Department of Product Development, Faculty of Agro-Industry, Kasetsart University, Bangkok, Thailand since 2007 until now. She received her Ph.D. in Food Technology from Chulalongkorn University, Thailand in 2007. Her research expertise is the development of functional ingredients and functional meat products, natural extracts and their utilization in food, chitosan and hydrocolloids in food, protein hydrolysate and functional peptides and interactions of food macromolecules with functional ingredients. Her research interest and future research is in the field of the study using in vitro gut model system.
References
- Calvo, M., Dado, D., & Santa-Maria, G. (2007). Influence of extraction with ethanol or ethyl acetate on the yield of lycopene β-carotene, phytoene and phytofluene from tomato peel powder. European Food Research and Technology, 224, 567–13. doi:10.1007/s00217-006-0335-8
- Chanput, W., Krueyos, N., & Ritthiruangdej, P. (2016). Anti-oxidative assays as markers for anti- inflammatory activity of flavonoids. International Immunopharmacology, 40, 170–175. doi:10.1016/j.intimp.2016.08.038
- Chung, R. W. S., Leanderson, P., Lundberg, A. K., & Jonasson, L. (2017). Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis, 262, 87–93. doi:10.1016/j.atherosclerosis.2017.05.008
- Dutta, A. (2017). Chapter 4 - Fourier transform infrared spectroscopy. In S. Thomas, R. Thomas, A. K. Zachriah, & R. K. Mishra (Eds.), Spectroscopic methods for nanomaterials characterization, 73–79. Elsevier.
- Hassas-Roudsari, M., Chang, P. R., Pegg, R. B., & Tyler, R. T. (2009). Antioxidant capacity of bioactives extracted from canola meal by subcritical water, ethanolic and hot water extraction. Food Chemistry, 114, 717–726. doi:10.1016/j.foodchem.2008.09.097
- Hemwimol, S., Pavasant, P., & Shotipruk, A. (2006). Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrasonic Sonochemistry, 13, 543–548. doi:10.1016/j.ultsonch.2005.09.009
- Honest, K. N., Zhang, H. W., & Zhang, L. (2011). Lycopene: Isomerization Effects on Bioavailability and Bioactivity Properties. Food Reviews International, 27, 248–58. doi:10.1080/87559129.2011.563392
- Jaeschke, D. P., Rech, R., Marczak, L. D. F., & Mercali, G. D. (2017). Ultrasound as an alternative technology to extract carotenoids and lipids from Heterochlorella luteoviridis. Bioresource Technology, 224, 753–757. doi:10.1016/j.biortech.2016.11.107
- John, J. J., Kuhn, S., Braeken, L., & Van Gerven, T. (2018). Effect of fluid properties on ultrasound assisted liquid-liquid extraction in a microchannel. Ultrasonics Sonochemistry, 42, 68–75. doi:10.1016/j.ultsonch.2017.11.003
- Kaulmann, A., & Bohn, T. (2014). Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutrition Research, 34, 907–929. doi:10.1016/j.nutres.2014.07.010
- Kha, T., Nguyen, M., Roach, P., & Stathopoulos, C. (2015). Ultrasound-assisted aqueous extraction of oil and carotenoids from microwave-dried gac (Momordica cochinchinensis Spreng) Aril. International Journal of Food Engineering, 11, 479–492. doi:10.1515/ijfe-2014-0220
- Konwarh, R., Pramani, S., Kalita, D., Mahanta, C. L., & Karak, N. (2012). Ultrasonication – A complementary ‘green chemistry’ tool to biocatalysis: A laboratory-scale study of lycopene extraction. Ultrasonics Sonochemistry, 19, 292–299. doi:10.1016/j.ultsonch.2011.07.010
- Kubola, J., & Siriamornpun, S. (2011). Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai Gac (Momordica cochinchinensis Spreng). Food Chemistry, 127, 1138–1145. doi:10.1016/j.foodchem.2011.01.115
- Lorand, T., Molnar, P., DELI, J., & Toth, G. (2002). FT-IR study of some seco- and apocarotenoids. Journal of Biochemical and Biophysical Methods, 53, 251–258. doi:10.1016/S0165-022X(02)00113-6
- Luengo, E., Condon-Abanto, S., Condon, S., Alvarez, I., & Raso, J. (2014). Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Separation and Purification Technology, 136, 130–136. doi:10.1016/j.seppur.2014.09.008
- Makon-Sebastien, N., Francis, F., Eric, S., Henri, V. P., Francois, L. J., Laurent, P., … Serge, C. (2014). Lycopene modulates THP1 and Caco2 cells inflammatory state through transcriptional and non transcriptional Processes. Mediators of Inflammation, (2014, 1–12. doi:10.1155/2014/507272
- Mason, T. J., Paniwnyk, L., Chemat, F., & Vian, M. A. (2011). Ultrasonic food processing. In A. Proctor (Ed.), Alternative to conventional food processing (pp. 387–414). UK: Cambridge.
- Murakami, K., Honda, M., Takemura, R., Fukaya, T., Kubota, M., Wahyudiono,, … Goto, M. (2017). The thermal Z-isomerization-induced change in solubility and physical properties of (all-E)-lycopene. Biochemical and Biophysical Research Communications, 491, 317–322. doi:10.1016/j.bbrc.2017.07.103
- Napolitano, M., Depascale, C., Wheeler-Jones, C., Botham, K. M., & Bravo, E. (2007). Effects of lycopene on the induction of foam cell formation by modified LDL. American Journal of Physiology - Endocrinology and Metabolism, 293, 1820–1827. doi:10.1152/ajpendo.00315.2007
- Phan-Thi, H., & Wache, Y. (2014). Isomerization and increase in the antioxidant properties of lycopene from Momordica cochinchinensis (Gac) by moderate heat treatment with UV–Vis spectra as a marker. Food Chemistry, 156, 58–63. doi:10.1016/j.foodchem.2014.01.040
- Poojary, M. M., & Passamonti, P. (2015). Optimization of extraction of high purity All-Trans-Lycopene from tomato pulp waste. Food Chemistry, 188, 84–91. doi:10.1016/j.foodchem.2015.04.133
- Schlucker, S., Szeghalmi, A., Schmitt, M., Popp, J., & Kiefer, W. (2003). Density functional and vibrational spectroscopic analysis of β-carotene. Journal of Raman Spectroscopy, 34(6), 413–419. doi:10.1002/jrs.v34:6
- Sun, Y., Liu, D., Chen, J., Ye, X., & Yu, D. (2011). Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-β-carotene from citrus peels. Ultrasonics Sonochemistry, 18, 243–249. doi:10.1016/j.ultsonch.2010.05.014
- Sun, Y., Ma, G., Ye, X., Kakuda, Y., & Meng, R. (2010). Stability of all-trans-β-carotene under ultrasound treatment in a model system: Effects of different factors, kinetics and newly formed compounds. Ultrasonics Sonochemistry, 17, 654–661. doi:10.1016/j.ultsonch.2009.12.005
- Vahur, S., Teearu, A., Peets, P., Joosu, L., & Leito, I. (2016). ATR-FT-IR spectral collection of conservation materials in the extended region of 4000-80 cm–1. Analytical and Bioanalytical Chemistry, 408, 3373–3379. doi:10.1007/s00216-016-9411-5
- Xu, Y., & Pan, S. (2013). Effects of various factors of ultrasonic treatment on the extraction yield of All-Trans-Lycopene from red grapefruit (Citrus Paradise Macf.). Ultrasonics Sonochemistry, 20(4), 1026–1032. doi:10.1016/j.ultsonch.2013.01.006
- Ye, J., Feng, L., Xiong, J., & Xiong, Y. (2011). Ultrasound-assisted extraction of corn carotenoids in ethanol. International Journal of Food Science & Technology, 46, 2131–2136. doi:10.1111/ifs.2011.46.issue-10
- Yeh, S. L., Wang, H. M., Chen, P. Y., & Wu, T. C. (2009). Interactions of beta-carotene and flavonoids on the secretion of pro-inflammatory mediators in an in vitro system. Chemico-Biological Interaction, 179, 386–393. doi:10.1016/j.cbi.2008.12.006
- Zhang, J. M., & An, J. (2007). Cytokines, inflammation and pain. International Anesthesiology Clinics, 45, 27–37. doi:10.1097/AIA.0b013e318034194e