?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was aimed at determining the effect of temperature and water activity (aw) on the radial growth of Aspergillus flavus and A. carbonarius isolated from dried chili and their production of aflatoxin B1 (AFB1) and ochratoxin A (OTA), respectively. The isolates were grown on a synthetic chili-based medium, and growth and mycotoxin production were studied following a central composite design. The diameter of each colony was measured, and mycotoxins were extracted from the medium after 7 days of incubation at each different combination of temperature and aw. The analysis of variance results showed that temperature, aw, and their interaction had significant effects on growth and mycotoxin production. Response surface analysis showed that both strains were able to grow within a wide range of temperatures (22.93–37.07°C) and aw values (0.885–0.984), but production of AFB1 and OTA was not detected at 37.07°C or at aw below 0.900. The most favorable growth conditions for A. flavus were within a temperature range of 25–30°C and aw higher than 0.970, and maximum AFB1 production occurred at 22.93°C and aw of 0.984. For A. carbonarius, optimum growth and OTA production were observed at 22.93°C and aw of 0.970–0.984. Our results may be useful for controlling the growth of post-harvest mold in chili and preventing mycotoxin contamination of dried chili.
PUBLIC INTEREST STATEMENT
Chili is susceptible to fungal growth, which can result in contamination of the product with mycotoxins. Aflatoxin B1 (AFB1) and ochratoxin A (OTA) are mycotoxins produced by certain species of Aspergillus that infect in crops, including chili. To prevent the adverse health effects associated with these mycotoxins, it is necessary to understand the environmental factors that influence the growth and production of mycotoxins by toxigenic fungi in food crops.
This study described the effects of temperature and water activity (aw) on Aspergillus spp. isolated from dried chili, particularly A. flavus and A. carbonarius. Growth of the fungi and their production of mycotoxins were evaluated on a synthetic chili powder medium, using various aw values and incubation at different growth temperatures. The response surface analysis was used to find the optimum conditions to prevent growth of and mycotoxin production by Aspergillus species.
1. Introduction
Mycotoxins are toxic secondary metabolites produced by certain fungal species, such as Aspergillus, Fusarium, Penicillium, and Alternaria, that causes disease in agricultural crops (Reverberi et al., Citation2010). These substances can harm animal and human health, and economic losses resulted from mycotoxin contamination of crops worldwide. The fungal species are common and widespread in the environment, and Aspergillus spp. are often found in grain crops grown under stressful conditions, such as heat and drought (Medina et al., Citation2017).
Aspergillus spp. produced aflatoxins, which are the most toxic of the known mycotoxins. These compounds are carcinogenic and mutagenic, and they are mainly produced by Aspergillus spp. belonging to section Flavi, especially A. flavus and A. parasiticus. The aflatoxins include types B1, B2, G1, and G2, with aflatoxin B1 (AFB1) being considered the most toxic (IARC, Citation1993). Some Aspergillus spp. in tropical and temperate climates also produced ochratoxin A (OTA). These species belong to Aspergillus section Nigri and include A. niger and A. carbonarius (Merla et al., Citation2018). The OTA that they produce can cause nephrotoxicity, hepatotoxicity, teratogenicity, and immunosuppression (Hope & Hope, Citation2012).
In addition to grain crops, Aspergillus spp. can infect chili peppers (Capsicum spp.), one of the most important spice crops produced and consumed in many countries. Aflatoxin and OTA contamination are frequently detected in dried chili, with reported occurrences in multiple countries including Thailand (Tansakul et al., Citation2013), Sri Lanka, Belgium (Yogendrarajah et al., Citation2014), Pakistan (Iqbal et al., Citation2017), Nigeria, Ghana (Frimpong et al., Citation2019), and Indonesia (Wikandari et al., Citation2020).
Mycotoxin-producing fungi can affect crops during both the pre-harvest and post-harvest stages. The main environmental factors influencing fungal growth and the accumulation of mycotoxins in food commodities are temperature and water activity (aw) (Gebremeskel et al., Citation2019; Mannaa & Kim, Citation2017). The aw describes the unbound water that is available in food for microbial growth (Bellí et al., Citation2004). The study of environmental conditions that contributed to the growth of fungi and mycotoxin production is crucial for controlling and limiting adverse health risks and evaluating the microbiological safety and quality of food. The aim of the present study was to determine the influence of temperature and aw on the radial growth of A. flavus and A. carbonarius isolates from ground dried chili and their respective production of AFB1 and OTA. Response surface methodology using a central composite design (CCD) was performed to understand the interaction effect of temperature and aw on the mycotoxigenic Aspergillus species.
2. Materials and methods
2.1. Fungal culture
Aflatoxigenic isolate A. flavus strain CH141 and ochratoxigenic isolate A. carbonarius strain CH112 from dried chili in Thailand were used in this experiment. Both fungal isolates were deposited in the Mycotoxin Laboratory of the Kasetsart University Research and Development Institute (KURDI), Bangkok, Thailand. Fungi were maintained in potato dextrose agar (PDA). Agar containing 3% chili powder extract was prepared by boiling 30 g of chili powder in distilled water for 30 min and then filtering the water through a triple layer of cheesecloth. The filtrate was made up to 1 L with distilled water and 1.5% agar was added (Marín et al., Citation2009). The aw of the chili medium was adjusted by adding glycerol to reach the required level (0.885, 0.900, 0.935, 0.970, and 0.984). After autoclaving, the aw value of medium was measured using a water activity meter (AquaLab 4TE, Decagon Device, Inc., WA, USA). The measurements were performed in triplicate at 25°C.
2.2. Assessment of fungal growth and mycotoxin production
Initially, A. flavus and A. carbonarius were grown on PDA plates at room temperature (30–32°C) for 7 days. A 5-mm agar plug was cut from each fungus, using a sterile cork borer, and aseptically placed at the center of a chili powder extract agar of different aw values. The inoculated plates were incubated at different temperatures (22.93°C, 25°C, 30°C, 35°C, and 37.07°C). Colony diameter was measured at 7 days of incubation. AFB1 and OTA in the culture medium were then analyzed.
AFB1 and OTA were extracted from the cultures and purified using AFLAPREP® and OCHRAPREP® immunoaffinity column (R-Biopharm AG, Germany), respectively, following the manufacturer instructions. The sample eluent was injected separately into HPLC with fluorescence detection (2690/95, Waters Corporation, Milford, MA, USA) for analysis of each mycotoxin. Chromatographic separation was carried out on the Symmetry® C18 (5 µm, 3.9 × 150 mm) (Waters Corporation) at a temperature of 35°C and a flow rate of 1 mL/min. The mobile phases used for AFB1 and OTA consisted of water, acetonitrile, and methanol (60:15:25) and 6% acetic acid, acetonitrile, and methanol (45:35:20), respectively. The excitation wavelength and emission wavelength were set at 365 and 445 nm, respectively, for detection of AFB1 and 350 and 470 nm, respectively, for OTA. To enhance the sensitivity for fluorescence detection of AFB1, a post-column derivatization using a photochemical reactor for enhanced detection (PHRED) system (Aura Industries Inc., San Diego, CA, USA) was performed. Quantification analysis was performed by measuring the peak area at the retention time of each mycotoxin and comparing these areas with the relevant standard calibration curve obtained by diluting AFB1 and OTA standards (Sigma-Aldrich, St. Louis, MO, USA) to 5 point concentrations.
2.3. Experimental design
A CCD was used to study the effect of temperature (X1) and aw (X2) on growth and mycotoxin production by the Aspergillus species. Each factor was examined at five different levels (−1.414, −1, 0, +1, +1.414) with five replicates at the center point. The coded values and the actual values of each factor are presented in Table . A total of 13 experimental runs were conducted in triplicate. The radial growth of both fungal species and the production of AFB1 by A. flavus and OTA by A. carbonarius were considered the response (Yi). The response results were used to fit the quadratic polynomial represented by the following equation:
Table 1. Factors and their levels used in CCD experiment
where Y is the response variable; β0, βi, βj, and βij are the regression coefficient variables for intercept, linear, quadratic, and interaction terms, respectively; Xi and Xj are the independent variable or investigated factor; and ε is an error associated with the model. Design Expert 11 (Trial Version, Stat-Ease Inc., Minneapolis, MN, USA) was used for the experimental design, data analysis, and model building.
3. Results
In this study, CCD was used to predict the effect of the interaction between the main environmental factors (temperature and aw) on radial growth and mycotoxin production of Aspergillus species. After cultures were grown on chili powder agar with different aw values under various incubation temperatures for 7 days, colony diameter and mycotoxin production were analyzed. The observed and predicted values are shown in Table . The maximum radial growth of A. flavus was observed in run 5 (30°C and aw of 0.984), while the minimum radial growth was observed for run 10 (35°C and aw of 0.900). The highest AFB1 production was achieved in run 12 (25°C and aw of 0.970). The fungus did not produce AFB1 when grown under the conditions of run 10 (35°C, 0.900 aw) and run 11 (37.07°C, 0.935 aw). The optimal condition for growth of A. carbonarius and OTA production was run 12 (25°C and aw of 0.970). At a temperature of 37.07°C and aw of 0.935 (run 11), the fungus shows the smallest colony diameter and no OTA production.
Table 2. Matrix of CCD for A. flavus and A. carbonarius growth and mycotoxin production
To evaluate the relationship between two factors associated with response variables, an analysis of variance (ANOVA) was performed using the experimental results (Table ). Based on radial growth and mycotoxin production by both Aspergillus species, a Fisher F-test with a very low probability value (P < 0.0001) demonstrated a high significance for the regression model at a >99% confidence level. The goodness of the fit of the model was checked by the determination coefficient (R2) which was higher than 0.980, indicating that the model could explain >98% of the variation in the predicted response of fungal growth and mycotoxin production. The values of the adjusted determination coefficient (Adj. R2), which exceeded 0.970, were also high, which supported the high significance of the model. A higher value of the predicted R2 (>0.920) validated an excellent correlation between the temperature and aw.
Table 3. Analysis of variance (ANOVA) of the quadratic model for CCD
The regression analysis results are summarized in Table . Almost all linear and quadratic terms of the independent variables’ temperature (X1 and X12) and aw (X2 and X22) and the interactions between them (X1X2) had a significant effect (P < 0.05) on radial growth of the two Aspergillus isolates and their mycotoxin production, except for X1X2 and X22 were not significant (P > 0.05) for radial growth of A. flavus and OTA production of A. carbonarius, respectively.
Table 4. Coefficients and test of significance for the quadratic model for CCD
Based on their significant effect (P < 0.05), the reduced quadratic equations with coefficient of factors are given below:
where YF (cm) and YAFB1 (ng/g) are the radial growth of and AFB1 produced by A. flavus, respectively; YC (cm) and YOTA (ng/g) are the radial growth of and OTA produced by A. carbonarius, respectively; and X1 and X2 are the coded values of temperature and aw, respectively.
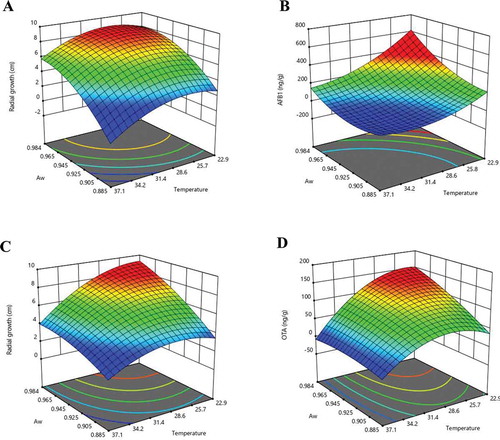
To explain the interaction between the investigated factors on the fungal growth and mycotoxin production, response surface graphs are provided in Figure . Table summarizes the conditions that can stimulate and inhibit the growth of A. flavus and A. carbonarius and their mycotoxin production.
Table 5. Optimal conditions of the interactive effect of temperature and aw for stimulating and inhibiting A. flavus and A. carbonarius growth and mycotoxin production in chili powder medium obtained from the response surface plots
4. Discussion
The present study assessed the influence of temperature and aw on the growth of mycotoxigenic Aspergillus species and their mycotoxin production in cultures using a chili powder medium. Data analyses used CCD and response surface analysis. Our results showed that both environmental factors had a significant effect on fungal growth and on AFB1 production by A. flavus and OTA production by A. carbonarius. In this work, A. flavus growth increased at temperatures ranging from 22.93°C to 30°C for all tested aw values, and the highest radial growth was observed at the highest aw (0.984). However, fungal growth decreased at higher temperatures ranging from 30°C to 37.07°C. The optimal temperatures and aw values for A. flavus growth in the present study were in accordance with previously reported ranges. For example, Bernáldez et al. (Citation2017) investigated A. flavus growth on maize-based medium and found that the tested fungus had a maximum growth rate at a temperature of 30°C and aw of 0.990. However, our results contrast with previous reports by Liu et al. (Citation2017) who found that the optimum temperature and aw for growth of A. flavus on shelled peanuts were 37°C and 0.980, respectively. Lv et al. (Citation2019) also reported that the optimal environmental conditions for the growth of A. flavus on paddy rice were a temperature of 37°C and aw of 0.940. In our study, no growth of A. flavus occurred at the lowest aw (0.885) when temperature ranged from 22.93°C to 37.07°C. These results agree with those obtained by Lasram et al. (Citation2016), who found that mycelial growth of A. flavus decreased significantly as aw declined and the fungus was not able to grow at aw < 0.900. Furthermore, Peromingo et al. (Citation2016) also demonstrated that several strains of A. flavus were not able to grow at aw of 0.850 on meat matrices over a period of 12 days. In our study, aw values of the medium were adjusted by adding glycerol. Because glycerol is non-ionic and has a limited effect of fungal development, it is commonly used as an agent to reduce the aw (Ramirez et al., Citation2004; Schmidt-Heydt et al., Citation2010). Other solutes used as aw depressor are NaCl and KCl, but these salts can cause a fungistatic effect by delaying germination time in Penicillium expansum and reducing the radial growth rate of P. expansum, P. roqueforti, and Paecilomyces niveus (Nguyen Van Long et al., Citation2017).
We found that AFB1 production increased as the temperature decreased and aw increased. The maximum AFB1 concentration was detected at 22.93°C and aw of 0.984. AFB1 production was absent at a temperature higher than 37.07°C and aw < 0.900. Similarly, Mousa et al. (Citation2011) reported that AFB1 production on paddy rice increased along with aw and high AFB1 production was observed at 25°C and 30°C. The same authors also reported on AFB1 production of A. flavus on polished and brown rice (Mousa et al., Citation2013). They demonstrated that AFB1 production occurred at temperatures ranging from 20°C to 40°C and it greatly increased with an increasing aw level. Lasram et al. (Citation2016) also reported that AFB1 production was not detected when A. flavus was grown at aw lower than 0.900 with a temperature of 20°C, 25°C, and 30°C.
In the case of A. carbonarius, the results showed that the maximum radial growth and OTA production occurred when the fungus was cultured at temperatures ranging from 22.93°C to 30°C, with the highest aw (0.984). Similar to our results, Bellí et al. (Citation2005) found that the optimum growth of A. carbonarius in a synthetic grape medium was at a temperature between 30°C and 35°C and aw of 0.980. Bragulat et al. (Citation2019) indicated that the optimum temperatures and aw for growth of A. carbonarius in synthetic grape juice medium were in the ranges of 25–35°C and 0.950–0.990 for aw, respectively. The highest concentration of OTA was detected at 15°C and 0.980–0.990 for aw. In the present study, the minimum growth and OTA production were observed at the highest temperature of 37.07°C with aw values ranging from 0.885 to 0.900. Similar optimum conditions for A. carbonarius growth have been reported for an isolate from green coffee by Palacios-Cabrera et al. (Citation2005). They indicated that A. carbonarius growth was greater at 30°C than 35°C, and it was significantly inhibited at a temperature of 41°C. According to Tassou et al. (Citation2009), two isolates of A. carbonarius did not grow or produce OTA in a synthetic grape juice medium at 40°C for all aw levels investigated (0.850–0.980). In addition, Alborch et al. (Citation2011) found that A. carbonarius can grow at a temperature range of 20–35°C and produce OTA on maize kernels at a temperature range of 15–35°C at aw of 0.920–0.980. Our results suggest that favorable conditions for fungal growth and mycotoxin production vary depending on the fungal stain and substrate.
5. Conclusion
The influence of temperature and aw on fungal growth and mycotoxin production by A. flavus and A. carbonarius was studied using CCD and a response surface analysis. The results showed that temperature, aw, and their interaction had a significant effect on growth and mycotoxin production. Response surface analysis was used to find the conditions for the significant factors that minimize and maximize fungal growth and mycotoxin production. Although all Aspergillus strains were able to grow under wide ranges of temperatures and aw values, they did not produce mycotoxins at a temperature of 37.07°C and aw <0.900. We suggest that the aw in harvested chili should be immediately reduced to be lower than 0.900 by an effective drying method to prevent fungal growth and mycotoxin production.
Competing interests
The authors declare no competing interests.
Acknowledgements
Scientific Equipment and Research Division, KURDI, Kasetsart University, Thailand, is acknowledged for kindly supporting laboratory facilities.
Additional information
Funding
Notes on contributors
Thanapoom Maneeboon
Chananya Chuaysrinule is a graduate student at the Department of Food Science and Technology, Faculty of Agro-Industry, Kasetsart University. She holds a BSc degree in Fisheries from the Department of Fisheries Product, Faculty of Fisheries from the same university. Her research interests are the isolation and identification of mycotoxin-producing fungi.
Warapa Mahakarnchanakul is an assistant professor at the Department of Food Science and Technology, Faculty of Agro-Industry, Kasetsart University. She received her PhD in Food Science at the University of Georgia (USA). Her major areas of interest include mycotoxin detection in food products, stress responses of foodborne pathogens, GMP/HACCP systems, and food safety risk management.
Thanapoom Maneeboon earned his BSc in Microbiology from the Faculty of Science, Ubon Ratchathani University and his MS degree in Microbiology from the Faculty of Science, Kasetsart University. He is a researcher at the Kasetsart University Research and Development Institute (KURDI). His research focuses on mycotoxins and related fungi as well as the microbial degradation of toxic substances.
References
- Alborch, L., Bragulat, M. R., Abarca, M. L., & Cabañes, F. J. (2011). Effect of water activity, temperature and incubation time on growth and ochratoxin A production by Aspergillus niger and Aspergillus carbonarius on maize kernels. International Journal of Food Microbiology, 147(1), 53–12. https://doi.org/10.1016/j.ijfoodmicro.2011.03.005
- Bellí, N., Marín, S., Sanchis, V., & Ramos, A. J. (2004). Influence of water activity and temperature on growth of isolates of Aspergillus section Nigri obtained from grapes. International Journal of Food Microbiology, 96(1), 19–27. https://doi.org/10.1016/j.ijfoodmicro.2004.03.004
- Bellí, N., Ramos, A. J., Coronas, I., Sanchis, V., & Marín, S. (2005). Aspergillus carbonarius growth and ochratoxin A production on a synthetic grape medium in relation to environmental factors. Journal of Applied Microbiology, 98(4), 839–844. https://doi.org/10.1111/j.1365-2672.2004.02469.x
- Bernáldez, V., Córdoba, J. J., Magan, N., Peromingo, B., & Rodríguez, A. (2017). The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus. LWT-Food Science and Technology, 83, 283–291. https://doi.org/10.1016/j.lwt.2017.05.030
- Bragulat, M. R., Abarca, M. L., Castellá, G., & Cabañes, F. J. (2019). Intraspecific variability of growth and ochratoxin A production by Aspergillus carbonarius from different foods and geographical areas. International Journal of Food Microbiology, 306, 108273. https://doi.org/10.1016/j.ijfoodmicro.2019.108273
- Frimpong, G. K., Adekunle, A. A., Ogundipe, O. T., Solanki, M. K., Sadhasivam, S., & Sionov, E. (2019). Identification and toxigenic potential of fungi isolated from Capsicum peppers. Microorganisms, 7(9), 303. https://doi.org/10.3390/microorganisms7090303
- Gebremeskel, A. F., Ngoda, P. N., Kamau-Mbuthia, E. W., & Mahungu, S. M. (2019). Prevalence and controlling mechanisms of mycotoxin. Cogent Food & Agriculture, 5(1), 1658978. https://doi.org/10.1080/23311932.2019.1658978
- Hope, J. H., & Hope, B. E. (2012). A review of the diagnosis and treatment of ochratoxin A inhalational exposure associated with human illness and kidney disease including focal segmental glomerulosclerosis. Journal of Environmental and Public Health, 2012, 835059. https://doi.org/10.1155/2012/835059
- IARC. (1993). Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. Aflatoxins. In WHO IARC monographs on the evaluation of carcinogenic risks to humans (Vol. 56, pp. 489–521).
- Iqbal, S. Z., Asi, M. R., Mehmood, Z., Mumtaz, A., & Malik, N. (2017). Survey of aflatoxins and ochratoxin A in retail market chilies and chili sauce samples. Food Control, 81, 218–223. https://doi.org/10.1016/j.foodcont.2017.06.012
- Lasram, S., Hamdi, Z., Chenenaoui, S., Mliki, A., & Ghorbel, A. (2016). Comparative study of toxigenic potential of Aspergillus flavus and Aspergillus niger isolated from barley as affected by temperature, water activity and carbon source. Journal of Stored Products Research, 69, 58–64. https://doi.org/10.1016/j.jspr.2016.06.002
- Liu, X., Guan, X., Xing, F., Lv, C., Dai, X., & Liu, Y. (2017). Effect of water activity and temperature on the growth of Aspergillus flavus, the expression of aflatoxin biosynthetic genes and aflatoxin production in shelled peanuts. Food Control, 82, 325–332. https://doi.org/10.1016/j.foodcont.2017.07.012
- Lv, C., Jin, J., Wang, P., Dai, X., Liu, Y., Zheng, M., & Xing, F. (2019). Interaction of water activity and temperature on the growth, gene expression and aflatoxin production by Aspergillus flavus on paddy and polished rice. Food Chemistry, 293, 472–478. https://doi.org/10.1016/j.foodchem.2019.05.009
- Mannaa, M., & Kim, K. D. (2017). Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology, 45(4), 240–254. https://doi.org/10.5941/MYCO.2017.45.4.240
- Marín, S., Colom, C., Sanchis, V., & Ramos, A. J. (2009). Modelling of growth of aflatoxigenic A. flavus isolates from red chilli powder as a function of water availability. International Journal of Food Microbiology, 128(3), 491–496. https://doi.org/10.1016/j.ijfoodmicro.2008.10.020
- Medina, A., Akbar, A., Baazeem, A., Rodriguez, A., & Magan, N. (2017). Climate change, food security and mycotoxins: Do we know enough? Fungal Biology Reviews, 31(3), 143–154. https://doi.org/10.1016/j.fbr.2017.04.002
- Merla, C., Andreoli, G., Garino, C., Vicari, N., Tosi, G., Guglielminetti, M. L., Moretti, A., Biancardi, A., Arlorio, M., & Fabbi, M. (2018). Monitoring of ochratoxin A and ochratoxin-producing fungi in traditional salami manufactured in Northern Italy. Mycotoxin Research, 34(2), 107–116. https://doi.org/10.1007/s12550-017-0305-y
- Mousa, W., Ghazali, F. M., Jinap, S., Ghazali, H. M., & Radu, S. (2011). Modelling the effect of water activity and temperature on growth rate and aflatoxin production by two isolates of Aspergillus flavus on paddy. Journal of Applied Microbiology, 111(5), 1262–1274. https://doi.org/10.1111/j.1365-2672.2011.05134.x
- Mousa, W., Ghazali, F. M., Jinap, S., Ghazali, H. M., & Radu, S. (2013). Modeling growth rate and assessing aflatoxins production by Aspergillus flavus as a function of water activity and temperature on polished and brown rice. Journal of Food Science, 78(1), M56–M63. https://doi.org/10.1111/j.1750-3841.2012.02986.x
- Nguyen Van Long, N., Rigalma, K., Coroller, L., Dadure, R., Debaets, S., Mounier, J., & Vasseur, V. (2017). Modelling the effect of water activity reduction by sodium chloride or glycerol on conidial germination and radial growth of filamentous fungi encountered in dairy foods. Food Microbiology, 68, 7–15. https://doi.org/10.1016/j.fm.2017.06.014
- Palacios-Cabrera, H., Taniwaki, M. H., Hashimoto, J. M., & Menezes, H. C. D. (2005). Growth of Aspergillus ochraceus, A. carbonarius and A. niger on culture media at different water activities and temperatures. Brazilian Journal of Microbiology, 36(1), 24–28. https://doi.org/10.1590/S1517-83822005000100005
- Peromingo, B., Rodríguez, A., Bernáldez, V., Delgado, J., & Rodríguez, M. (2016). Effect of temperature and water activity on growth and aflatoxin production by Aspergillus flavus and Aspergillus parasiticus on cured meat model systems. Meat Science, 122, 76–83. https://doi.org/10.1016/j.meatsci.2016.07.024
- Ramirez, M. L., Chulze, S. N., & Magan, N. (2004). Impact of osmotic and matric water stress on germination, growth, mycelial water potentials and endogenous accumulation of sugars and sugar alcohols in Fusarium graminearum. Mycologia, 96(3), 470–478. https://doi.org/10.1080/15572536.2005.11832946
- Reverberi, M., Ricelli, A., Zjalic, S., Fabbri, A. A., & Fanelli, C. (2010). Natural functions of mycotoxins and control of their biosynthesis in fungi. Applied Microbiology and Biotechnology, 87(3), 899–911. https://doi.org/10.1007/s00253-010-2657-5
- Schmidt-Heydt, M., Rüfer, C. E., Abdel-Hadi, A., Magan, N., & Geisen, R. (2010). The production of aflatoxin B1 or G 1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotoxin Research, 26(4), 241–246. https://doi.org/10.1007/s12550-010-0062-7
- Tansakul, N., Limsuwan, S., Böhm, J., Hollmann, M., & Razzazi-Fazeli, E. (2013). Aflatoxins in selected Thai commodities. Food Additives & Contaminants: Part B, 6(4), 254–259. https://doi.org/10.1080/19393210.2013.812148
- Tassou, C. C., Natskoulis, P. I., Magan, N., & Panagou, E. Z. (2009). Effect of temperature and water activity on growth and ochratoxin A production boundaries of two Aspergillus carbonarius isolates on a simulated grape juice medium. Journal of Applied Microbiology, 107(1), 257–268. https://doi.org/10.1111/j.1365-2672.2009.04203.x
- Wikandari, R., Mayningsih, I. C., Sari, M. D. P., Purwandari, F. A., Setyaningsih, W., Rahayu, E. S., & Taherzadeh, M. J. (2020). Assessment of microbiological quality and mycotoxin in dried chili by morphological identification, molecular detection, and chromatography analysis. International Journal of Environmental Research and Public Health, 17(6), 1847. https://doi.org/10.3390/ijerph17061847
- Yogendrarajah, P., Jacxsens, L., De Saeger, S., & De Meulenaer, B. (2014). Co-occurrence of multiple mycotoxins in dry chilli (Capsicum annum L.) samples from the markets of Sri Lanka and Belgium. Food Control, 46, 26–34. https://doi.org/10.1016/j.foodcont.2014.04.043