Abstract
Low-input cultivated pastures to feed cattle have dominated land use after forest clearing for decades in the western Brazilian Amazon. This study was undertaken to help understand the inherent nutrient supply dynamics underwriting cattle performance on three farms in the state of Acre. We assessed soil chemical and physical properties associated over time with different land uses following forest clearing. This information permitted specifying a conceptual model of nutrient stocks and flows under the observed grazing system, which produced insights about the dynamics of soil nutrient degradation. Above ground forage mass, topsoil nutrient concentrations and soil bulk density were measured. Land covers were Brachiaria spp. grasses, a grass-Pueraria phaseoloides mix, cropland and forest. Most soil nutrient parameters initially decreased after clearing, gradually recovering over time with grass-only pastures; however, 20 yr-old pastures had 20% less forage mass. Most pasture system nutrients on these farms resided in topsoil and roots, where large stocks of mature forage supported soil fertility with recycled nutrients from litter. Estimates of partial topsoil nutrient balances were negative. This suggested that corresponding nutrient stocks and the accumulation of forage mass were probably maintained primarily through the sum of inflows from cattle excreta, the subsoil, soil organic matter, and litter mineralization with scant input of commercial fertilizer. Therefore, herd management to increase animal system productivity via higher stocking rates on vegetatively younger forage requires monitoring of nutrient stocks and flows and fertilization that assures replenishment of the nutrients extracted. Otherwise, rapid depletion of soil nutrient stocks will lead to system degradation and failure.
PUBLIC INTEREST STATEMENT
Pasture-based cattle production remains the primary long-term use of deforested lands in the western Brazilian Amazon due primarily to growing regional demand for animal products and limited agricultural alternatives. Although pasture productivity in these systems have been maintained over time, our study suggests that current and former soil fertility management practices result in persistent, slow rates of soil nutrient losses. System intensification would accelerate soil nutrient losses without substantial nutrient additions to the system (e.g., via fertilizer or manure). The long-term viability of pasture-based grazing in the western Brazilian Amazon requires recognition of, and adaptation to, the factors contributing to nutrient depletion, and is informed by research evaluating pasture system nutrient status and employing a conceptual model structure like the one arising from this study.
Competing Interests
The authors declare no competing interests.
1. Introduction
Cattle production from grass and mixed grass-legume pastures is the primary use of cleared forestland in the western Brazilian Amazon. Low soil fertility, high rainfall and distant markets hinder a more diversified agriculture. Nearly seven million hectares in the states of Acre and Rondônia were deforested during the quarter century from 1988 to 2015 (INPE, Citation2016b). More than 75% of these deforested lands were cultivated with pasture species variously undergoing processes of degradation (IBGE, Citation2006; INPE, Citation2016a) to produce beef and milk (Valentim & Andrade, Citation2009). Principal species were Brachiaria brizantha, B. humidicola, Panicum maximum, and Cynodon nlemfuensis. Susceptible to spittlebug (Deois incompleta) (Gonçalves, Cruz-Oliveira, & Dutra, Citation1996) the predominant B. decumbens pastures of the 1970s and 1980s were replaced primarily by the spittlebug-resistant Marandu cultivar of B. brizantha. A decade later a pasture death syndrome arising from poor adaptation to low oxygen conditions during the rainy season in low permeability soils afflicted these Marandu pastures (Andrade & Valentim, Citation2007). In Acre these pastures often incorporate the herbaceous legume Pueraria phaseoloides (tropical kudzu) or forage peanut (Arachis pintol Cv. Belmonte). Legumes help increase animal productivity potentials and production per unit area through greater total forage intake and improved dietary quality (Valentim & Andrade, Citation2005a, Citation2005b).
Changes in soil properties following deforestation and subsequent pasture performance, often preceded by short-term cropping, have been studied mostly in the eastern Amazon (Buschbacher, Uhl, & Serrão, Citation1987; Fearnside & Barbosa, Citation1998; Reiners, Bowman, Parsons, & Keller, Citation1994). This region is characterized by soils with high clay content, a 7-mo rainy season, and a grass cover (e.g., B. brizantha Cv. Marandu) ill-adapted to soils of low permeability. Eastern Amazonian pastures are noted for their large soil nutrient stocks (except P) at establishment. Subsequently, however, nutrient stocks shrank to nearly undetectable amounts with concomitant losses in ground cover. With plant nutrient requirements unmet forage yield fell precipitously within five years (Serrão, Falesi, de Veiga, & Teixeira, Citation1978). Besides lost ground cover other forms of degradation included weed invasion, regrowth of unpalatable native grasses and secondary forest, soil compaction and surface sealing. In 2014, 23% of the 76 million hectares of deforested lands in the Brazilian Amazon was populated by secondary vegetation following pasture degradation. Of the remaining 48 million hectares of pastureland, about one-fifth was undergoing degradation in varying degrees (INPE, Citation2016a).
However, these eastern region outcomes neither represent nor characterize the entire Brazilian Amazon. Besides soil type pastureland persistency is influenced by multiple factors varying by location and management practice including method of establishment, fertilization, and local climate (Valentim & Andrade, Citation2009). The specific causes of degradation in the eastern Amazon are unknown because establishment history went mostly undocumented (e.g., bulldozer or manual clearing, specific stocking rates, burning frequency and associated soil-plant nutrient relationships). Degradation outcomes in this region have been associated with extensive management approaches based on high rates of stocking with continuous grazing without sufficient fertilization to replenish the extracted nutrients (Dias-Filho, Citation2011; Rao et al., Citation2015). Recent studies indicate that grazing more intensively by rotating animals among smaller paddocks may be ecologically more sustainable (Instituto Internacional para Sustentabilidade, Citation2015; Rao et al., Citation2015). Others have similarly posited that higher yield potentials of beef and milk are possible from sustainable intensification practices with pasturelands restored (Latawiec, Strassburg, Valentim, Ramos, & Alves-Pinto, Citation2014). Strassburg et al. (Citation2014) indicated that actual productivity of cultivated pasturelands is about one-third of its potential. They suggested that tapping one-half of the inherent productivity potential would satisfy the domestic Brazilian demand for beef until 2040 (plus provisions of food crops, wood products and biofuels) without further sacrificing natural ecosystems. Such productivity gains could also mitigate up to 14.3 Gt CO2 Eq.
Cattle system characteristics and productivity constraints in the western Amazon, including appropriate management of adapted grasses (e.g., Brachiaria spp.) and legumes, likely differ from other parts of the Amazon basin. Besides differences in soil mineralogy, depth, texture and organic matter content, soils proximate to the Andes frequently contain greater amounts of Ca, Mg and K than in other regions (Fernandes et al., Citation1997). Few studies have estimated the nutrient status of tropical pasture biomass and its relationship with land productivity (e.g., de Moraes, Cerri, Volkoff, & Bernoux, Citation1996; Krishnaswamy & Richter, Citation2002). None have quantified nutrient stocks and flows in grazing systems of the western Amazonia. In our study to assess cattle production potentials in Acre (Rueda et al., Citation2003) the dietary supply of metabolizable energy (ME) for animal growth from Brachiaria-Pueraria pastures was consistently more limiting than metabolizable protein. During the rainiest months grasses contained more neutral detergent fiber, neutral detergent insoluble protein and least ME, which restricted weight gain by 20% compared to other times of the year. Higher stocking densities on judiciously fertilized grass-legume pastures were identified as a means to improve net margins through more intensive beef production. The arising query is how to achieve this outcome.
Therefore, the purpose of this western Brazilian Amazon study was: (1) to measure soil nutrient concentrations and bulk density in the topsoils of forest, cropland and cultivated pastures at varying intervals since forest clearing, (2) to estimate nutrient stocks and flows and partial topsoil nutrient balances in crop-livestock systems, and (3) to provide insights about performance potentials of low-input Brachiaria spp. pastures in supporting cattle production over time under traditional and alternative grazing scenarios.
2. Materials and methods
2.1. Site description
Study sites consisted of three farms within 80 km of Rio Branco, Acre, Brazil (07º 07ʹ S and 66º 30ʹ W). Predominant soil types were Ultisols and Oxisols with mostly flat terrain approximately 200 masl. Acre is situated in the moist, humid tropics with an average annual temperature of 25 ºC and 84% relative humidity. Average annual precipitation from 1981 to 2010 was 1940 mm (Duarte, Citation2015) with most rainfall occurring October through April.
Farm management histories were obtained by interviewing farm owners (Table ). Collaborators were owners of: (1) a small farm (90 ha) in the Pedro Peixoto settlement with a dual-purpose (milk and beef) cattle system, and cultivating maize and rice, (2) a beef cattle farm (1600 ha) in Xapuri, and (3) a beef farm (3000 ha) along the Transacreana highway. Each farm had similar proportions of total land holdings in pasture (42% to 55%; Table ). Pasture grasses on the largest farm were sown immediately after clearing and the burning of felled biomass. Pastures on other farms were established following three years of cropping with maize or rice. Commercial fertilizer was not utilized except for a few paddocks on the largest farm. Weeds were controlled manually and with biennial burning of the oldest paddocks. Spittlebug infestation, which first occurred in B. decumbens in the mid-1990s, was managed by burning and with higher stocking rates to reduce herbage accumulation. Since the time of our fieldwork (1999 to 2000) spittlebug-resistant grasses (Andrade & Assis, Citation2008) and grass-legume pastures (Valentim & Andrade, Citation2004) have been adopted by farmers. Insecticides to control spittlebug attack in the remaining B. decumbens pastures on large farms also reduced pasture burning to control weeds. The average stocking rate for each farm was two animal units (AU) per hectare, where one AU is the live weight equivalent of 450 kg.
Table 1. Management practices on collaborating cattle farms in Acre, Brazil
2.2. Soil and forage sampling
Topsoil (≤ 10 cm) samples (n = 96) from 20 site locations were collected from October 1999 to June 2000 to estimate physical and chemical composition. Samples represented three forest locations (one per farm), three locations cropped with maize or rice (one per farm), and 14 paddocks (5 to 240 ha paddock−1) sown with Brachiaria spp. or grass-kudzu associations with varying intervals, or ages, since clearing.
Forage samples were collected in 28-d intervals throughout the study period from five paddocks (one on the largest farm and two on each of the other farms) to determine forage dry matter availability and chemical composition of the forage biomass. Monitored paddocks included two with B. decumbens (14 and 20 yr since forest clearing), two with B. brizantha Cv. Marandu (4 and 8 yr since forest clearing), and one where B. decumbens was associated with about 30% kudzu. Forage mass was estimated by the cutting-quadrants method (‘T Mannetje, Citation2000). Grasses with average height of about 80 cm were cut 20-cm above ground and green leaves were separated from stems and senescent materials. Following Van Soest (Citation1994), samples to determine likely nutritive values of consumed forage were collected by mimicking the grazing behavior of animals by simultaneously collecting samples similar to those selected by them.
2.3. Laboratory analysis
Soil samples were air dried, sieved with a 2 mm screen, and analyzed for contents of Ca, Mg, Na, Al, K, P and C at the Agroforestry Research Center laboratory (Embrapa-Acre). Bulk density was also determined at depths up to 5 cm and 5 to 10 cm using 90 cm3 cylinders (Kiehl, Citation1979). Soil acidity (pH) was determined with a 1:2.5 soil:water suspension and 1M KCl using a combination glass electrode. Calcium, Mg and Al were extracted with 1N KCl and quantified by atomic absorption for Ca and Mg and by titration for Al. Mehlich dilute double-acid solution (Mehlich-1) was used to extract P, K and Na for analysis by flame emission spectrophotometer (Mehlich, Citation1953). Organic carbon was determined by the volumetric method using 0.4N K bichromate (Silva, Citation1999).
Forage samples (500 g) were separated into leaves and stems, dry-ashed for 4 h in a muffle furnace at 500 ºC and ground to pass a 1-mm screen in a Wiley mill. Concentrations of Ca, P, Mg and K were extracted with acid solution (1.5N HNO3 + 0.5N HCl) and estimated with a Thermo Jarrell Ash IRIS Radial Spectrometer (Sirois, Reuter, Laughlin, & Lockwood, Citation1994). Acid detergent lignin content was also determined (AOAC, Citation1990).
2.4. Above ground nutrient stocks
The total above ground stock of nutrients per unit of land comprised nutrients in forage mass and live animals. Average above ground forage nutrient mass was the product of average forage mass per hectare at a given point in time and average nutrient concentration in whole plants. The nutrient stock of live animals was based on average live weight and the average stocking rate. The nutrient content of plant litter was not determined.
Annual consumption and excretion of minerals by herds feeding on grass and grass-legume pastures were estimated using version 4.0 of the Cornell Net Carbohydrate and Protein System model (Fox et al., Citation2004; Tedeschi & Fox, Citation2016), assuming an average daily forage dry matter intake of 2.5% of animal body weight consisting of 100% B. brizantha for steers and a mix of 30% P. phaseoloides and 70% B. decumbens for lactating cows. This model estimates minerals excreted in feces and urine by subtracting the model-estimated quantities absorbed from the predicted daily intake. Quantities of absorbed nutrients included those required for body maintenance and product synthesis (milk production, tissue accretion, and fetal growth during the third trimester of gestation). Five partial topsoil nutrient balances were then estimated as the difference between nutrients ingested and those excreted, with and without fertilization, and with and without assumed manure and fertilizer nutrient losses. Although losses would differ by element, for simplicity loss calculations were assumed to be uniform for all elements. Three low-input partial nutrient balance scenarios (2 AU ha−1 stocking rate, where 1 AU = 450 kg live weight; scenarios A, B, C) and two intensified scenarios (4 AU ha−1 stocking rate; scenarios D, E) were estimated.
A: feces + urine—ingested
B: feces + urine + fertilizer—ingested, where fertilizer is split-applied at 100 kg
N (ha*yr)−1, 88 kg P (ha*yr)−1 and 84 kg K (ha*yr)−1
C: same as B, except with 50% nutrient losses assumed from fertilizer and excreta
D: same as A at higher stocking rate
E: same as C at higher stocking rate
The nutrient stock for a cattle herd with average stocking rate of 2 AU ha−1 was calculated as the sum of products of animal numbers and their carcass weights (steers = 250 kg, cows = 225 kg), and expected nutrient concentrations in soft tissues (muscle and fat), bone and offal (Nour & Thonney, Citation1987, Citation1988). Nutrients in offal were estimated as a function of live weight (Apple, Davis, & Stephenson, Citation1999). Nutrient exports from beef systems included nutrients contained in sold steers, culled cows, and mortality losses. Nutrient exports from the dual-purpose system comprised those in milk sold, culled cows, weaned calves, and mortality losses. Estimated average annual culling rates (cows = 5%; steers = 0.4%), mortality rates (calves = 6%; adults = 2%) and milk sales (1350 kg cow–1 yr−1) were provided by the collaborating farmers. Nutrient losses from burning, volatilization, de-nitrification, leaching and runoff were important unmeasured outflows.
2.5. Below ground nutrient stocks
The amount of soil in a hectare furrow slice to 10-cm depth was calculated by multiplying the soil volume in one hectare by its bulk density. Nutrient stocks (kg ha−1) were then determined as the product of topsoil amount and nutrient concentration in topsoil. Soil nutrients below 10-cm depth were not measured. Root biomass was approximated as 1.5 times the above ground pasture biomass (Rao, Kerridge, & Macedo, Citation1996). Mineral concentration was assumed equal to that in pasture biomass, except for an assumed value of 0.33% N for roots (Urquiaga, Cadisch, Alves, Boddey, & Giller, Citation1998). Deeper soil strata were not measured, although the subsoil constitutes a key nutrient stock in the system.
2.6. Statistical analysis
Chemical and physical characteristics of topsoil were analyzed using JMP PRO 12.0.1 (SAS Institute Inc, Citation2015) accounting for land cover (forest, cash crop, B brizantha, B. decumbens, or an association of Brachiaria spp. grass and kudzu) and time interval since forest clearing. Soil physical and chemical parameters were evaluated individually. Candidate fixed effects were combinations of land cover, interval since forest clearing, and clay concentration to reflect soil texture. Farm was treated as a random effect for soil chemical and physical models, and ignored when it accounted for <10% of total residual variance. Mean differences (P ≤ 0.05) among land cover–time interval combinations were identified using Tukey’s adjustment for multiple comparisons. Model residuals were assessed for normality and log or square root transformations were applied to improve model fit. Geometric means and 95% confidence intervals are reported for transformed data.
3. Results
3.1. Soil chemical and physical properties
Chemical and physical properties (Supporting Information Table 1) revealed low fertility and moderately acid topsoils. Soils were silty loams with clay concentrations of 180 g kg−1 to 270 g kg−1, which are typical of highly weathered tropical soils. Topsoil concentrations of Ca, Mg, Na, and C increased with clay concentration (soil texture effect), while the concentration of P and soil pH decreased. Farm was treated as a random effect in assessing Ca, Na, pH, and bulk density (Supporting Information Table 2). Mean differences in soil chemical and physical characteristics due to land cover and time interval since clearing were detected for C, P, Ca, K, Na, and bulk density (Figure ; Figure ; Figure ; Supporting Information Table 2). Overall, differences were not detected between forest and other combinations of land cover-time after clearing for most soil chemical properties. Exceptions included: (1) higher C concentration in forest than eight-yr-old grass + kudzu and (2) concentrations of P in paddocks with 10 to 20 yr-old B. brizantha exceeded those for most other land covers, including forest and older B. decumbens pastures. Consistent with the study of Acre soils by Leonidas (Citation1998), total C was lowly associated with most fertility parameters in our study (r < 0.4). Pairwise correlations were higher between C and Ca (r > 0.6) and P and Na (r > 0.4).
Figure 1. Back-transformed soil C (left) and soil P (right) least squares (geometric) means with 95% confidence intervals for land covers with different time intervals, or ages, since forest clearing. Land cover–age combinations in each panel not connected by the same letter differ significantly (P ≤ 0.05). Grayscale shades represent different land covers
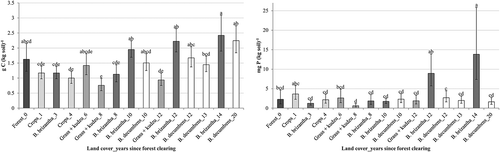
Figure 2. Back-transformed soil Ca (top left) and Mg (top right), K (bottom left), and Na (bottom right) least squares (geometric) means with 95% confidence intervals for land covers with different time intervals, or ages, since forest clearing. Land cover–age combinations in each panel not connected by the same letter differ significantly (P ≤ 0.05). Grayscale shades represent different land covers
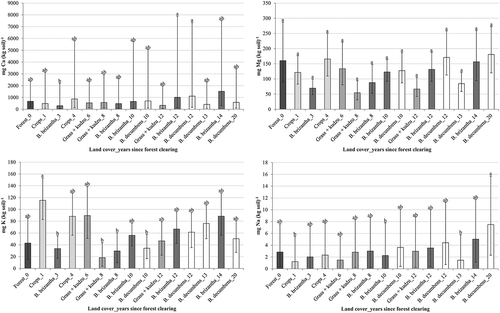
Figure 3. Bulk density least squares means with 95% confidence intervals for land covers with different time intervals, or ages, since forest clearing for soil depths of 0 to 5 cm (left) and 5 to 10 cm (right) below ground level. Land cover–age combinations in each panel not connected by the same letter differ significantly (P ≤ 0.05). Grayscale shades represent different land covers
Most soil chemical properties for topsoils under B. brizantha increased with time since clearing (Figures and ). A similar pattern was observed under B. decumbens for concentrations of C, K, Mg and Na (Figures and ). Consistent with topsoil C, soil cations (Figure ) reflected nutrient maintenance or accretion in older pastures relative to recently cleared land. However, highest topsoil K concentrations were observed under crops and the grass–kudzu association. Ash from forest burning increases initial availability of K, Mg, Ca, and P (Brady & Weil, Citation2008), which may explain elevated topsoil K for recently converted croplands (2.7 times more K than for uncleared land). The bulk density of forest soil to a depth of 5 cm was significantly less than for most other land cover-time combinations (Figure ).
3.2. Forage mass and nutritive value
Monthly forage mass from grasses ranged from 2.2 to 3.7 Mg DM ha−1 from the end of the dry season to the end of the subsequent rainy season. Cattle selected forage from the top 20 cm of plants. This portion of the sward, which contained 85% leaves and 15% stems, constituted about 40% of the total plant biomass. Consequently, these selectively-grazed pastures accumulated large stocks of mature biomass, particularly from growth during the 7-mo rainy season (October through April).
The Ca content of grasses (Table ) was within the boundaries (1.0 to 3.7 g kg−1) for unrestricted plant growth (Rao et al., Citation1996; Rao, Miles, & Granobles, Citation1998). Concentrations of Mg (Table ) surpassed requirements for B. brizantha and kudzu, which generally range from 1.0 to 3.0 g kg−1 (Whitehead, Citation2000), but were less than the critical value for unconstrained growth of B. decumbens (Rao et al., Citation1996, Citation1998). Grasses contained 50% more K than kudzu, also exceeding critical values for unrestricted growth.
Table 2. Concentrationsa of macronutrients and lignin in the grazed portion (top 20 cm) of the plant for Brachiaria decumbens, Brachiaria brizantha and Pueraria phaseoloides on three farms in Acre, Brazil
3.3. Partial nutrient balances, nutrient stocks and flows in the pasture-based cattle system
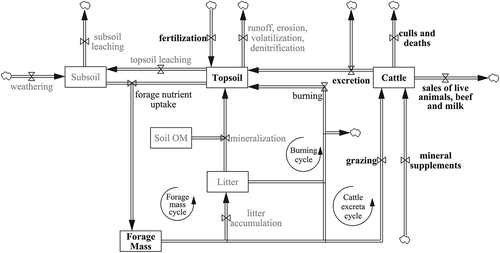
A conceptual presentation of relationships among key nutrient stocks and flows of this pasture-based cattle system is in Figure . This framework aids understanding the structure undergirding this system over time. Three stocks of nutrients were estimated from parameters measured in this study: topsoil, forage mass, and cattle. Although not measured empirically, other stocks—nutrients in subsoil, forage litter and soil organic matter—also contributed to the overall system framework. Five partial topsoil nutrient balance scenarios (Table ) were calculated from estimated nutrient inflows into the topsoil stock (i.e., from fertilization and by excretion of manure and urine) and from the outflow (nutrient uptake) through forage plants.
Table 3. Estimated annual quantities per hectare of nutrients ingested and excreted in cattle systems in Acre, Brazil
Figure 4. Generic nutrient stocks and flows in cultivated western Amazonian grazing systems. Boxes represent stocks or accumulations of nutrients in the system. Valves and arrows represent flows of nutrients between the stocks, and into and out of the system. Clouds represent nutrient sources or sinks. Parameters measured or estimated in calculations are emboldened. Parameters neither measured nor estimated are in gray
Topsoil balances were negative for all nutrients measured in the low-input system (2 AU ha−1 without chemical fertilizer), including assumed nil nutrient losses from excretion (Table , Scenario A). These balances would be more negative without chemical fertilizer and 50% nutrient losses (especially N). Fifty percent nutrient losses from excreta and fertilizer would yield negative balances for all nutrients considered except for P.
Annual nutrients consumed in forage (i.e., the grazing inflow to the cattle stock) were estimated about 20 kg ha−1 each for P and Mg, 105 to 144 kg ha−1 of N and 173 to 192 kg ha−1 of K (Table ). Estimated annual consumption of Ca was about 15 kg from B. brizantha and more than twice as much (38 kg) when grazing a grass-legume mixture. About 80% of ingested P, 95% of N, and 50% of Ca were estimated returnable to pasture by excreta, which agrees with other findings (Boddey, Rao, & Thomas, Citation1996).
4. Discussion
4.1. Soil chemical and physical properties
Land cover over time since forest clearing influenced all topsoil properties (Supporting Information Table 2). Following the initial drop in topsoil nutrients with clearing neither differences nor time pattern were detected for most parameters, suggesting that topsoil nutrients under grass-only pastures were maintained or may have increased slowly over time. There was an initial loss of soil C within 10 yr after clearing for crops and pastures, consistent with the soil OM losses expected after intensive burning (Kennard & Gholz, Citation2001). Furthermore, C contents of Acre soils have been lower than the overall Amazon average. This may result from continuous cycles of drying and moistening with associated conditions favoring mineralization of soil organic matter (Melo, Citation2003).
Thus, in addition to biomass burning the soil structure likely contributed to initial losses of C relative to other soils during the transition from forest cover. Soil C was later recovered in older grass-only cases, exceeding the amounts observed in soils with crops or grass-kudzu pastures. Soil C concentration under the oldest pure grass pastures did not differ from forest soils (Figure ; Supporting Information Table 2). This suggests that soil organic matter and the amounts contributed by cattle excreta and the litter from above- and below-ground sources replenished topsoil C over time.
Greater K concentrations under grass-only pasture compared to the forest soils in this study suggest the capacity to retain or even augment K after burning. The maintenance (or accretion) of nutrients in pastures is attributable to the ash from burning accumulated herbage, to the slow decomposition of unburned woody debris, to weathering, and to pasture root systems transferring subsoil nutrients to the surface (Amézquita, Thomas, Rao, Molina, & Hoyos, Citation2004; Serrão & Toledo, Citation1990).
Greater bulk density has been associated with OM losses after clearing and intensive fires (Kennard & Gholz, Citation2001). Pasture root penetration was likely unimpaired. Bulk densities were mostly less than 1.45 g cm–3 at both topsoil depths (Figure ), which is the approximate threshold when plant growth is compromised in heavy, moist soils (Brady & Weil, Citation2008). Similar densities have been reported in this region (1 to 1.6 g cm−3) for various land covers with largest values occurring at greatest depths (Amaral, Brown, & Melo, Citation2001; Melo, Citation2003). Greater bulk density in pasture topsoil is frequently attributed to compaction by trampling, especially under sparse land cover. Our results suggest that modest compaction occurred directly following forest clearing, but did not worsen under pasture relative to cropland (Figure ).
4.2. Forage mass and nutritive value
Forage mass in pastures grazed by 2 to 4 AU ha−1 in the humid tropics of South America ranged from 0.5 to 5.5 Mg DM ha−1 mo−1 for B. decumbens and 1.3 to 3.5 Mg DM ha−1 mo−1 for B. brizantha (Costa, Citation1997; Jannotti, Citation1997). These location similarities plus ours suggest that certain pasturelands are under low grazing pressure. Pastures in the eastern Brazilian Amazon also have been underutilized (Muchagata & Brown, Citation2003). Andrade, Garcia, Valentim, and Pereira (Citation2006a) reported that the dry season carrying capacity of a mixed pasture (Panicum maximum x P. infestum Cv. Massai and Arachis pintoi Ac 01) was half of that obtained during the rainy season (1.8 AU ha−1 vs. 3.6 AU ha−1), accentuating the impacts of seasonal rainfall on pasture growth and carrying capacity. Rotationally grazed pastures may be more efficiently utilized when herbage accumulations are low under higher stocking rates (Andrade, Garcia, Valentim, & Pereira, Citation2006b).
The youngest pasture in this study (B. brizantha Cv. Marandu) accrued only 20% more forage mass than the oldest pasture (B. decumbens), demonstrating that a 20 yr-old B. decumbens pasture without fertilization can remain productive when lightly stocked and resistant to spittlebug attacks. This modest difference between the youngest and oldest pastures may have been partly due to the poor adaptation of B. brizantha Cv. Marandu to the low permeability soil conditions, as subsequently established (Dias-Filho, Citation2002; Dias-Filho & Carvalho, Citation2000). Accumulated forage mass for B. brizantha was greatest during the period of low rainfall, which differed from B. decumbens. Brachiaria decumbens is moderately tolerant of water-logging, which frequently occurs in Acre soils (Dias-Filho & Carvalho, Citation2000). Marandu intolerance to these conditions is a factor driving replacement by B. brizantha Cv. Xaraés, which is better-adapted to water-logging (Andrade & Assis, Citation2008). Drought tolerance of B. brizantha is known to exceed that of B. decumbens and B. humidicola (Fisher & Kerridge, Citation1996).
Although requirements are similar, grasses extract K more readily than the legumes growing with them (Whitehead, Citation2000) and forage mass can be maintained with herbage K concentrations near 10 g kg−1 (Cherney, Cherney, & Bruulesma, Citation1998). Herbage P concentrations exceeded critical levels (Rao et al., Citation1996), suggesting that P was not deficient in either grass or kudzu plant tissues (Table ) despite low amounts in topsoil (Figure ). The P concentration in grass was 40% to 60% greater than in kudzu, possibly because deep-rooted Brachiaria grasses extract P from greater depths, including less available forms (Garcia-Montiel et al., Citation2000). The concentration of P in kudzu (1.7 g kg—1) was less than the amount required by temperate perennial legumes (2.1 g kg−1 to 3.1 g kg−1) (Davis, Citation1991). Evidence from the Amazon region indicates that nitrogen is the most limiting soil nutrient for high pasture productivity (Andrade, Valentim, Pereira, & Ferreira, Citation2010).
4.3. Nutrient stocks, flows and partial balances
Negative partial balances indicate that most topsoil nutrients were, or expected to be, lost over time. Therefore, forage growth must have been underwritten with nutrients from subsoil stocks, mineralization of organic matter and litter, nitrogen deposition, and ash. In our case magnitudes of these stocks and inflows to the topsoil are uncertain, as are the losses due to burning, runoff, erosion, volatilization and de-nitrification. Lower yields in older pastures suggest that balances were slightly negative, at least for the most limiting soil nutrients. Furthermore, although topsoil nutrient stocks were maintained (or increased) over this forest-to-pasture time horizon (Figure ; Figure ; Figure ), yield declines likely reflect outflows that exceed inflows, signifying active pathways of nutrient depletion in the subsoil stock and organic matter.
Much organic N is rapidly mineralized after passage through the animal (Haynes & Williams, Citation1993). The amounts of nutrients in manure and urine vary with dry matter intake, nutrient content of the diet and physiological status of animals. Nutrients in urine are in plant available form (K) or quickly mineralized (N and S) with greater potential losses from volatilization and leaching than those in feces (Nguyen & Goh, Citation1992). Bouman and Nieuwenhuyse (Citation1999) estimated that N recovery from excreta is no more than 30%. Therefore, our negative partial topsoil N balances assuming 50% losses from excreta (Table ; scenario C) are probably underestimates.
Annual mineral extractions per animal in live weight gain of steers or milk production in these Acre cattle systems are depicted in the sales outflow from the cattle stock in Figure . These pasture system exports included approximately 1.5 kg P ha−1 and 2 kg Ca ha−1 (Table ). Most nutrients in these systems reside below ground (Table ) in topsoil stocks and in below-ground biomass (forage mass stock in Figure ). Above ground biomass contained about 47% and 30% of the stocks of P and K, respectively. Phosphorus in topsoil was about 3 kg ha−1 (Table ), portending limitation even with low grazing pressure. This low stock of topsoil P was similar to findings by Feldpausch, Rondon, Fernandes, Riha, and Wandelli (Citation2003), who found soil P of 8.3 kg ha−1 at a greater 45 cm depth in degraded pasture near Manaus, Brazil. Without P fertilization about 90% of Amazonian topsoils have been considered to be of low productivity potential (Cochrane & Sanchez, Citation1982). Over 95% of the Ca and Mg pools in this study were apparently below ground (topsoil and roots). Most N likely resided in the above-ground forage and cattle nutrient stocks.
Table 4. Estimated quantities of minerals extracted annually in the live weight gain of steers and in the milk produced by dual-purpose cows in Acre, Brazil
Table 5. Estimated stocks of major nutrients per hectare in topsoila and rootsb and in above ground green biomass of Brachiaria brizantha and live weight of cattle in Acre, Brazil
At a stocking rate of 2 AU ha−1 estimated annual exports in sales of milk and beef, mortality losses and culled animals were approximately 4 to 7 kg N ha−1 and 3 kg P ha−1 (Table ; outflows from the cattle stock in Figure ). The estimated removal of N exceeded other nutrient exports in the beef cattle system. Milk production accounted for high Ca export from the dual-purpose system. Consequently, the low-intensity grazing management in these cattle systems indicates slower nutrient removal than by cropping with cassava, maize, rice, beans or coffee. For example, rice cropping annually removes 9, 13, 2 and 0.4 kg ha−1 of P, K, Ca and Mg. Cassava, banana, cacao, and coconut annually remove 13 to 20 kg P ha−1, while cassava, banana, oil palm, and passion fruit annually extract 12 to 21 kg Ca ha−1 (Fernandes et al., Citation1997), at least six times more than these cattle systems. Except P, the quantities of nutrients leaving these systems in animal products are relatively small proportions of the total nutrient stocks in soil, animals and above ground biomass (Tables and ).
Table 6. Estimated nutrients leaving cattle systemsa in milk and animals in Acre, Brazil for a stocking rate of two animal unitsb (AU) per hectare
4.4. Unmeasured nutrient stocks and flows
Litters from above- and below-ground forage masses constitute another important nutrient stock in pasture-based cattle systems (Figure ). This unmeasured stock arose especially from the unpalatable 60% of standing biomass. A study of B. humidicola pastures in eastern Brazil (Rezende et al., Citation1999) indicated a litter stock of about 16 Mg DM ha−1 yr−1 containing 7 g N kg−1 DM with a 28-d half-life. .
Rates of litter mineralization (also unmeasured) are affected by chemical composition, especially N, lignin, C:N ratios, and secondary compounds (i.e., tannins and other polyphenols). Less N is released when plant materials contain less than 25 g N kg−1 (Palm, Gachengo, Delve, Cadisch, & Giller, Citation2001). Kudzu contains twice as much N and three times more lignin (Table ) than grasses (Tedeschi, Fox, Pell, Lanna, & Boin, Citation2002). The lignin:N ratios in our study (3:1 to 4.7:1) indicated low net mineralization (Thomas & Asakawa, Citation1993) and slow decomposition with greater potential total N released for kudzu than for grass. In our study a slowly decomposing, or passive, pool comprising mature leaves, stems and roots would be expected to contain less N and more lignin than found in selectively-grazed forage pools (Rezende et al., Citation1999; Vanlauwe, Nwoke, Sanginga, & Merckx, Citation1996). Although we did not measure them, subsoil nutrients likely play importantly in the persistency of pasture-based cattle systems. For low grazing pressure scenarios with good ground cover and well-distributed excreta, most nutrients, especially N, are expected from litter, biological N fixation, fertilization, and the subsoil. Other small quantities obtain from the mineralization of soil OM (soil OM stock in Figure ) and atmospheric N (Eickhout, Bouwman, & van Zeijts, Citation2006).
In recent years, environmental governance policies under the Brazilian Forest Code have been aimed at reducing deforestation and the use of fire (Valentim, Citation2015). Herbicide control has been largely substituted for burning, although accidental fires still occur, particularly when rainfall is low (Cicone, Citation2016). Based on forage mass and nutrient composition, we estimated annual losses of 31, 26, 5, 3 and 3 kg ha−1 of K, N, P, Ca and Mg from traditional biennial burning of B. brizantha pastures with 3.3 Mg ha−1 of standing biomass. The estimated total annual P thus removed by burning is about one-third of the estimated amount residing in topsoil and root stocks, which constitutes a potentially large drain from the topsoil stock of P.
4.5. Potentials for system intensification
There are several opportunities to enhance nutrient cycling and raise productivity potentials of Acre cattle systems. Among these higher stocking rates with seasonal rotational grazing and electric fencing (used by some farmers) is fundamental. Nutrient cycling via the cattle excretion and forage mass cycles (Figure ) is subject to management through stocking rate, manure distribution, and plant species that determine the supplies and composition of decomposable litter and excreta. Higher stocking rates (e.g., 3 to 4 AU ha−1) are expected to increase the flow of nutrients through the cattle excretion cycle while restricting the accumulation of mature plant biomass and litter via the forage mass cycle. Correspondingly, more forage nutrients would be expected to be consumed by animals.
Managing risk is also part of system intensification. In the absence of adequate inorganic fertilization larger negative partial topsoil nutrient balances (Table , scenarios D and E) and depletion of soil nutrient stocks are more likely over time with more intensive grazing. Thus, without additional nutrient inputs more intensive grazing in Acre systems portends more rapid nutrient depletion and concomitant land degradation. Therefore, appropriate pastureland management requires accurate quantification and monitoring of expected nutrient balances and stock-flow dynamics in order to steward the system over time. Key considerations include nutrient balance outcomes from alternative stocking and herd management scenarios. This requires efficacious use of external inputs (e.g., inorganic fertilizer to obtain a greater dietary forage supply to support animal and herd performance (McRoberts et al., Citation2016, Citation2018). Although liberal fertilization and higher stocking rates in the Acre region could improve economic returns from beef production (Rueda et al. (Citation2003), our analysis points to the further need for careful control of topsoil nutrient stocks to avoid their degradation and concomitant system decline.
5. Implications and conclusions
Pasture-based cattle production remains the primary long-term use of deforested lands in Acre, especially owing to growing regional demand for animal products and limited agricultural options. More profitable cattle enterprises will be those becoming more productive per unit area from greater supplies of dietary forages providing animals with more metabolizable energy. Achieving this outcome involves higher stocking that is seasonally adjusted, herd grazing by groups of animals differentiated by their nutritional requirements, effective landscape distribution of excreta, and supplemental applications of chemical fertilizer harmonized with the amounts of nutrients exported.
The risks of such intensification include accelerated land degradation in the absence of sufficient nutrient inflows (e.g., through fertilization) to replenish system outflows in animal products. Grass-legume mixtures can enrich diets, also incorporating more N to ensure pasture survival with modest additions of external inputs. Efficacious decision making will benefit from ex ante assessments, including dynamic simulation modeling (e.g., McRoberts, Nicholson, Blake, Tucker, and Díaz Padilla (Citation2013)), to help assure incentives to farmers by comparing attractive options. The resulting best bets should be supplemented by experimentation targeting animal and herd productivity, monitoring of nutrient stocks and flows, and the expected net economic returns from candidate technologies and management choices (Faminow, Citation1998).
Our study suggests that current and former soil fertility management practices result in negative partial nutrient balances and persistent, slow rates of soil degradation. Therefore, absent substantial additional inputs more intensive grazing is expected to accelerate this process. The long-term viability of pasture-based grazing in the western Brazilian Amazon requires recognition of, and adaptation to, the factors contributing to the depletion of nutrient stocks. The conceptual stock-flow model presented in this study helps to inform about these nutrient supply dynamics.
Supplemental Material
Download MS Word (33.5 KB)Acknowledgements
We especially thank the staff of Centro de Pesquisa Agroflorestal do Acre, Brazil (Embrapa) for facilitating and partially financing this study. The authors also acknowledge the financial support of Cornell International Institute for Food, Agriculture and Development, Consejo Nacional de Ciencia y Tecnología (México), and Instituto Nacional de Investigaciones Forestales y Agropecuarias (México). We are grateful for the valuable advice of S. Vosti and C. L. Carpentier; their insights and help were fundamental to the planning of this research. Special thanks to Dr. Jailton da Costa Carneiro, Jordâo Santos de Melo and José Elcio Feitoza for their technical assistance. Special recognition and gratitude is given to the three collaborating farm owners. Their interest, support and hospitality were essential to the success of the study.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
Notes on contributors
K. C. McRoberts
Robert’s Blake’s research group leads international research and training programs that emphasize management of and net economic returns from the livestock component of mixed farming systems in developing countries, especially Latin America and Africa. Teaching, training, outreach, development and research constitute an integral strategy. Program objectives are to train scientists by investigating options to improve productivity, net economic returns and sustainability of the livestock component of farming systems in developing countries. Parallel objectives are to disseminate this information to students, trainees, international collaborators and other decision makers.
References
- ‘T Mannetje, L. (2000). Measuring Biomass of Grassland Vegetation. In L. ‘T Mannetje & R. M. Jones (Eds.), Field and laboratory methods for grassland and animal production research (pp. 151–19). Wallingford, UK: CAB International.
- Amaral, E. F. D., Brown, I. F., & Melo, A. W. F. D. (2001). Efeito de diferentes usos da terra nas características do solo no Estado do Acre. Embrapa Acre, Boletim de Pesquisa, 30. Rio Branco, Acre, Brasil: Embrapa Acre. p. 23.
- Amézquita, E., Thomas, R. J., Rao, I. M., Molina, D. L., & Hoyos, P. (2004). Use of deep-rooted tropical pastures to build-up an arable layer through improved soil properties of an Oxisol in the Eastern Plains (Llanos Orientales) of Colombia. Agriculture Ecosystems & Environment, 103, 269–277. doi:10.1016/j.agee.2003.12.017
- Andrade, C. M. S., & Assis, G. M. L. (2008). Capim-xaraés: Cultivar de gramínea forrageira recomendada para pastagens no Acre. Documentos, 112. Rio Branco, Acre, Brasil: Embrapa, Acre. p. 34.
- Andrade, C. M. S., Garcia, R., Valentim, J. F., & Pereira, O. G. (2006a). Grazing management strategies for massaigrass-forage peanut pastures. 3. Definition of sward targets and carrying capacity. Revista Brasileira de Zootecnia, 35, 352–357. doi:10.1590/S1516-35982006000200004
- Andrade, C. M. S., Garcia, R., Valentim, J. F., & Pereira, O. G. (2006b). Grazing management strategies for massaigrass-forage peanut pastures: 2. productivity, utilization and sward structure. Revista Brasileira de Zootecnia, 35, 343–351. doi:10.1590/S1516-35982006000200003
- Andrade, C. M. S., & Valentim, J. F. (2007). Síndrome da morte do capim-brizantão no Acre: Características, causas e soluções tecnológicas. Embrapa Acre, Documentos, 105. Rio Branco, Acre, Brasil: Embrapa Acre. doi:10.1094/PDIS-91-4-0467B
- Andrade, C. M. S., Valentim, J. F., Pereira, J. B. M., & Ferreira, A. S. (2010). Yield and botanical composition of a mixed grass-legume pasture in response to maintenance fertilization. Revista Brasileira de Zootecnia, 39, 1633–1640. doi:10.1590/S1516-35982010000800003
- AOAC. (1990). Official methods of anlaysis (15th ed.). Arlington, VA: Association of Official Analytical Chemists.
- Apple, J. K., Davis, J. C., & Stephenson, J. (1999). Influence of body condition score on by-product yield and value from culled beef cows. Journal of Animal Science, 77, 1670–2679.
- Boddey, R. M., Rao, I. M., & Thomas, R. J. (1996). Nutrient Cycling and Environmental Impacts of Brachiaria Pastures. In J. W. Miles, B. L. Maass, & C. B. Do Valle (Eds.), Brachiaria: Biology, agronomy and improvement (pp. 72–86). Cali, Colombia: CIAT, Embrapa.
- Bouman, B. A. M., & Nieuwenhuyse, A. (1999). Exploring options for sustainable beef cattle ranching in the humid tropics: A case study for the Atlantic zone of Costa Rica. Agricultural Systems, 59, 145–161. doi:10.1016/S0308-521X(98)00087-0
- Brady, R. C., & Weil, R. R. (2008). The nature and properties of soils. Upper Saddle River, NJ: Pearson Education, Inc.
- Buschbacher, R. J., Uhl, C., & Serrão, E. A. S. (1987). Large-Scale development in Eastern Amazonia: Pasture management and environmental effects near Paragominas, Para. In C. F. Jordan (Ed.), Amazonian rain forests. Ecosystem disturbance and recovery (pp. 90). New York: Springer-Verlag.
- Carpentier, C. L., Vosti, S. A., & Witcover, J. (2000). Intensified production systems on western Brazilian Amazon settlement farms: could they save the forest? Agriculture Ecosystems & Environment, 82, 73–88
- Cherney, J. H., Cherney, D. J. R., & Bruulesma, T. W. (1998). Potassium management. In J. H. Cherney & D. J. R. Cherney (Eds.), Grass for Dairy Cattle (pp. 137–160). Wallingford, UK: CAB International.
- Cicone, R. (2016). Is the past decade of persistent drought in Brazil a new normal? Washington, D.C.: ISCIENCES, L.L.C.
- Cochrane, T. T., & Sanchez, P. A. (1982). Land resources, soils and their management in the Amazon region: An estate of knowledge report. In S. B. Hecht (Ed.), Amazonia: Agriculture and land use research (pp. 137–209). Cali, Colombia: CIAT.
- Costa, N. D. L. (1997). Produtividade e manejo de pastagens de Brachiaria humidicola no Tropico Umido sulamericano. Documentos 37. Porto Velho: Embrapa-CPAF Rondônia. p. 1–41.
- Davis, M. R. (1991). The comparative phosphorus requirements of some temperate perennial legumes. Plant and Soil, 133, 17–30. doi:10.1007/BF00011895
- de Moraes, J. F. L., Cerri, C. C., Volkoff, B., & Bernoux, M. (1996). Soil properties under Amazon forest and changes due to pasture installation in Rondônia, Brazil. Geoderma, 70, 63–81. doi:10.1016/0016-7061(95)00072-0
- Dias-Filho, M. B. (2002). Tolerance to flooding in five Brachiaria brizantha accessions. Pesquisa Agropecuária Brasileira, 37, 439–447. doi:10.1590/S0100-204X2002000400003
- Dias-Filho, M. B. (2011). Degradação de pastagens: Processos, causas e estratégias de recuperação (4a.Edição ed.). Belém: Embrapa.
- Dias-Filho, M. B., & Carvalho, C. J. R. D. (2000). Physiological and morphological responses of Brachiaria spp. to flooding. Pesquisa Agropecuária Brasileira, 35, 1959–1966. doi:10.1590/S0100-204X2000001000006
- Duarte, A. F. (2015). A vulnerabilidade social como causa fundamental das alagações recorrentes no estado do Acre, Amazônia Ocidental. In: R. N. Natal (Ed.), Proceedings of the VI Simposio Internacional de Climatologia, Anais.
- Eickhout, B., Bouwman, A. F., & van Zeijts, H. (2006). The role of nitrogen in world food production and environmental sustainability. Agriculture Ecosystems & Environment, 116, 4–14. doi:10.1016/j.agee.2006.03.009
- Faminow, M. D. (1998). Cattle, deforestation and development in the Amazon. New York: CAB International.
- Fearnside, P. M., & Barbosa, R. I. (1998). Soil carbon changes from conversion of forest to pasture in Brazilian Amazonia. Forest Ecology and Management, 108, 147–166. doi:10.1016/S0378-1127(98)00222-9
- Feldpausch, T. R., Rondon, M. A., Fernandes, E. C. M., Riha, S. J., & Wandelli, E. (2003). Carbon and nutrient accumulation in secondary forests regenerating from degraded pastures in central Amazônia. Ecological Applications, 14, 164–176. doi:10.1890/01-6015
- Fernandes, E. C. M., Biot, Y., Castilla, C., Canto, A. D. C., Matos, J. C., Garcia, S., … Wandeli, E. (1997). The impact of selective logging and forest conversion for subsistence agriculture and pastures on terrestrial nutrient dynamics in the Amazon. Ciencia e cultura, 49, 34–47.
- Fisher, M. J., & Kerridge, P. C. (1996). The agronomy and physiology of Brachiaria species. In J. W. Miles, B. L. Maass, & C. B. Do Valle (Eds.), Brachiaria: Biology, agronomy and improvement (pp. 43–52). Cali, Colombia: CIAT.
- Fox, D. G., Tedeschi, L. O., Tylutki, T. P., Russell, J. B., Van Amburgh, M. E., Chase, L. E., … Overton, T. R. (2004). The net carbohydrate and protein system for evaluating herd nutrition and nutrient excretion. Animal Feed Science and Technology, 112, 29–78. doi:10.1016/j.anifeedsci.2003.10.006
- Garcia-Montiel, D. C., Neill, C., Melillo, J., Thomas, S., Steudler, P. A., & Cerri, C. C. (2000). Soil phosphorus transformations following forest clearing for pasture in the Brazilian Amazon. Soil Science Society of America Journal, 64, 1792–1804. doi:10.2136/sssaj2000.6451792x
- Gonçalves, C. A., Cruz-Oliveira, J. R. D. C., & Dutra, S. (1996). Renovação e utilização de pastagens na engorda de bovinos em Porto Velho, Rondônia, Brasil. Pasturas Tropicales, 18, 24–33.
- Haynes, R. J., & Williams, P. H. (1993). Nutrient cycling and soil fertility in the grazed pasture ecosystem. Advances in Agronomy, 49, 119–199.
- IBGE. (2006). Censo Agropecuario 2006. Brasil, Grandes Regiões e Unidades da Federação: Instituto Brasileiro de Geografia e Estatística.
- INPE. (2016a). Projeto terraclass. São José dos Campos, SP: Author.
- INPE. (2016b). Taxas anuais do desmatamento - 1988 até 2015. Projeto PRODES: Monitoramento da Floresta Amazônica por Satélite. São José dos Campos, SP: Author.
- Instituto Internacional para Sustentabilidade. (2015). Análise econômica de uma pecuária mais sustentável. Rio de Janeiro: Author.
- Jannotti, W. I. (1997). Produção de leite em pastagem de capim Bráquiaria (Brachiaria decumbens) sob duas ofertas diarias de forragem (M.S. thesis). Universidade Federal de Viçosa.
- Kennard, D. K., & Gholz, H. L. (2001). Effects of high- and low-intensity fires on soil properties and plant growth in a Bolivian dry forest. Plant and Soil, 234, 119–129. doi:10.1023/A:1010507414994
- Kiehl, E. J. (1979). Manual de Edafologia. São Paulo: Ceres.
- Krishnaswamy, J., & Richter, D. D. (2002). Properties of advanced weathering-stage soils in tropical forests and pastures. Soil Science Society of America Journal, 66, 244–253. doi:10.2136/sssaj2002.2440
- Latawiec, A. E., Strassburg, B. B. N., Valentim, J. F., Ramos, F., & Alves-Pinto, H. N. (2014). Intensification of cattle ranching production systems: Socioeconomic and environmental synergies and risks in Brazil. Animal, 8, 1255–1263. doi:10.1017/S1751731114001566
- Leonidas, F. C. D. (1998). Alterações Físicas e Químicas do Solo sob Pastagem na Amazõnia Ocidental, Submetido a diferentes Períodos de Utilização (M.S. thesis). Universidade Federal de Paraíba, Centro de Ciencias Agrárias, Campus III.
- McRoberts, K. C., Ketterings, Q. M., Parsons, D., Hai, T. T., Quan, N. H., Ba, N. X., … Cherney, D. J. R. (2016). Impact of forage fertilization with urea and composted cattle manure on soil fertility in sandy soils of south-central Vietnam. International Journal of Agronomy, Article ID 4709024. doi:10.1155/2016/4709024
- McRoberts, K. C., Nicholson, C. F., Blake, R. W., Tucker, T. W., & Díaz Padilla, G. (2013). Group Model Building to Assess Rural Dairy Cooperative Feasibility in South-Central Mexico. International Food and Agribusiness Management Review, 16, 55–98.
- McRoberts, K. C., Parsons, D., Ketterings, Q. M., Hai, T. T., Quan, N. H., Ba, N. X., … Cherney, D. J. R. (2018). Urea and composted cattle manure affect forage yield and nutritive value in sandy soils of South-Central Vietnam. Grass and Forage Science, 73, 132–145. doi:10.1111/gfs.2018.73.issue-1
- Mehlich, A. (1953). Determination of P, Ca, Mg, K, Na, and NH4. North Carolina Soil Test Division (Mimeo 1915).
- Melo, A. W. F. (2003). Avaliação do estoque e composição isotópica do carbono do solo no Acre (Master’s Master’s). Escola Superior de Agricultura Luiz de Queiroz.
- Muchagata, M., & Brown, K. (2003). Cows, colonists and trees: Rethinking cattle and environmental degradation in Brazilian Amazonia. Agricultural Systems, 76, 797–816. doi:10.1016/S0308-521X(02)00015-X
- Nguyen, K., & Goh, M. (1992). Nutrient cycling and losses based on a mass balance model in grazed pastures receiving long-term superphosphate applications in New Zealand: 2. Sulphur. The Journal of Agricultural Science (Camb.), 119, 107–122. doi:10.1017/S0021859600071598
- Nour, A. Y. M., & Thonney, M. L. (1987). Carcass soft tissue and bone composition of early and late maturing steers fed two diets in two housing types and serially slaughtered over a wide weight range. The Journal of Agricultural Science, 109, 345–355. doi:10.1017/S0021859600080771
- Nour, A. Y. M., & Thonney, M. L. (1988). Minerals of carcass soft tissue and bone of serially slaughtered cattle as affected by biological type and management. The Journal of Agricultural Science, 111, 41–49. doi:10.1017/S0021859600082794
- Palm, C. A., Gachengo, C. N., Delve, R. J., Cadisch, G., & Giller, K. E. (2001). Organic inputs for soil fertility management in tropical agroecosystems: Application of an organic resource database. Agriculture Ecosystems & Environment, 83, 27–42. doi:10.1016/S0167-8809(00)00267-X
- Rao, I., Peters, M., Castro, A., Schultze-Kraft, R., White, D., Fisher, M., … Rudel, T. (2015). LivestockPlus—The sustainable intensification of forage-based agricultural systems to improve livelihoods and ecosystem services in the tropics. Tropical Grasslands-Forrajes Tropicales, 3, 59–82. doi:10.17138/TGFT(3)59-82
- Rao, I. M., Kerridge, P. C., & Macedo, M. C. M. (1996). Nutritional Requirements of Brachiaria and Adaptation to Acid Soils. In J. W. Miles, B. L. Maass, & C. B. Do Valle (Eds.), Brachiaria: Biology, Agronomy and Improvement (pp. 53–71). Cali, Colombia: CIAT, Embrapa, CNPGC.
- Rao, I. M., Miles, J. W., & Granobles, J. C. (1998). Differences in tolerance to infertile acid soil stress among germplasm accessions and genetic recombinants of the tropical forage grass genus Brachiaria. Field Crops Research, 59, 43–52. doi:10.1016/S0378-4290(98)00106-3
- Reiners, W. A., Bowman, A. F., Parsons, W. F. J., & Keller, M. (1994). Tropical rainforest conversion to pasture: Changes in vegetation and soil properties. Ecological Applications, 4, 363–377. doi:10.2307/1941940
- Rezende, C. D. P., Cantarutti, R. B., Braga, J. M., Gomide, J. A., Pereira, J. M., Ferreira, E., … Boddey, R. M. (1999). Litter deposition and disappearance in Brachiaria pastures in the Atlantic forest region of the South of Bahia, Brazil. Nutrient Cycling in Agroecosystems, 54, 99–112. doi:10.1023/A:1009797419216
- Rueda, B. L., Blake, R. W., Nicholson, C. F., Fox, D. G., Tedeschi, L. O., Pell, A. N., … Carneiro, J. D. C. (2003). Production and economic potentials of cattle in pasture-based systems of the western Amazon region of Brazil. Journal of Animal Science, 81, 2923–2937. doi:10.2527/2003.81122923x
- SAS Institute Inc. (2015). JMP PRO 12.0.1 for Windows. Cary, NC: SAS Inst.
- Serrão, E., Falesi, I., de Veiga, J., & Teixeira, N. (1978) Productivity of cultivated pastures on low fertility soils in the Amazon of Brazil. In: P. Sanchez & L. Tergas (Eds.), Proceedings of the Seminar Pasture Production in Acid Soils of the Tropics. Cali, Colombia.
- Serrão, E. A. S., & Toledo, J. M. (1990). The search for sustainability in Amazonian pastures. In A. B. Anderson (Ed.), Alternatives for deforestation: Steps towards sustainable use of the Amazonian rain forest (pp. 195). New York: Columbia University Press.
- Silva, F. C. D. (1999). Manual de análises químicas de solos, plantas e fertilizantes (pp. 370). Brasilia, DF: Embrapa.
- Sirois, P. K., Reuter, M. J., Laughlin, C. M., & Lockwood, P. J. (1994). A method for determining macro and micro elements in forages and feeds by inductively coupled plasma atomic emission spectrometry. The Spectroscopist, 3, 6–9.
- Strassburg, B. B. N., Latawiec, A. E., Barioni, L. G., Nobre, C. A., Silva, V. P., Valentim, J. F., … Assad, E. D. (2014). When enough should be enough: Improving the use of current agricultural lands could meet production demands and spare natural habitats in Brazil. Global Environmental Change, 28, 84–97. doi:10.1016/j.gloenvcha.2014.06.001
- Tedeschi, L. O., & Fox, D. G. (2016). The ruminant nutrition system. An applied model for predicting nutrient requirements and feed utilization in ruminants. Acton, MA: XanEdu.
- Tedeschi, L. O., Fox, D. G., Pell, A. N., Lanna, D. P. D., & Boin, C. (2002). Development and evaluation of a tropical feed library for the Cornell Net Carbohydrate and Rrotein System model. Scientia Agricola, 59, 1–18. doi:10.1590/S0103-90162002000100001
- Thomas, R. J., & Asakawa, N. M. (1993). Decomposition of leaf litter from tropical forage grasses and legumes. Soil Biology and Biochemistry, 25, 1351–1361. doi:10.1016/0038-0717(93)90050-L
- Urquiaga, S., Cadisch, G., Alves, B. J. R., Boddey, R. M., & Giller, K. E. (1998). Influence of decomposition of roots of tropical forage species on the availability of soil nitrogen. Soil Biology and Biochemistry, 30, 2099–2106. doi:10.1016/S0038-0717(98)00086-8
- Valentim, J. F. (2015). Environmental governance and technological innovations for sustainable development in the Amazon. In J. D. Needell (Ed.), Emergent Brazil: Key perspectives on a new global power (pp. 219–240). Gainesville, FL :University Press of Florida.
- Valentim, J. F., & Andrade, C. M. S. (2004). Perspectives of grass-legume pastures for sustainable animal production in the tropics. Proceedings of the reunião annual da sociedade brasileira de zootecnia, Campo Grande, Matto Grosso de Sul, Brasil.
- Valentim, J. F., & Andrade, C. M. S. (2005a). Forage peanut (Arachis pintoi): A high yielding and high quality tropical legume for sustainable cattle production systems in the western Brazilian Amazon. Proceedings of the international grassland congress, (p. 329). Dublin, Ireland.
- Valentim, J. F., & Andrade, C. M. S. (2005b). Tropical kudzu (Pueraria phaseoloides): Successful adoption in sustainable cattle production systems in the western Brazilian Amazon. Proceedings of the international grassland congress (pp. 328). Dublin, Ireland.
- Valentim, J. F., & Andrade, C. M. S. (2009). Tendências e perspectivas da peccuária bovina na Amazônia Brasileira. Amazônia: Ciência & Desenvolvimento, 4, 9–32.
- Van Soest, P. J. (1994). Nutritional Ecology of the Ruminant. Ithaca, NY: Cornell University Press.
- Vanlauwe, B., Nwoke, O. C., Sanginga, N., & Merckx, R. (1996). Impact of the residue quality on the C and N mineralization of leaf and root residues of three agroforestry species. Plant and Soil, 183, 221–231. doi:10.1007/BF00011437
- Whitehead, D. C. (2000). Nutrient elements in grassland: Soil-plant-animal relationships. Wallingford, Oxon, UK: CAB International.

