Abstract
In this study, Limoniastrum feei extracts were evaluated for management of Fusarium oxysporum f. sp. albedinis (Foa) through antifungal tests and cellulases inhibition (cellulases are cell wall degrading enzymes “CWDE”). Antifungal activity evaluation was realized by direct bioautography. Cellulases inhibition test was realized by contact bioautography. In addition, a phytochemical screening has been done for these extracts on TLC plates (extraction with solvents of increasing polarity: hexane, ethyl acetate, dichloromethane, and methanol). Phytochemical screening was intended to know which metabolite is responsible for the antifungal activity through correlation with antifungal and anti-cellulases effect. The phytochemical study revealed the presence of saponins, flavonoids, tannins, coumarins, and alkaloids in these extracts. The different extracts showed high variability in their phenolic and flavonoids contents as well as antifungal capacity, as the case of ethyl acetate extract. Detection of inhibition zones was revealed with ethanolic Gram’s iodine. Inhibition zones were determined as dark brown spots. The results of enzymatic activity -by contact bioautography technique-showed that certain extracts of L. feei present a significant effect on cellulases of Foa. The best effect was presented by methanolic extract of aerial part (inhibition zone diameter in chromatogram is higher than 2 mm). Best antifungal effect was presented by compounds with high probability to be phenolic compounds.
PUBLIC INTEREST STATEMENT
Date palm (Phoenix dactylifera L.) constitutes importance in the social and economic life of the Algerian Sahara. It represents the food, shade, garden, and refuge for the Saharan people. In South-West of Algeria, the cultivation of the date palm development and the safeguarding of the already existing date palms constitute a major concern of these oases’farmers while its cultivation is threatened by several diseases such as Fusariosis or Fusarium wilt disease. The vascular wilt of the date palm is caused by a soil fungus: Fusarium oxysporum f. sp. albedinis.
In this work, we evaluated the effect of Limoniastrum feei extracts (an endemic medicinal plant) against the fungus causing vascular disease by using the bioautography technique. To our knowledge, there are few studies used this technique in the antifungal tests. The results showed that certain extracts of Limoniastrum feei present a significant effect against Fusarium oxysporum f. sp. albedinis.
Competing Interests
The authors declares no competing interests.
1. Introduction
Due to the notable medicinal value of Limoniastrum feei, it was considered of interest to carry out phytochemical and antimicrobial investigations of this species (Belboukhari & Cheriti, Citation2005). The other uses of L. feei are as an antibacterial, for treatment of bronchitis and stomach infection (Belboukhari & Cheriti, Citation2007). Also, antioxidant and antiradical-scavenging activity were evaluated (Keffous et al., Citation2016). A previous investigation revealed that methanol extract from L. feei leaves contained potential antifungal activity against Candida albicans and antibacterial effects against E. coli (Belboukhari & Cheriti, Citation2009). These results prompted us to test in vitro several extracts of L. feei against Fusarium oxysporum f. sp. albedinis.
In South-West of Algeria, the culture of the date palm development and the safeguarding of the already existing date palms constitute a major concern of the farmers of these oases (Benabdelkader, Malek, & Draoui, Citation2011). However, its culture is threatened by several pests and diseases such as vascular fusariosis. The vascular wilt of the date palm (Phoenix dactylifera L.), locally known as Bayoud disease, is caused by a soil fungus: Fusarium oxysporum f. sp. albedinis (Killian and Maire) Gordon (Kettout & Rahmania, Citation2010).
Many Fusarium species are serious plant pathogens, causing symptoms such as necrotic lesions, rot, and wilt. Since its first signal before 1870, Bayoud disease has killed approximately 20 million date palm trees in Morocco and Algeria (Boulenouar, Marouf, & Cheriti, Citation2014). The only way to fight this disease is to prevent its propagation to other date-growing areas in the region and farther fields (Boulenouar, Marouf, & Cheriti, Citation2011). It is known that the appearance of a disease is the result of several factors called “factor of pathogenicity “. These factors are mainly enzymes and toxins (Freeman & Maymon, Citation2000). The most significant enzymes from the pathogenicity point of view of Fusarium oxysporum f. sp. albedinis are the CWDE (Cell Wall Degrading Enzymes). Among these enzymes, the cellulases, which hydrolyze the cellulose (El Modafar, Tantaoui, & El Boustani, Citation2000).
In this context, the cellulases can be the target of certain factors and/or substances. Therefore, the objective of this study was to evaluate the effect of L. feei extracts on the Fusarium oxysporum f. sp. albedinis cellulases, degradation enzymes of walls of the host plant (Phoenix dactylifera L). The plant was used to evaluate their extracts with four solvents (hexane, ethyl acetate, dichloromethane, and methanol), using the bioautography technique.
2. Materials and methods
2.1. Plant materials and extract preparation
The aerial parts and roots of L. feei were collected from Taghit, in Bechar, South-West of Algeria. Plant material was identified and a voucher specimen was conserved at the phytochemical herbarium of Phytochemistry and Organic Synthesis Laboratory under accession No CA99/14. The plant material was dried in a shaded area. After grinding, the material was stored at room temperature. The extraction was prepared by 10 g of the plant parts in 100 ml of solvents (ethyl acetate, dichloromethane, hexane or methanol) during two (02) hours (using heat reflux extraction). The residues are weighed (after filtration and evaporation). The extract was stored at 4°C until use.
2.2. Thin layer chromatography (TLC)
The extracts of each solvent were subjected to TLC. The used solvent system was ethyl acetate: heptane (75:25). Spots were detected on TLC under UV light.
2.3. Phytochemical screening on TLC plate
The extracts obtained were then subjected to phytochemical analysis to detect the chemical constituents present in each extract. The screening was done according to Pascual, Carretero, Slowing, and Villar (Citation2002).
2.3.1. Test for flavonoids
The TLC plates containing the extracts are sprayed with an ethanolic solution with 5% aluminum chloride (AlCl3). The appearance of the greenish-yellow color, with the revelation by UV 365 ηm, indicates the presence of flavonoids.
2.3.2. Test for coumarins
The TLC separation plates are sprayed, immediately, with the ethanolic solution of KOH. The appearance of red color, in the visible, indicates the presence of anthraquinones, or the yellow color, with the revelation by UV 365 ηm, which indicates the presence of anthrones or furanocoumarins.
2.3.3. Test for tannins
The TLC plates containing the extracts are sprayed with a methanolic solution (80%) of ferric chloride 1 % (FeCl3). The appearance of black-blue color indicates the presence of gallic tannins, or greenish-brown indicates the presence of catechin tannins.
2.3.4. Test for alkaloids: “marquis reagent”
Formaldehyde solution (10 ml) with 100 ml of sulfuric acid (H2SO4) was prepared. The TLC separation plates are sprayed immediately. The color change of the spots indicates the presence of alkaloids or aromatic hydrocarbons.
2.3.5. Test for saponins
Froth test for saponins (foam) was used. 2g of the sample was weighed into Erlenmeyer flask in which 100 ml of sterile distilled water was added and boiled for 15 min. The mixture was filtered and 2.5 ml of the filtrate was added to 10 ml of sterile distilled water in a test tube.
The test tube was shaken vigorously for about 30 seconds. It was then allowed to stand for half an hour. The height of the foam was measured in each tube. The formation of a 1 cm layer of foam indicates the presence of saponins.
2.4. Antifungal screening by direct bioautography (thin-layer chromatography-direct bioautography)
In order to screen for and identify compounds with the antifungal activity present in the plant extracts, direct bioautography was used as described by Boulenouar et al. (Citation2011). This method, based on the direct immersed and growing of a fungal spore suspension on a developed TLC chromatogram. 80µg/µl of each extract was applied to the TLC plate of silica gel 60 F254 (7 × 1.5 cm). After developing the chromatogram using a suitable eluent for efficient separation and location of the UV absorbing spot, chromatograms were then moved directly into Petri dishes containing 20 ml of spores for 10 seconds, where the concentration is 2.107 spores/ml. Bioautograms were examined for fungal growth at regular intervals until TLC plates were completely covered with a lawn of mycelial growth. Then, they are added to other sterile Petri dishes containing cotton wet with distilled water to provide moisture. These are incubated for 4 days at 21°C. The plates were immersed in a 2 mg/ml solution of p-iodonitrotetrazolium violet (INT) and further incubated for 1 hour at 21°C. Rf zones on the plates were compared to that of reference plates to find the Rf of the active compound.
2.5. Cellulases production by Fusarium oxysporum f. sp. albedinis
Fusarium oxysporum f. sp. albedinis was cultivated on a liquid medium at 21°C and incubated for 96 h under moderate agitation. For the inoculation of the cultures, a suspension of spores (4.3 X 105 spores/ml) was used in sterile distilled water added to 2 ml of Tween 80. The filtrate containing cellulases was put in tubes (5 mL in each tube). The tubes were conserved at −18°C until use.
2.6. Contact bioautography
The use of a TLC support to screen for potential plant-derived enzyme inhibitors is a rapid method that is relatively free of disturbances due to the solvent (Pascual et al., Citation2002). In contact bioautography, antimicrobials compound diffuse from the chromatogram to the inoculated agar plate. The test by contact bioautography used in this study was described by Choma and Jesionek (Citation2015) with slight modifications. The test has been realized on cellulose agar medium (2% cellulose, 1.5% agar). Ten milliliters of the medium have been poured in each Petri dish and left to solidify. The plant’s extracts have undergone TLC. Each chromatogram—after evaporation of the eluent- was put face down on the cellulose agar plate for 2 hours to promote diffusion. The filtrate containing cellulases previously prepared was poured on the cellulose agar plates after removal of the chromatogram and incubated at 21°C for 2 hours. The detection of inhibition zones was done using Gram’s iodine. Gram’s iodine was used as described by Kasana, Salwan, Dhar, Dutt, and Gulati (Citation2008). Plates were flooded with Gram’s iodine (3% KI and 2% I2 in 70 % ethanol) for 5 minutes then washed with distilled water. Gram’s iodine formed a dark brown color with cellulose but not with hydrolyzed cellulose, giving a distinct, clear, and prominent zone in 3 to 5 minutes (Kasana et al., Citation2008). The inhibition zones were visible as dark brown zones. The evaluation of cellulases activity inhibition was expressed as the diameter of inhibition zones (mm) and the Rf values to reflect the inhibitors. The control tests passed all the protocol without extracts or filtrate (the filtrate has been replaced by the culture medium). The tests were realized in triplicate (the standard errors were less than 10%).
3. Results
3.1. Thin-layer chromatography (TLC)
Thin-layer chromatography was used as a preliminary method for compositional analysis of the crude extracts. Table groups obtained Rf values. According to the chromatograms obtained by TLC, all the parts of the plant contain several chemical compounds (several spots).
Table 1. Rf values of TLC in different extracts
TLC analysis (EtOAc: Hep, 75:25) revealed four constituents in the hexane extract (yield 1.22%, Rf 0.57, 0.85, 0.90, 0.95), five constituents in the ethyl acetate extract (yield 2.83%, Rf 0.35, 0.57, 0.85, 0.90, 0.95), and five constituents in the methylene chloride (DCM) extract (yield 1.58%, Rf 0.35, 0.57, 0.85, 0.90, 0.93), and two constituents in the methanolic extract (yield 11.88%, Rf 0.35, 0.90).
The TLC analyses indicated that the extracts of L. feei were composed of a mixture of secondary metabolites. Rahmani, Belboukhari, and Cheriti (Citation2014) reportedly obtained the Rf values of L. feei extract by ethyl acetate in the comparable range of 0.36–0.77, eluted with another solvent system, not as that used in the present study.
3.2. Phytochemical screening of the plant extracts
The characterization reactions revealed several chemical groups in the different extracts. The use of solvents with varying polarity in the extraction procedure is determined for the successful isolation of compounds with different ranges of polarity.
In the present study, phytochemical screening for all extracts of L. feei aerial part and root showed a significant indication of the presence of metabolites. Saponins, flavonoids, tannins, coumarins, and alkaloids, were found to be present in the majority of the plant extracts (Table ).
Table 2. Phytochemical screening of various extracts of Limoniastrum feei
The phytochemical screening of two parts of L. feei on flavonoids, tannins, and coumarins, show the presence of flavonoids in ethyl acetate, hexane and methanol extract and total absence in dichloromethane, but the tannins derivative are present in all parts. It is also observed that the extracts of ethyl acetate are the richest in tannins and coumarins by contribution to other extracts e.g., methanolic and hexane extracts.
The revelation of the alkaloids on the TLC plate provided Table .
According to Table , we observe: Colors change in most parts of the spots; we can say that these are alkaloids or aromatic hydrocarbons. The different colors are shown in all the extracts (MeOH, DCM, Hex, and EtOAc).
A previous study by Rahmani et al. (Citation2014) reported the presence of eleven polyphenol compounds in the extract of the aerial part of L. feei. In another study, two molecules of the saponins family were extracted and identified by Belboukhari and Cheriti (Citation2009) (Figure ), from the stems of L. feei, namely:
2α,3 β,23-Trihydroxy-30-acetylolean-12-ene (1) (C32H52O5) and 1-O-[α -L-Rhamnopyranosyl-(1)-6-O-acetylβ -D-galactopyranosyl]-1 β,3 β,22 ξ − 26-tetrahydroxyfurost-5(6)-ene-26-Oβ -D-glucopyranosid (2) (C50H82O19).
Figure 1. Saponins from the stems of L. feei (1 and 2) (Belboukhari & Cheriti, Citation2009).
1:2α,3β,23-Trihydroxy-30-acetylolean-12-ene (C32H52O5)
2:1-O-[α-L-Rhamnopyranosyl-(1)-6-O-acetylβ -D-galactopyranosyl]-1 β,3 β,22 ξ − 26-tetrahydroxyfurost-5(6)-ene-26-Oβ -D-glucopyranosid(C50H82O19)
![Figure 1. Saponins from the stems of L. feei (1 and 2) (Belboukhari & Cheriti, Citation2009).1:2α,3β,23-Trihydroxy-30-acetylolean-12-ene (C32H52O5)2:1-O-[α-L-Rhamnopyranosyl-(1)-6-O-acetylβ -D-galactopyranosyl]-1 β,3 β,22 ξ − 26-tetrahydroxyfurost-5(6)-ene-26-Oβ -D-glucopyranosid(C50H82O19)](/cms/asset/8d8f5982-a01b-4976-9ed2-d67556a7a9da/oafa_a_1726083_f0001_b.gif)
3.3. Antifungal activity of the plant extracts
3.3.1. Direct bioautography
Frequently, TLC-Direct Bioautography is used as a bio-guiding method to destine to substances with biological activity that can be further analyzed by spectroscopic methods to obtain information on their structure (Choma & Jesionek, Citation2015).
Strong growth inhibition against Fusarium oxysporum f. sp. albedinis has been shown by a methanolic extract that was assayed using direct bioautography on the TLC plate (Table ).
Table 3. Characterization of alkaloids on the TLC plate
Table 4. Result of the direct bioautography of the plant extracts
3.3.2. The cellulases inhibition by contact bioautography
The results of the cellulases inhibition are presented in Table . Various parameters are to be taken into account for good comprehension and evaluation of these results. For the tests carried out by TLC, the evaluation of effects is made by comparison between extracts.
Table 5. Effect of L. feei extracts on Fusarium oxysporum f. sp. albedinis cellulases using contact bioautography
4. Discussion
Bioautography is notably significant to avoid the time-consuming isolation of inactive compounds (Dewanjee, Gangopadhyay, Bhattacharya, Khanra, & Dua, Citation2015). TLC bioautographic methods combine chromatographic separation and in situ activity determination facilitating the localization and target-directed isolation of active constituents in a mixture (Suleiman, McGaw, Naidoo, & Eloff, Citation2010). In this study, the evaluation of L. feei extracts on the causal agent of Bayoud “Fusarium oxysporum f. sp. albedinis” (Foa), was realized using new principles added to contact bioautography. Some studies made at the Phytochemistry and Organic Synthesis Laboratory (POSL, Bechar University, Algeria) have demonstrated that this plant contains secondary metabolites with biological activities (Belboukhari & Cheriti, Citation2005, Citation2007, Citation2009).
Three extracts of L. feei are revealed active on cellulases with the appearance of the dark zones. The methanol extract of the aerial part of L. feei showed a remarkable result with inhibition zone diameter greater than 2 mm. Indeed, the dichloromethanic extracts showed no antifungal activity on Foa.
In this study, the tests were carried out using crude extracts; then a positive result is the component of two parameters: the intrinsic activity of the active products and their relative quantity in the extract. Among the 8 tests carried out, only one extract presented a good effect on the cellulases of Foa (Ø > 2mm). These results prove the resistance of Foa against the extracts of L. feei. Boulenouar, Marouf, Cheriti, and Belboukhari (Citation2012) determined this resistance against several treatments (Boulenouar et al., Citation2012).
El Hassni et al. (Citation2007) revealed that the strategies of the fight against Foa are very limited that proves its high resistance (El Hassni et al., Citation2007). Several studies showed that the studied plant contains several secondary metabolites very significant with pharmacological properties (Belboukhari & Cheriti, Citation2005, Citation2009; Boulenouar et al., Citation2014). Belboukhari and Cheriti (Citation2009) showed by spectroscopic analyses the presence of the saponins in the leaves and the stems of L. feei. This plant is rich in phenolic compounds. The antimicrobial activity of L. feei extract was studied by Belboukhari and Cheriti (Citation2005); the methanolic extracts of L. feei leaves were revealed active against certain bacteria and two yeasts. By comparing these findings with the obtained results, the methanolic extracts of the aerial part gave a remarkable result (Ø > 2 mm) and confirm the presence of antimicrobial compounds in L. feei.
The analytical investigations reveal that the ethyl acetate extract of the aerial part of L. feei showed the highest phenolic compounds. In addition, the presence of flavonoids in L. feei (Belboukhari & Cheriti, Citation2007; Rahmani et al., Citation2014) may be responsible for the antifungal activity.
Belboukhari and Cheriti (Citation2005) revealed no effect of ethyl acetate extract from leaves and twigs of L. feei on two fungi: Candida albicans and Saccharomyces cerevisiae. The difference with our results is possibly due to the difference between species biology (Foa, C. albicans and S. cerevisiae) and/or to the difference between plants parts used for extraction.
The effect of the extracts used in this study was shown by Boulenouar et al. (Citation2014) but by the disc diffusion technique. The results of the effect against Foa showed that these extracts have a detectable effect at least in two tests (Boulenouar et al., Citation2014). Confirming the presence of antifungal substances despite the difference between the techniques used. The significant effect of the various parts of the plant can be related to the difference in the components present in the various parts. This difference can depend on the chemical nature of the substances or on the mechanism of action. There are substances with antifungal activity against Foa but not on its cellulases, this reflects the complexity of pathogenicity mechanism. On the other hand, substances inhibiting cellulases play a crucial role in antifungal activity against Foa because of importance of these enzymes in invading date palm through roots.
By comparing the results obtained using contact bioautography on the cellulases of Foa and the other results using other techniques. Inhibition of cellulases activity is not always related to the inhibition of the growth. Foa produces several toxins; these mycotoxins play a very significant role in the pathogenicity of this fungus. Therefore the different researches carried out previously against Foa reveals active substances that influence one or more of these mycotoxins by the modification of their metabolism or their effects (Boulenouar, Marouf, & Cheriti, Citation2009; El Hadrami, El Idrissi-Tourane, El Hassni, Daayf, & El Hadrami, Citation2005).
Phytochemical analysis was intended to know which metabolite is responsible for the antifungal activity. According to the TLC profiling, we can suppose that inhibition is caused by flavonoids present in ethyl acetate and methanolic extracts of aerial parts of L. feei (Rf: 0.35, 00) respectively and in hexanic extract of roots of L. feei (Rf: 0.54).
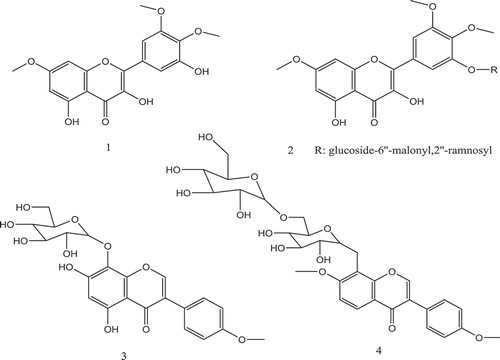
Belboukhari and Cheriti (Citation2007) contributed to the study of the characterization of flavonoids extracted from the stems of L. feei; they identified four flavonoids (Figure ). We can suppose that one or more of these four flavonoids have antifungal activity against Foa and its cellulases.
Figure 2. Flavonoids from the stems of L. feei (1–4) (Belboukhari & Cheriti, Citation2007).
(1): 6, 3ʹ, 4ʹ-Tri-methoxy 3, 5, 5ʹ-trihydroxy flavonol.
(2): 3-(6”-malonyl 2”-ramnosyl glucosil) 6, 3ʹ, 4ʹ-tri-methoxy 5, 5ʹ-dihydroxy flavonol.
(3): Tetraacetate 7-dihydroxy-4ʹ-Methoxy 8-O-β-glucopyranoside isoflavone.
(4): Tetraacetate 7, 4ʹ-diMethoxy 8-O-β-glucopyranoside isoflavone
From the results obtained by El Haci, Mazari, Atik-Bekkara, Hassani, and Gherib (Citation2017), polar organic extracts: methanol, ethanol, and acetone of L. feei presented considerable levels of phenols and flavonoids. These levels ranged from 127.07 to 262.11 mg GAE/g and 84 to 157.88 mg CEQ/g, respectively (El Haci et al., Citation2017).
The flavonoids are a group of substances, which are responsible for the inhibition of many enzymes. The phytochemical screening showed a variety of phytoconstituents with the prevalence of phenolic compounds (El Haci et al., Citation2017). Various studies were carried out on the structure-activity relationship of different polyphenols in a glance with their antifungal activity, but in spite of their great number, this relationship is not really clear (Munari, Citation2006).
The absence of an effect for an extract tested on a given biological target always does not exclude the presence of active substances (case of synergy). In certain cases also, the concentration of these substances can be very weak so that we can detect their activity on TLC plates.
Recently, the fungicidal activities of plant extracts have been extensively reported (Balouiri, Sadiki, & Ibnsouda, Citation2016). However, little research has been performed on the fungicidal activity of L. feei extracts on the pathogen F. oxysporum, which poses the greatest threat to date palm by causing Fusarium wilt.
The potential medicinal uses of L. feei are supported by the presence of the above-mentioned phenolics and flavonoids activities.
5. Conclusions
Based on our study, L. feei could be used as a source of fungicides for the control of Foa. This study indicated that the extracts of L. feei not only demonstrated antifungal activity against Fusarium oxysporum f. sp. albedinis, but also could inhibit the cellulases enzymes of this phytopathogen. The results obtained shed light on the possibility to use some of these extracts (representing the best effects) against Foa by proceeding with further advanced studies. The antifungal activity of L. feei is strongly correlated with the qualitative and quantitative variations in their phenolic constituents.
Additional information
Funding
Notes on contributors

Abdelkrim Cheriti
Abdelkrim CHERITI, Born in El-Bayadh (Algeria), 25 November 1963. Received his Ph.D. in the chemistry of natural products from ENSSPICAM & Pharmacy faculty of Marseille (France) in 1992. Professor of Chemistry (Algeria). Contributor in numerous papers in international and national journals; Editor in chief of Phytochem & BioSub Journal, Associate Editor of Annales of TMB University and Director of Al Ouloum collection. Coordinator of various research projects in the field of natural products, bioactive and pharmaceutical substances from Saharan medicinal plants. Founder and Director of the Phytochemistry and Organic Synthesis Laboratory (POSL). The main purpose of POSL is scientific research and development of knowledge and techniques concerning the field of Phytochemistry and the environment. Our present study is a continuation of our research efforts in the context of medicinal plants valorization and to explore the natural compounds for their antifungal activity.
References
- Balouiri, M., Sadiki, M., & Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 6(2), 71–12. doi:10.1016/j.jpha.2015.11.005
- Belboukhari, N., & Cheriti, A. (2005). Antimicrobial activity of aerial part crude extracts from Limoniastrum feei. Asian Journal of Plant Sciences, 4(5), 496–498. doi:10.3923/ajps.2005.496.498
- Belboukhari, N., & Cheriti, A. (2007). Flavonoids of Limoniastrum feei. Research Journal of Phytochemistry, 1(2), 74–78. doi:10.3923/rjphyto.2007.74.78
- Belboukhari, N., & Cheriti, A. (2009). Analysis and isolation of saponins from Limoniastrum feei by LC-UV. Chemistry of Natural Compounds, 45(5), 756. doi:10.1007/s10600-009-9455-2
- Benabdelkader, M., Malek, A., & Draoui, B. (2011). Perspective du pompage éolien appliqué à l’irrigation du palmier dattier dans la région de Béchar. Revue des Energies Renouvelables, 14(3), 381–395.
- Boulenouar, N., Marouf, A., & Cheriti, A. (2009). Effect of some poisonous plants extracts on Fusarium oxysporum f. sp. albedinis. Journal of Biological Sciences, 9(6), 594–600. doi:10.3923/jbs.2009.594.600
- Boulenouar, N., Marouf, A., & Cheriti, A. (2011). Antifungal activity and phytochemical screening of extracts from Phoenix dactylifera L. cultivars. Natural Product Research, 25(20), 1999–2002. doi:10.1080/14786419.2010.536765
- Boulenouar, N., Marouf, A., & Cheriti, A. (2014). Direct bioautography for antifungal. Measurement “case of bayoud disease. PhytoChem & BioSub Journal, 8, 33–37.
- Boulenouar, N., Marouf, A., Cheriti, A., & Belboukhari, N. (2012). Medicinal plants extracts as source of antifungal agents against fusarium oxysporum f. sp. albedinis. Journal of Agricultural Science and Technology, 14, 659–669.
- Choma, I., & Jesionek, W. (2015). TLC-direct bioautography as a high throughput method for detection of antimicrobials in plants. Chromatography, 2(2), 225–238. doi:10.3390/chromatography2020225
- Dewanjee, S., Gangopadhyay, M., Bhattacharya, N., Khanra, R., & Dua, T. K. (2015). Bioautography and its scope in the field of natural product chemistry. Journal of Pharmaceutical Analysis, 5(2), 75–84. doi:10.1016/j.jpha.2014.06.002
- El Haci, I. A., Mazari, W., Atik-Bekkara, F., Hassani, F., & Gherib, M. (2017). Assessment of the cytotoxic activity and the reduction power of limoniastrum feei girard (Batt.): A medicinal plant from Algerian Sahara. Oriental Pharmacy and Experimental Medicine, 17(2), 143–150. doi:10.1007/s13596-017-0270-1
- El Hadrami, A., El Idrissi-Tourane, A., El Hassni, M., Daayf, F., & El Hadrami, I. (2005). Toxin-based in-vitro selection and its potential application to date palm for resistance to the bayoud Fusarium wilt. Comptes Rendus Biologies, 328(8), 732–744. doi:10.1016/j.crvi.2005.05.007
- El Hassni, M., El Hadrami, A., Daayf, F., Chérif, M., Barka, E. A., & El Hadrami, I. (2007). Biological control of bayoud disease in date palm: Selection of microorganisms inhibiting the causal agent and inducing defense reactions. Environmental and Experimental Botany, 59(2), 224–234. doi:10.1016/j.envexpbot.2005.12.008
- El Modafar, C., Tantaoui, A., & El Boustani, E. (2000). Effet de L’acide Caféoylshikimique des Racines du Palmier Dattier sur L’activité et la Production des. Phytopathologische Zeitschrift, 148(2), 101–108. doi:10.1046/j.1439-0434.2000.00472.x
- Freeman, S., & Maymon, M. (2000). Reliable detection of the fungal pathogenfusarium oxysporum f. sp. albedinis, causal agent of bayoud disease of date palm, using molecular techniques. Phytoparasitica, 28(4), 341–348. doi:10.1007/BF02981829
- Kasana, R. C., Salwan, R., Dhar, H., Dutt, S., & Gulati, A. (2008). A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’s iodine. Current Microbiology, 57(5), 503–507. doi:10.1007/s00284-008-9276-8
- Keffous, F., Belboukhari, N., Sekkoum, K., Djeradi, H., Cheriti, A., & Aboul-Enein, H. Y. (2016). Determination of the antioxidant activity of Limoniastrum feei aqueous extract by chemical and electrochemical methods. Cogent Chemistry, 2(1), 1186141. doi:10.1080/23312009.2016.1186141
- Kettout, T. A., & Rahmania, F. (2010). Identification par CG-SM de l’acide phénylacétique produit par Fusarium oxysporum f. sp. albedinis, agent causal du bayoud. Comptes Rendus Biologies, 333(11–12), 808–813. doi:10.1016/j.crvi.2010.10.001
- Munari, C. (2006). Investigation phytochimique de plantes alpines: Etude d’espèces du genre Oxytropis (Fabaceae) et isolement de composés antifongiques et antiradicalaires à partir d’Oxytropis fetida (Vill.) DC., Potentilla grandiflora L. (Rosaceae) et Vaccinium uliginosum ssp. University of Geneva. Retrieved from https://nbn-resolving.org/urn:nbn:ch:unige-33548
- Pascual, M., Carretero, M., Slowing, K., & Villar, A. (2002). Simplified screening by TLC of plant drugs. Pharmaceutical Biology, 40(2), 139–143. doi:10.1076/phbi.40.2.139.5849
- Rahmani, S., Belboukhari, N., & Cheriti, A. (2014). Phytochemical investigation of bioactive extract from endemic medicinal plant Limoniastrum feei (Girard) Batt (Plumbaginaceae). Asian Journal of Chemistry, 26(2), 365. doi:10.14233/ajchem.2014.15395
- Suleiman, M. M., McGaw, L., Naidoo, V., & Eloff, J. (2010). Detection of antimicrobial compounds by bioautography of different extracts of leaves of selected South African tree species. African Journal of Traditional, Complementary and Alternative Medicines, 7, 1. doi:10.4314/ajtcam.v7i1.57269
