Abstract
Continuous agricultural and other activities of human being have caused changes in the chemistry of global climate, as a consequence many biotic and abiotic stresses that reduce photosynthetic capacity of plants have emerged. Therefore, the aim of this review is to review photosynthesis limiting stresses under climate change scenarios and role of chlorophyll fluorescence on this stress. Different research findings indicate that UV-B radiation cause changes in biological processes of plants such as damage to the internal structure of photosynthesis or control its cellular process. Other type of stress is drought stress, which inhibits photosynthetic electron transport through Photosystem II and damages the oxygen evolving complex of PSII. On the other way, high night temperature stresses are increasing, and it can cause constant suppression of net CO2 assimilation rate in both C3 and C4 plants. Photoinhibition is considered as reactive oxygen species induced reduction of the primary acceptor of PSII plastoquinone (QA) or change in recombination between acceptor and donor side of P S I I. For assessing the effect of stress on photosynthesis, chlorophyll fluorescence is mostly used parameter and also it is an effective indicator of photosynthesis limiting stress and their mechanisms.
PUBLIC INTEREST STATEMENT
Photosynthesis is the most important biological process in plants and tweaking it allows us to increase crop productivity but gradually as a result of climate change events photosynthetic efficiency in plants has been affected by different stresses. This review gives mechanisms of such photosynthesis limiting stresses and the effect of such stress conditions on chlorophyll fluorescence which is frequently used as an indicator of photosynthetic activity. It also provides us hints on future research for crop yield improvement by modulating chlorophyll fluorescence.
1. Introduction
Global climate is the result of a complex interplay of various atmospheric conditions and their interaction. Agricultural and other activities of human being have caused changes in the chemistry of the climate, as a consequence many biotic and abiotic stress that reduce photosynthetic capacity of plants have occurred (Houghton et al., Citation2001; Reicosky et al., Citation2000). Photosynthesis is a complex process of redox reaction that occurs when the light harvesting complex absorbs photonic energy and transfers it to photosystem reaction centers (Baker, Citation2008). However, stresses such as UV-B, drought, high temperature, and high light (photoinhibtion) can cause a reduction of the electron transport chain (ETC), which leads to photooxidation of photosystem II (C.H. Foyer et al., Citation2012; Kangasjarvi et al., Citation2012; Rochaix, Citation2011). Due to increased industrialization and resulting chloroflurocarbon release in to the atmosphere the earth’s protective stratospheric ozone layer has been depleted (Dentener et al., Citation2001).
Nowadays, continued depletion of the ozone layer is main concern because this layer absorbs the harmful Ultraviolet-B radiation (280–320 nm), and it avoids its damage on plant photosynthetic process. However, collapse of ozone molecule within the stratosphere currently reduce UV-B absorption by stratospheric ozone layer and that result high UV-B absorption by our planet earth, when stratospheric ozone layer reduced by 1% UV-B radiation reaching in to the earth surface increase by 31.8% (M. M. Caldwell & Flint, Citation1994; McKenzie et al., Citation2003). This high UV-B radiation mainly affect plant photosynthesis (Lidon, Citation2012; Lidon & Ramalho, Citation2011; Lidon et al., Citation2012).
Nowadays, drought is one of most known factor that inhibits crop photosynthesis, a n d its one of the most causes of crop loss throughout the world, reducing yields of main crop plants by 50% (Bray et al., Citation2000; Wang et al., Citation2003). This yield reduction was due to drought stress effects on plant photosynthesis, since lack of water damage basic organizational structures of photosynthesis and in turn inhibit carbon assimilation (Ali & Ashraf, Citation2011; Golldack et al., Citation2011), the main reason for low carbon assimilation was due to stomatal limitation by drought stress (Degl’Innocenti et al., Citation2009; Misson et al., Citation2010).
As climate is continued changing worldwide temperature is estimated to continuously increase. According to (IPCC, Citation2014) report global mean temperature has increased by 0.8°C in the 20th century and it is predicted to increase by 3–5°C in 21st century. Photosynthesis is the most sensitive to high temperature stress (Sharkey & Schrader, Citation2006). High temperature affects photosynthesis by causing reduction of the oxidation property of PSII acceptors and diminishes the efficiency of photosynthetic electron transport of both photosystem PSII and photosystem PSI (Mathur et al., Citation2014). However, night temperatures are increasing as a result of climate change and it damage thylakoid membrane (Ristic & Prasad, Citation2007). Also, high night temperature reduce photosystem II quantum yield (Yang et al., Citation2002; Pradhan et al., Citation2012), and this result reduction in total photosynthetic efficiency of crop plants (Prasad et al., Citation2008).
The other types of stress are photoinhibition which is defined as light-induced inhibition of photosystem II activity (Murata et al., Citation2007). However, the extent of photoinhibition depends on the balance between photo damage and repair capacities of PSII (Demmig-Adams et al., Citation2012).
Normally, chlorophyll fluorescence is used to study over all photosynthetic process in plants (Murchie & Lawson, Citation2013), because it is user friendly and non-invasive. Many researches are done on chlorophyll fluorescence and its role to supply information’s related to photosynthetic process of plants (Guo & Tan, Citation2015; Kalaji et al., Citation2017; Ruban, Citation2016; Stirbet et al., Citation2018). Therefore, the aim of this review is to assess photosynthesis limiting stresses under climate change scenarios and role of chlorophyll fluorescence on this stress.
2. Photosynthesis limiting stresses
2.1. Increasing Ultraviolet-B radiation
Ultraviolet-B radiation was studied by two methods such as attenuation and exclusion study as shown in (Figure ). Ultraviolet-B radiation (280–320 nm) quantity in the solar spectrum was very small but it affects plants at molecular, cellular, and organism level (M. M. Caldwell et al., Citation2007; Jenkins, Citation2009). Actually, UV-B radiation is absorbed by large molecules such as nucleic acids, proteins, and lipids. Therefore, it can cause change in biological process of plants by damaging internal structure of photosynthesis or controlling their cellular process (Jenkins, Citation2009; Tian & Yu, Citation2009).
UV-B radiation effect on internal structure of photosynthesis was altering general photosynthetic process (Reddy et al., Citation2003), water metabolism (Fuhrer & Booker, Citation2003), partitioning the carbon from growth pools to secondary metabolic pathways (Bassman, Citation2004), and its effect observed externally in plants, such as tissue chlorosis and necrosis, change in leaf ultrastructure and anatomy (Jenkins, Citation2009; Lidon & Ramalho, Citation2011; Tevini, Citation2004). Also, several studies showed reduction of stomatal conductance in response to UV-B radiation (Lidon & Ramalho, Citation2011; Pal et al., Citation1999), and the reduction of stomatal conductance result in CO2 limitation in many crops (Lidon & Ramalho, Citation2011; Zhao et al., Citation2003). Also, U V- B affects the balance of mesophyll cell because of its damage at both biochemical and biophysical level (Lidon & Ramalho, Citation2011). On the other hand, UV-B affect ribulose-1,5-bisphosphate carboxylase/oxygenase content and activities (Correia et al., Citation1999; Savitch et al., Citation2001), also, the RuBP regeneration capacities (Allen et al., Citation1997; Savitch et al., Citation2001). This effect was because of changes in proteins by photooxidation, reactive oxygen species and free radicals produced by UV-B radiation (C. R. Caldwell, Citation1993; Foyer et al., Citation1994). However, UV-B radiation mainly affects the chloroplast that leads to weakening of the photosynthetic function (Allen et al., Citation1998; Lidon et al., Citation2012), and also it may totally suppress chlorophyll synthesis(Kulandaivelu et al., Citation1991). As shown in (Figure ) UV-B radiation was high at highland area and the pigment anthocyanin increase as a mechanism of plants to protect the damage caused by UV-B radiation.
Many studies showed that photophosphorylation process is most sensitive part from thylakoid membrane on exposure to UV-B radiation (Bolink et al., Citation2001; Correia et al., Citation1999; Lidon et al., Citation2012; Savitch et al., Citation2001). On PSII, UV-B radiation function as either the reaction center or producing dissipative sinks for excitation energy (Lidon & Ramalho, Citation2011). The water oxidizing complex seems to be UV-B sensitive (Lidon et al., Citation2012), since, the Mn cluster of water oxidation was the most easily broken part of the electron transport chain, UV-B absorption by the protein matrix may cause conformational changes and can cause inactivation of the Mn cluster.
Figure 1. Mechanisms of UV-B study on different agro ecosystems: effects of UV-B radiation on (a) natural ecosystems, (b) native plants, such as Gunnera magellanica and Blechnum penna- marina, (c) field crops, such as (d) soybean, (e) barley, (f) tomato, (g) the model plant Arabidopsis thaliana.
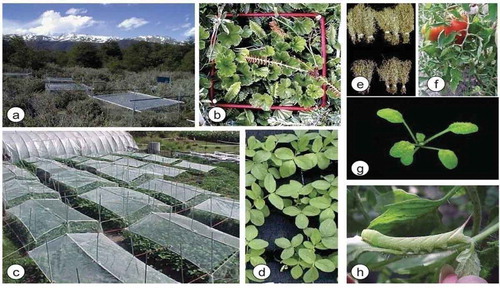
Figure 2. Pigment change (a: Highland; b: Lowland) as indicator of pigment anthocyanin synthesis in response to UV-B radiation.

2.2. Increasing drought stress
Drought affects photosynthesis by reducing carbon and nitrogen assimilation capacities, as a result of stomatal and non-stomatal effects of drought, because plants respond to drought by a rapid closure of stomata (Mwanamwenge et al., Citation1999; Yordanov et al., Citation2000, Citation2003). Many studies indicate that photosynthetic rate decline under drought stress was due to low CO2 concentration at the acceptor site of ribulose-1,5 bisphosphate carboxylase/oxygenase (Bunce, Citation2009; Lawlor & Tezara, Citation2009)), or hold back of photosynthetic enzyme synthesis mainly rubisco (HauptHerting & Fock, Citation2000), also, ATP synthesizing ability (Nogués & Baker, Citation2000; Tezara et al., Citation1999). Moreover, drought stress inhibits photosynthetic electron transport through PSII and damages the oxygen evolving complex of PSII (Lu & Zhang, Citation1999; Skotnica et al., Citation2000), and as a result of drought vegetative growth become low as shown (Figure ).
Drought reduce vegetative growth due to its effect of photosynthetic pigment reduction, since drought reduces total chlorophyll content and the reduction was attributed to ultrastructural deformation of plastids, which in turn causes unraveling of PSII that captures photons (Yang et al., Citation2001), and causing reduction of its efficiency in electron transfer (Kannan & Kulandaivelu, Citation2011). This decline was due to stress-induced destruction of pigment forming biosynthetic pathways or its degradation. The decrease in chlorophyll content is generally observed fact under drought stress condition (Bijanzadeh & Emam, Citation2010; Din et al., Citation2011).
Many findings indicate that drought first cause stomatal limitation, then change photosynthetic reaction mechanism’s (Flexas et al., Citation2008; Zlatev & Yordanov, Citation2004). Such changes are gas exchange mechanisms, carboxylation efficiency and increase in CO2 compensation point that result in fluctuations of CO2 carves of photosynthesis (Zlatev & Yordanov, Citation2004). Drought showed a reduction i n both the initial slope of those curves (Zlatev & Yordanov, Citation2004), at the initial time CO2 curve and carboxylation efficiency of Rubisco was at its maximal and rate of photosynthesis increase. These indicate the potential of the leaves to restore RuBP, since drought stress led reduction of both Rubisco carboxylation efficiency and RuBP regeneration capacity. However, Photosynthesis mainly based on a balance between Rubisco carboxylation capacity, RuBP utilization and its regeneration (Baker et al., Citation1997; Nogués & Baker, Citation2000). On the other hand, drought hinder biochemical processes of photosynthesis by changing ionic or osmotic conditions, which reduce ATP syntheses (HauptHerting & Fock, Citation2000; Tezara et al., Citation1999). According to the conclusions of (Liu et al., Citation2006; Zlatev, Citation2009) photosynthesis is reduced under drought stress condition because of its effect on biochemical processes related to the PSII reaction, and the associated disconnection of non-cyclic photophosphorylation (Yordanov et al., Citation2000). This disconnection affects a balance between generation and utilization of electrons, resulting in reduction of electron transport rate. This reduction could be due to a dehydration effect on rubisco that reflect an increase in rubisco hydrolysis, since the amount and activities of rubisco manly determine photosynthesis (Lawlor, Citation2002).
Figure 3. Impact of drought stress on the vegetative growth of rice.

2.3. High night temperature stress
Scientists reported that night temperature increased by 1.13°C from 1979 to 2003 in Philippines (Peng et al., Citation2004). Global air circulation model predicts 1.4–5.8°C rise in temperature globally because of expected increase in concentrations of greenhouse gases by the end of the 21st century (IPCC, Citation2007). Much of this increase in temperature is due to an increase in night temperature. Because of increased cloudiness and less radiant heat loss, night temperatures are expected to increase at a faster rate than day temperatures (Alward et al., Citation1999). Also, (Dai et al., Citation2001) reported that many climate models predict significant increase of night temperature compared with day temperature, which narrows the diurnal temperature range.
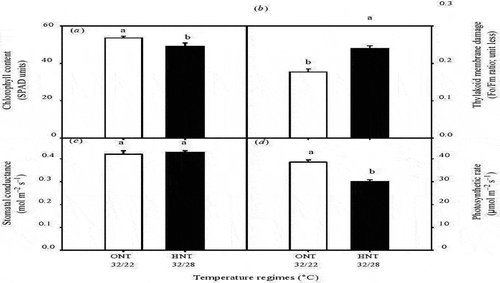
High night temperature reduce crop production by limiting photosynthetic efficiency (Loka & Oosterhuis, Citation2010; Turnbull et al., Citation2002), and increasing respiration rate (Mohammed & Tarpley, Citation2009). However, (Turnbull et al., Citation2002) indicated that photosynthesis and respiration respond independently to night temperature but related by their effect on leaf carbon status. High night temperature consistently suppress net CO2 assimilation rate of both C3 and C4 plants (Bange & Milroy, Citation2004; Sao et al., Citation2013; Zhang et al., Citation2010). Also, it affects photosynthesis by causing leaf chlorophyll damage (Prasad & Djanaguiraman, Citation2011). (Ristic & Prasad, Citation2007) reported that high night temperature damage the thylakoid membrane, which result to chlorophyll loss and decrease the efficiency of photosystem II (PSII) (Pradhan et al., Citation2012; Yang et al., Citation2002), and totally reduce crop photosynthesis (Prasad et al., Citation2008). According to (Figure ) high night temperature reduce chlorophyll content, photosynthesis rate and increase thylakoid membrane damage.
At the cellular level, high night temperature cause a decrease in antioxidant activity and lead to an increase of reactive oxygen species and its oxidative damage mainly lipid peroxidation and membrane damage (Sairam et al., Citation2000). Increased reactive oxygen species concentration at high night temperature was a result of decreased antioxidant activity (Djanaguiraman et al., Citation2010; Prochazkova et al., Citation2001). This reactive oxygen species cause blockage of PSII reaction center and electron flow, and all these changes lead to reduction of photosynthesis (Djanaguiraman et al., Citation2010). According to (Schrader et al., Citation2004) permeability of thylakoid membrane increase under high night temperature, which leads to proton leakage, in turn cause reduction of adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) synthesis, and end up with reduction of photosynthesis.
Figure 4. Effect of optimum and high night temperature, (optimum night temperature (ONT) white bars, 32oC day maximum temperature and 22oC night minimum temperature), and (high night temperature (HNT) black bars), 32 oC day maximum temperature and 28oC night minimum temperature) on (a) chlorophyll content, (b) thylakoid membrane damage (F0/Fm ratio), (c) stomatal conductance (molm–2 s–1), and (d) photosynthetic rate (mmo lm–2 s–1) in sorghum leaves.
2.4. Photoinhibition of photosystem II
The inhibition of PSII activity under continuous exposure to high Light is commonly known as photoinhibition (Murata et al., Citation2007). According to (Murata et al., Citation2007; Takahashi & Badger, Citation2011) the primary sources of PSII photodamage is the light-dependent distraction of oxygen-evolving complex (OEC), and which result the release of manganese ions. When LHCII absorbs high light than it used up in photochemistry, photoinhibition surges exponentially that cause severe impairment to PSII (Nishiyama & Murata, Citation2014; Tikkanen & Aro, Citation2014). Besides, (Nishiyama & Murata, Citation2014) reported that superoxide anion radical or singlet oxygen is produced when more electrons are released in the electron transport chain (ETC) than used by the Calvin cycle. Findings of (Allakhverdiev et al., Citation2004; Allakhverdiev & Murata, Citation2004; Ohnishi & Murata, Citation2006) indicate that PSII photoinhibition, ROS, such as superoxide radicals and singlet oxygen produced as a result of high light (photoinhibtion).
Studies done by (Murata et al., Citation2012) give another analysis of the photoinhibition mechanism, he reported that reactive oxygen species do not damage PSII reaction centers directly but inhibit the repair of PSII by inhibiting protein synthesis. The same findings indicate that photodamage to PSII happens by two successive steps: (1) the light dependent destruction of the Mn cluster of the oxygen evolving complex, and (2) the inactivation of the PSII reaction centers by the light that has been absorbed by chlorophyll (Ohnishi et al., Citation2005). However, (Cazzaniga et al., Citation2013) indicated that under high photoinhibtion non -photochemical quenching (NPQ) act as photoprotecter by causing reduction in concentration of chlorophyll excited states (Chl*) in PSII and activate a heat dissipation channel. Moreover, nonphotochemical quenching (NPQ) initiation during photoinhibtion was as a result of alterations in the distribution and molecular direction of chlorophyll proteins in the thylakoid membrane (Herbstova et al., Citation2012; Johnson et al., Citation2011).
Hypothesis on the impact of stresses other than light on PSII activity indicate that stresses increase the rate of photoinhibition of PSII (Adir et al., Citation2003; Melis, Citation1999). Nowadays, this hypothesis has been disproved many researchers showed that the repair mechanism of PSII is more sensitive to other stresses than the process of photodamage itself (Kangasjarvi et al., Citation2012; Nishiyama & Murata, Citation2014). Photosystem II is the most sensitive component to photoinhibtion in the thylakoid membrane. Therefore, the principal result of other stress is to cause PSII prone to photoinhibition (Nishiyama et al., Citation2001). However, unlike to PSII, PSI is not regularly damaged by photoinhibtion, due to its very efficient mechanism of protecting from photoinhibition (Sonoike, Citation2011; Tikkanen & Aro, Citation2014).
3. Role of chlorophyll fluorescence on photosynthesis limiting stresses
3.1. Chlorophyll fluorescence and PSII photochemistry
Chlorophyll a fluorescence is induced when a photosynthetic sample is moved from darkness to high light intensity (Papageorgiou et al., Citation2007). It is divided into two phases: (1st), the fast induction phase or the OJIP phase, where O is origin, P is peak and J–I are the intermediate phases and (2nd), the slow induction phase, where, P is peak, S is steady, M maximum, and T is the terminal. The high-speed induction kinetics expresses the primary photochemistry of PSII. However, the slow kinetics is a complex phase primarily associated to the interactions with processes in the thylakoids membrane, and reductive carbon cycle of the stroma (Krause & Weis, Citation1991). In other way, the slow induction phase is emitted from photosynthetic samples in the red infrared region for a short time after the fast fluorescence has decay. The fast phase of the transient gives important information about reduction of electron acceptors during electron transport chain (ETC). On the other hand, it is difficult to interpret the slow transient phase, because a number of different processes such as NPQ, ATP synthesis, and the Calvin cycle are involved on this phase (Stirbet & Govindjee, Citation2011). However, the slow fluorescence emission of PSII is thought as good approach to study light-induced electron transfer, since electron movement is not visible by conventional spectroscopic (Goltsev et al., Citation2009). Many researchers have analyzed the slow fluorescence methods to evaluate plant performance under stressful environment (Zhang et al., Citation2007; Valikhanov et al., Citation2002).
According to (Zhang et al., Citation2007) PSII efficiency under normal and stress conditions can be determined by fast chlorophyll a transient (Baker, Citation2008; Gururani et al., Citation2013, Citation2015).
Analysis of fast chlorophyll a transients has a potential give us fascinating facts pertaining to the change and modification of the photosynthetic machinery at different stress conditions. Measuring changes of fast chlorophyll a fluorescence transients become a widely used technique for assessing different stresses. An increase of fluorescence from F0 (minimum) to Fm (maximum) in dark adapted plants exposed to a strong saturating light pulse (3000–12,000 mmol photons m2 s-1, 200–1000 ms), and at high resolution of (0–200 ms) is known as apolyphasic OJIP transient, it has three phases: OJ (0–3 ms), JI (3–30 ms), and IP (30–200 ms). Latest findings indicate that the increase of fluorescence minimum (F0) and maximum (Fm) show the reduction in the amount of quinine (QA), the 1st electron acceptor of PSII (Schansker et al., Citation2014). The OJIP or JIP test studied by (Strasser et al., Citation2004) is the main descriptive model used to explain OJIP transients (Baker, Citation2008; Baker & Rosenqvist, Citation2004). The model works by comparing the photosynthetic activities of stressed and normally growing plants it is noninvasive tool for analyzing the effects of different stresses on photosystem II and photosynthetic efficiency (Goltsev et al., Citation2012; Ranjan et al., Citation2014). Generally, chlorophyll a fluorescence transients provide very important information about PSII photochemistry and electron transport chain (ETC), and the acceptance of this analytical method on plant photosynthetic research was increasing (Stirbet & Govindjee, Citation2011).
3.2. Chlorophyll fluorescence on high night temperature stress
High night temperature stress reduces both the ratio of acceptors such as plastoquinone (QA) to reaction centers (RC) and the ratio of reduced acceptors plastoquinone (QB) to (QA). Also, maximum quantum yield of PSII decrease and minimal fluorescence value increase (Brestic & Zivcak., Citation2013; Chen et al., Citation2009). However, high night temperature stress also influences the shape of the O–J–I–P curve, decreasing (Fm) and increasing (Fo). The increase in (Fo) may be due to the release of LHC II from the PSII complex, inactivation of PSII photochemical reaction, an inhibition of electron flow or reduction of electron transfer from QA to QB (Mathur et al., Citation2011), and the decrease in (Fm) was due to denaturation of chlorophyll proteins (Yemane et al., Citation1997). Also, (Figure ) indicate that high night temperature reduce photochemical quenching, increase non-photochemical quenching, decrease PSII quantum yield and electron transport rate.
The K peak at 300 s is a well-known symptoms of high night temperature stress, and it is used to indicate the separation of the oxygen evolving complex (OEC) and electron movement between pheophytin and primary electron acceptor (QA) (Laza´r, Citation2006; Strasser et al., Citation2000). The direct cause of the chlorophyll florescence curve peak (K) is the discharge of electrons from P680 to PSII acceptors, which over compensates the invasion of electrons from the donor side of PSII to P680, and an increase in the FK/FJ ratio (Srivastava & Strasser, Citation1995). This process indicates that the high night temperature stress is inhibiting the donation of electrons by the oxygen evolving complex (OEC). Since, the PSII complex performs splitting of water molecules and releasing molecular oxygen through an oxygen evolving complex (OEC) (Gururani et al., Citation2012; Tikkanen & Aro, Citation2014), and the released electrons are then transported to the PSI complex via electron transport chain (ETC) from plastocyanin to ferredoxin (Nellaepalli et al., Citation2014).
Figure 5. Effect the of optimum and high night temperature [optimum night temperature (ONT) white bars, 32oC day maximum temperature and 22oC night minimum temperature], and [high night temperature (HNT) black bars], 32oC day maximum temperature and 28oC night minimum temperature) on (a) PSII quantum yield, (b) electron transport rate (mmol m–2 s–1), (c) photochemical quenching, and (d) nonphotochemical quenching in sorghum leaves.
![Figure 5. Effect the of optimum and high night temperature [optimum night temperature (ONT) white bars, 32oC day maximum temperature and 22oC night minimum temperature], and [high night temperature (HNT) black bars], 32oC day maximum temperature and 28oC night minimum temperature) on (a) PSII quantum yield, (b) electron transport rate (mmol m–2 s–1), (c) photochemical quenching, and (d) nonphotochemical quenching in sorghum leaves.](/cms/asset/17fc125e-598d-4165-a9b4-43d31bea3a5d/oafa_a_1785136_f0005_b.gif)
3.3. Chlorophyll fluorescence on drought stress
Among the photosynthetic component, PSII is highly resistant to drought compared to PSI and it is affected by drought only at extreme drought conditions (Lauriano et al., Citation2006). Chlorophyll fluorescence protects PSII and PSI from drought stress by regulating the energy distribution between photosystems and activating alternative electron sinks (Zivcak et al., Citation2013). It enhances the resistance of PSII to drought stress by causing vanishing of the K band from the OJIP transient (Oukarroum et al., Citation2012). However, the fluorescence increase during the primary 2–3 s is said to primary photochemistry and it is has been proposed that stimulated L and K bands are often used as a tools to evaluate potential to avoid drought stress (Oukarroum et al., Citation2007). The L band affects the excitation energy transfer within PSII units, commonly known as connectivity or grouping (Strasser & Stirbet, Citation1998). The K band has been related to a dissociation of the oxygen evolving complex (Guisse et al., Citation1995).
The measurement of OLKJIP fluorescence transients and their analysis using the JIP test could be used as indicators for drought stress tolerance and physiological stability. However, the most generally used parameter from the chlorophyll fluorescence OJIP transient is the performance index (PI), which provides quantitative information about the overall state of plants and their vigor. Performance index (PI) is the product of three independent characteristics: (1) the concentration of reaction centers per chlorophyll, (2) a parameter regarding primary photochemistry and (3) a parameter regarding electron transport (Strasser et al., Citation2004). Performance index (PI) is sensitive to changes in antenna properties, trapping efficiency or electron transport beyond QA. For example, the PI of wheat decline if prolonged drought stress occur after post anthesis. Besides, the drought tolerance of wheat genotypes expected from PI values recorded in drought stress also correlated with the drought tolerance (Zivcak et al., Citation2008). The most drought tolerant and sensitive races of barley and great millet from Egypt were identified using the PI and chlorophyll fluorescence fast induction curve (Jedmowski et al., Citation2013). These studies show that drought tolerant and drought sensitive cultivars are often differentiated at the extent of PSII, as shown in (Table ).
Table 1. Chlorophyll fluorescence in leaves of control and drought stressed bean plants
3.4. Chlorophyll fluorescence on UV-B and photoinhibtion stress
The first important indicator derived from Kautskey curve was Fv/Fm ratio (Krause, Citation1988), and it is a good indicator of the PSII photoinhibtion by UV-B and other stresses (Krause & Weis, Citation1991). The ratio of Fv/Fm [(Fm-Fo)/Fm indicate maximal photochemical efficiency of PSII and also it indicate functional loss of PSII reaction center’s (Öquist et al., Citation1992). Fv/Fm values are between 0.75 and 0.85, and it is related to quantum yield of photochemistry. If this ratio decline it is considered as photoinhibtion and UV-B stress occurred, it is as a result of two different processes (Öquist et al., Citation1992). 1st PSII Photochemistry rate constant decrease due to damage of photochemistry reaction Centre and an increase in the rate constant of non radiative dissipation of excitation energy. This decrement of PSII photochemistry cause a n increase in initial fluorescence at open PSII (Fo), and maximum fluorescence at closed PSII (Fm) (Kitajim and Butler, 1975). However, the decrease in Fv/Fm ratio is not related to the amount of deactivated PSII reaction centers (Park et al., Citation1996).
Certainly, Fv/Fm ratio is considered as an indicator of PSII photoinactivation but this ratio decrement was not due to the closure of PSII reaction centers alone but also other processes participate with charge separation mainly thermal dissipation of absorbed light (Malnoë, Citation2018). Chlorophyll fluorescence quenching analysis has given an advance in the detection of PS II photoinhibtion (Bolhàr-Nordenkamp et al., Citation1993). Quenching analysis separate both photochemical and nonphotochemical processes in the quenching of variable fluorescence, by inducing a short closure of all PSII reaction Centre’s by saturating light pulse (Baker, Citation2008; Schreiber et al., Citation1995).
The low amount of fluorescence due to photochemistry and the charge separation is called photochemical quenching. However, concerning photochemistry the most useful parameters derived from quenching analysis is efficiency of PSII (Genty et al., Citation1989). Informally, similar to PSII another parameter obtained from quenching
Analysis is coefficient of photochemical quenching, qP, that shows the proportion of open PSII reaction centers (Maxwell & Johnson, Citation2000). On the other hand, PSII provides information on the electron transport rate and, unlike to Fv/Fm ratio it can determine dark adapted conditions of photoinhibtion. However, PSII decline as a result of inactivation of PSII reaction centers due to photoprotection (Krause et al., Citation1990), or may be a mechanism that adjusts the efficiency of PSII to photosynthetic photon flux density (Critchley, Citation1994), but at high light condition PSII also decline based on the activity of energy consuming biochemical reactions of CO2 assimilation (Genty et al., Citation1989). Besides, the proportion of light energy diverted to photochemistry quenching analysis controls the amount of light energy dissipated by other mechanisms such as non-photochemical quenching (Logan et al., Citation2014). Similarly, quenching analysis helps to identify the non-photochemical quenching (NPQ), that signifies rapid process and reversible thermal dissipation of absorbed light energy in the PSII antenna (Horton & Ruban, Citation2005; Ruban et al., Citation2012).
Non-photochemical quenching (NPQ) is a dissipation mechanism in to heat and many components are involved such as the energy dependent (qE), zeaxanthin-dependent (qZ) and photoinhibitory quenching (qI) (Derks et al., Citation2015). However, qE and qZ are essential for photoprotection but qI represent the photoinhibitory damage to PSII reaction centres (Ruban et al., Citation2012), but qE is most effective part at photo protecting PSII reaction centers from damage (Nilkens et al., Citation2010). In surplus light conditions, a transthylakoidal proton gradient (1pH) is generated, activating qE (Noctor et al., Citation1993). That can cause acidification of thylakoid lumen and it result in protonation of PSII subunits protein, then activates Violaxanthinepo-oxidase, which in turn changes violaxanthin (Vio) in to zeaxanthin (Zea) (Demming-Adams, Citation1990). Both protonation of PSII subunits protein (Psbs) and (Zea) cause an increase in the sensitivity of LHCII to the lumen protons inducing qE (Horton et al., Citation2000; Ruban et al., Citation2012). qZ coefficient is formed within 10–30 min, and it is independent to psbs and1pH, even strictly dependent to Zeaepoxidation and encourages conformational change of miner antenna protein CP26. However, qE and qZ changes the LHCII (CitationDall’Osto et al., ; Nilkens et al., Citation2010).
The final quenching coefficient qI is relatively slower to relax and losses its number of active PSII reaction centers at time of photoinhibtion (Derks et al., Citation2015). However, this independent portion named as qH (Malnoë et al., Citation2017), that includes different processes. Some are photoprotecting antenna and has similar mechanisms with qE and qZ. These mechanisms act in a different way to dissipate the excess excitation energy (Malnoë et al., Citation2017). The qH coefficient gives information on the decline of Fv/Fm ratio and it can result a high Fo due to inactivation of PSI reaction centers or antenna detachment, also both Fo and Fm decline if qH is present.
4. Conclusion
Our world is rapidly becoming industrialized and this result emission of toxic chemical compounds in to the atmosphere that deplete protective stratospheric ozone layer. As a result, Ultraviolet-B radiation absorption by the earth increase, then it reduces photosynthetic capacity of plants by directly damaging photosynthetic structures and process. In addition, other stress such as photoinhibtion, high temperature and drought stresses gets advantage and affects the photosynthetic capacity in integrated way. Nowadays, these stresses are reducing photosynthesis and affecting crop production and productivity in general. Therefore, controlling this photosynthetic limiting stress works must be done on such area: 1. Employing chlorophyll fluorescence to know which part of photosynthetic process is affected by this stress. 2. Breeding must be done by identifying good performing genes by chlorophyll fluorescence.
Competing interest
There is no competing interest.
Additional information
Funding
Notes on contributors
Simeneh Tamrat Alemu
My Name is Simeneh Tamrat Alemu, I have MSc degree in Horticulture with specialization of stress physiology (UV-B and light Quality). Currently, I am working as a lecturer at department of Dry land crop and Horticultural science, college of Dry land Agriculture and natural resources, Mekelle University, Mekelle, Ethiopia. My research interest was stress physiology of plants and horticultural crops improvement.
References
- Adir, N., Zer, H., Shochat, S., & Ohad, I. (2003). Photoinhibition: A historical perspective. Photosynthesis Research, 1(76), 343–18. https://doi.org/10.1023/A:1024969518145
- Ali, Q., & Ashraf, M. (2011). Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: Growth, photosynthesis, water relations and oxidative defense mechanism. Journal of Agronomy and Crop Science, 7(197), 258–271. https://doi.org/10.1111/j.1439-037X.2010.00463.x
- Allakhverdiev, S. I., & Murata, N. (2004). Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of Photosystem II in Synechocystis sp. PCC 6803. Biochimica et biophysica acta, 1657(2004), 23–32. https://doi.org/10.1016/j.bbabio.2004.03.003
- Allen, D. J., McKee, I. F., Farage, P. K., & Baker, N. R. (1997). Analysis of the limitation to CO2 assimilation to exposure of leaves of two Brassica napus cultivars to UV-B. Plant Cell & Environment, 5(20), 633–640. https://doi.org/10.1111/j.1365-3040.1997.00093.x
- Allen, D. J., Nogués, S., & Baker, N. R. (1998). Ozone depletion and increased UV-B radiation: Is there a real threat to photosynthesis? Journal of Experimental Botany, 49(328), 1775–1788. https://doi.org/10.1093/jxb/49.328.1775
- Alward, R. D., Detling, J. K., & Milchunas, D. G. (1999). Grassland Vegetation Changes and Nocturnal Global Warming. Science, 5399(283), 229–231. https://doi.org/10.1126/science.283.5399.229
- Baker, N., Nogues, R. S., & Allen, D. J. (1997). Photosynthesis and photoinhibition. In P. J. Lumsden (Ed.), Plants and UV-B: Responses to environmental change (pp. 95–111). Cambridge University Press.
- Baker, N. R. (2008). Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annual Review of Plant Biology, (2008(59), 89–113. https://doi.org/10.1146/annurev.arplant.59.032607.092759
- Baker, N. R., & Rosenqvist, E. (2004). Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. Journal of Experimental Botany, 55(403), 1607–1621. https://doi.org/10.1093/jxb/erh196
- Bange, M. P., & Milroy, S. P. (2004). Impact of short-term exposure to cold night temperature on early development of cotton (Gossypium hirsutum L.). Australian Journal of Agricultural Research, 6(55), 655–664. https://doi.org/10.1071/AR03221
- Bassman, J. H. (2004). Ecosystem consequences of enhanced solar ultraviolet radiation: Secondary plant metabolites as mediators of multiple trophic interactions in terrestrial communities. Journal of Photochemistry and Photobiology, 79(5), 382–398. https://doi.org/10.1562/si-03-24.1
- Bijanzadeh, E., & Emam, Y. (2010). Effect of defoliation and drought stress on yield components and chlorophyll content of wheat. Pakistan Journal of Biological Sciences: PJBS, 14(15), 699–705. https://doi.org/10.3923/pjbs.2010.699.705
- Bolhàr-Nordenkamp, F. H. R., Öquist, G., & Long, S. P. (1993). Chlorophyll fluorescence as a tool in photosynthesis research. In D. O. Hall, J. M. O. Scurlock, & Leegood (Eds.), Photosynthesis and Production in a Changing Environment (pp. 193–206). Springer.
- Bolink, E. M., van Schalkwijk, I., Posthumus, F., & van Hasselt, P. R. (2001). Growth under UV-B radiation increases tolerance to high-light stress in pea and bean plants. In J. Rozema, Y. Manetas, & L. O. Björn (Eds.), Responses of plants to UV -B radiation. advances in vegetation science, 154(18).
- Bray, E. A., Bailey-Serres, J., & Weretilnyk, E. (2000). Responses to abiotic stresses. In W. Gruissem, B. Buchnnan, & R. Jones (Eds.), Biochemistry and Molecular Biology of Plants (pp. 1158–1249). American Society of Plant Physiologists.
- Brestic, M., ., & Zivcak., M. (2013). PSII Fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: Protocols and applications. Molecular stress physiology of plants. Springer.
- Bunce, J. A. (2009). Use of the response of photosynthesis to oxygen to estimate mesophyll conductance to carbon dioxide in water-stressed soybean leaves. Plant, Cell & Environment, 7(32), 875–881. https://doi.org/10.1111/j.13653040.2009.01966.x
- Caldwell, C. R. (1993). Ultraviolet-induced photodegradation of cucumber (Cucumis sativus L.) microsomal and soluble protein tryptophanyl residues in vitro. Plant Physiology, 3(101), 947–953. https://doi.org/10.1104/pp.101.3.947
- Caldwell, M. M., Bornman, J. F., Ballare, C. L., Flint, S. D., & Kulandaivelu, G. (2007). Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochemical & Photobiological Sciences, 3(6), 252–266. https://doi.org/10.1039/B700019G
- Caldwell, M. M., & Flint, S. D. (1994). Stratospheric ozone reduction, solar UV-B radiation and terrestrial ecosystems. Climatic Change, 1(28), 375–394. https://doi.org/10.1007/BF01104080
- Cazzaniga, S., Dall’Osto, L., Kong, S. G., Wada, M., & Bassi, R. (2013). Interaction between avoidance of photon absorption, excess energy dissipation and zeaxanthin synthesis against photooxidative stress in Arabidopsis. The Plant Journal : For Cell and Molecular Biology, 4(76), 568–579. https://doi.org/10.1111/tpj.12314 Epub 2013 Oct 3
- Chen, L. S., Li, P., & Cheng, L. (2009). Comparison of thermo tolerance of sun-exposed peel and shaded peel of ‘Fuji’apple. Environmental and Experimental Botany, 66(1), 110–116. https://doi.org/10.1016/j.envexpbot.2008.12.017
- Correia, C. M., Areal, E. L. V., Torres-Pereira, M. S., & Torres-Pereira, J. M. G. (1999). Intra specific variation in sensitivity to ultraviolet-B radiation in maize grown under field conditions. II. Physiological and biochemical aspects. Field Crops Research, 2-3(62), 97–105. https://doi.org/10.1016/S0378-4290(98)00164-6
- Critchley, C. (1994). D1 protein turnover: Response to photodamage or regulatory mechanism? In N. R. Baker & J. R. Bowyer Jr. (Eds.), Photoinhibition of Photosynthesis (pp. 195–204). Bios Scientific.
- Dai, A., Wigley, T. M. L., Boville, B. A., Kiehl, J. T., & Buja, L. E. (2001). Climates of the twentieth and twenty-first centuries simulated by the NCAR climate system model. Journal of Climate, 4(14), 485–519. https://doi.org/10.1175/1520-0442(2001)014
- Dall’Osto, L., Caffarri, S., & Bassi, R. A. (2005) A mechanism of non-photochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP 26. The Plant Cell, (17)2005(2005), 1217–1232. https://doi.org/10.1105/tpc.104.030601
- Degl’Innocenti, E., Hafsi, C., Guidi, L., & Navari-Izzo, F. (2009). The effect of salinity on photosynthetic activity in potassiumdeficient barley species. Journal of Plant Physiology, 166(18), 1968–1981. https://doi.org/10.1016/j.jplph.2009.06.013 Epub 2009Jul 14
- Demmig-Adams, B., Cohu, C. M., Muller, O., & Adams, W. W. 2012). Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynthesis Research, 13(1–3):75–88. https://doi.org/10.1007/s11120-012-9761-6. Epub 2012 Jul 12. ., (), . doi:…
- Demming-Adams, B. (1990). Carotenoids and Photo protection: A role for the Xanthophylls Zeaxanthin. Biochimica et biophysica acta, 1(1020), 1–24. https://doi.org/10.1016/0005-2728(90)90088-L
- Dentener, F., Derwent, R., Dlugokencky, E., Holland, E., Isaksen, I., Katima, J., Kirchhoff, V., Matson, P., Midgley, P., & Wand, M. (2001). Atmospheric chemistry and greenhouse gases. In J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden, X. Dai, K. Maskell, & C. A. Johnson (Eds.), Climate Change. (2001). the Scientific Basis. Contributions of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (pp. 881). Cambridge University Press.
- Derks, A., Schaven, K., & Bruce, D. (2015). Diverse mechanisms for photo protection in photosynthesis. Dynamic regulation of photosystem 2 excitation in response to rapid environmental change. Biochimica et biophysica acta, 4-5(1847), 468–485. https://doi.org/10.1016/j.bbabio.2015.02.008
- Din, J., Khan, S. U., Ali, I., & Gurmani, A. R. (2011). Physiological and agronomic response of canola varieties to drought stress. Journal of Animal and Plant Sciences, 1(21), 78–82.
- Djanaguiraman, M., Prasad, P., & Seppanen, M. (2010). Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiology And Biochemistry: Ppb / Societe francaise de physiologie vegetale, 12(48), 999–1007. https://doi.org/10.1016/j.plaphy.2010.09.009 Epub 2010 Oct 1
- Fathi, A., & Barari, D. T. (2016). Effect of Drought Stress and its Mechanism in Plants. International Journal of Life Science, 1(10), 1–6. https://doi.org/10.3126/ijls.v10i1.14509
- Flexas, J., Ribas-Carbo, M., Diaz-Espejo, A., Galmes, J., & Medrano, H. (2008). Mesophyll conductance to CO2: Current knowledge and future prospects. Plant, Cell & Environment, 31(5), 602–612. https://doi.org/10.1111/j.1365-3040.2007.01757.x
- Foyer, C. H., Lelandais, M., & Kunert, K. J. (1994). Photo oxidative stress in plants. Physiologia Plantarum, 92(4), 696–717. https://doi.org/10.1111/j.1399-3054.1994.tb03042.x
- Foyer, C. H., Neukermans, J., Queval, G., Noctor, G., & Harbinson, J. (2012). Photosynthetic control of electron transport and the regulation of gene expression. Journal of Experimental Botany, 4(63), 1637–1661. https://doi.org/10.1093/jxb/ers013
- Fuhrer, J., & Booker, F. (2003). Ecological issues related to ozone: Agricultural issues. Journal of Environment International, 29(1), 141–154. https://doi.org/10.1016/S0160-4120(02)00157-5
- Genty, B., Briantais, J. M., & Baker, N. B. (1989). The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et biophysica acta, 990(1), 87–92. https://doi.org/10.1016/S0304-4165(89)80016-9
- Golldack, D., Lü King, I., & Yang, O. (2011). Plant tolerance to drought and salinity: Stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Reports, 8(30), 1383–1391. https://doi.org/10.1007/s00299-011-1068-0 Epub 2011 Apr 8
- Goltsev, V., Zaharieva, I., Chernev, P., Kouzmanova, M., Kalaji, H. M., Yordanov, I., Krasteva, V., Alexandrov, V., Stefanov, D., & Allakhverdiev, S. I. (2012). Drought-induced modifications of photosynthetic electron transport in intact leaves: Analysis and use of neural networks as a tool for a rapid non-invasive estimation. Biochimica et biophysica acta, 8(1817), 1490–1498. https://doi.org/10.1016/j.bbabio.2012.04.018 Epub 2012 May 15
- Goltsev, V., Zaharieva, I., Chernev, P., & Strasser, R. J. (2009). Delayed fluorescence in photosynthesis. Photosynthetic Research, 25(101), 217–232. https://doi.org/10.1007/s11120-009-9451-1
- Guisse, B., Srivastava, A., & Strasser, R. J. (1995). Effects of high temperature and water stress on the polyphasic chlorophyll a fluorescence transient of potato leaves. In P. Mathis (Ed.), Photosynthesis: From light to biosphere (pp. 913–916). Kluwer Academic Publishers.
- Guo, Y., & Tan, J. (2015). Recent advances in the application of chlorophyll fluorescence from photosystem 2. Photochemistry and Photobiology, 1(91), 1–14. https://doi.org/10.1111/php.12362
- Gururani, M. A., Upadhyaya, C. P., Strasser, R. J., Woong, Y. J., & Park, S. W. (2012). Physiological and biochemical responses of transgenic potato plants with altered expression of PSII manganese stabilizing protein. Plant Physiology And Biochemistry : Ppb / Societe francaise de physiologie vegetale, 1(58), 182–194. https://doi.org/10.1016/j.plaphy.2012.07.003 Epub 2012 Jul 11
- Gururani, M. A., Upadhyaya, C. P., Strasser, R. J., Yu, J. W., & Park, S. W. (2013). Evaluation of abiotic stress tolerance in transgenic potato plants with reduced expression of PSII manganese stabilizing protein. Plant Science : An International Journal of Experimental Plant Biology, 1(198), 7–16. https://doi.org/10.1016/j.plantsci.2012.09.014 Epub 2012 Oct 5
- Gururani, M. A., Venkatesh, J., Ganesan, M., Strasser, R. J., Han, Y. J., Kim, J. I., Lee, H. Y., & Song, P. S. (2015). In vivo assessment of cold tolerance through chlorophyll-a fluorescence in transgenic zoysia grass expressing mutant phytochrome A. PloS One, 5(10), 1–17. https://doi.org/10.1371/journal.pone.0127200
- HauptHerting, S., & Fock, H. P. (2000). Exchange of oxygen and its role in energy dissipation during drought stress in tomato plants. Physiologia Plantarum, 4(110), 489–495. https://doi.org/10.1111/j.1399-3054.2000.1100410.x
- Herbstova, M., Tietz, S., Kinzel, C., Turkina, M. V., & Kirchhoff, H. (2012). Architectural switch in plant photosynthetic membranes induced by light stress. Proceedings of the National Academy of Sciences, 49(109), 20130–20135. https://doi.org/10.1073/pnas.1214265109 Epub 2012 Nov 19
- Horton, P., & Ruban, A. V. (2005). Molecular design of the photosystem 2 light harvesting antenna: Photosynrhesis and photoprotection. Journal of Experimental Botany, 411(56), 365–373. https://doi.org/10.1093/jxb/eri023
- Horton, P., Ruban, A. V., & Wentworth, M. (2000). Allosteric regulation of the light harvesting system of photosystem 2. Philosophical Transactions of the Royal Society B, 13(355), 61–1370. https://doi.org/10.1098/rstb.2000.0698
- Houghton, J. T., Ding, D., Griggs, D. J., Noguer, M., van der Linden, P. J., Dai, X., Maskell, K., & Johnson, C. A. (2001). The scientific basis, contribution of working group I to the third assessment report of the intergovernmental panel on climatic change (IPCC). Cambridge University Press.
- IPCC. (2007). Climate change 2007: The physical basis summary for policy makers. Cambridge.
- IPCC. (2014). Impacts, adaptation, and vulnerability. Part A: Global and sectorial aspects. contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press.
- Jedmowski, C., Ashoub, A., & Bru¨ggemann, W. (2013). Reactions of Egyptian landraces of Hordeumvulgare and Sorghum bicolor to drought stress, evaluated by the OJIP fluorescence transient analysis. Acta Physiologiae Plantarum, 35(2), 345–354. https://doi.org/10.1007/s11738-012-1077-9
- Jenkins, G. I. (2009). Signal transduction in responses to UV-B radiation. Annual Review of Plant Biology, 1(60), 407–431. https://doi.org/10.1146/annurev.arplant.59.032607.092953
- Johnson, M. P., Goral, T. K., Duffy, C. D., Brain, A. P., Mullineaux, C. W., & Ruban, A. V. (2011). Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. The Plant Cell, 4(23), 1468–1479. https://doi.org/10.1105/tpc.110.081646 Epub 2011 Apr 15
- Kalaji, H. M., Schansker, G., Brestic, N., Bussotti, F., Calatayud, A., & Ferroni, L. (2017). Frequently asked questions about chlorophyll fluorescence. Photosynthesis Research, 1(132), 13–66. https://doi.org/10.1007/s11120-016-0318-y
- Kangasjarvi, S., Neukermans, J., Li, S., Aro, E. M., & Noctor, G. (2012). Photosynthesis, photorespiration and light signalling in defence responses. Journal of Experimental Botany, 4(63), 1619–1636. https://doi.org/10.1093/jxb/err402 Epub 2012 Jan 25
- Kannan, N. D., & Kulandaivelu, G. (2011). Drought induced changes in physiological, biochemical and phytochemical properties of Withania somnifera Dun. Journal of Medicinal Plants Research, 5(16), 3929–3935.
- Krause, G. H. (1988). Photoinhibtion of photosynthesis. An evaluation of damaging and protective mechanisms. Physiologia Plantarum, 3(74), 566–574. https://doi.org/10.1111/j.1399-3054.1988.tb02020.x
- Krause, G. H., Somersalo, S., Zumbush, E., Weyers, B., & Laasch, H. (1990). On The Mechanism of photoinhibition in chloroplast. Relationship between changes in fluorescence and activity of photosystem 2. Journal of Plant Physiology, 4(136), 472–479. https://doi.org/10.1016/S0176-1617(11)80038-6
- Krause, G. H., & Weis, E. (1991). Chlorophyll fluorescence and photosynthesis: The basics. Annual Review of Plant Biology and Annual Review of Plant Physiology, 1(42), 313–349. https://doi.org/10.1146/annurev.pp.42.060191.001525
- Kulandaivelu, G., Neduchezhian, N., & Annamalainathan, K. (1991). Ultraviolet-B (280– 320 nm) radiation induced changes in photochemical activities and polypeptide components of C3 and C4 chloroplasts. Photosynthetica, 2(25), 333–339.
- Lauriano, J. A., Ramalho, J. C., Lidon, F. C., & Ce´umatos, M. (2006). Mechanisms of energy dissipation in peanut under water stress. Photosynthetica, 44(3), 404–410. https://doi.org/10.1007/s11099-006-0043-4
- Lawlor, D. W. (2002). Limitation to photosynthesis in water stressed leaves: Stomata vs. metabolism and the role of ATP. Annals of Botany, 7(89), 871–885. https://doi.org/10.1093/aob/mcf110
- Lawlor, D. W., & Tezara, W. (2009). Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Annals of Botany, 4(103), 561–579. https://doi.org/10.1093/aob/mcn244
- Laza´r, D. (2006). The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Functional Plant Biology : FPB, 33(1), 9–30. https://doi.org/10.1071/FP05095
- Lidon, F. C. (2012). UV-B irradiation in rice: Interaction between micronutrients accumulation and the photosynthetic performance. Journal of Plant Interactions, 7(1), 19–28. https://doi.org/10.1080/17429145.2011.574214
- Lidon, F. C., & Ramalho, J. C. (2011). Impact of UV-B irradiation on photosynthetic performance and chloroplast membrane components in Oryza sativa L. Journal of Photochemistry and Photobiology B: Biology, 3(104), 457–466. https://doi.org/10.1016/j.jphotobiol.2011.05.004 Epub 2011 May 30
- Lidon, F. C., Teixeira, M., & Ramalho, J. C. (2012). Decay of the chloroplast pool of as corbate switches on the oxidative burst in UV-B irradiated rice. Journal of Agronomy and Crop Science, 2(198), 130–144. https://doi.org/10.1111/j.1439037X.2011.00489.x
- Liu, N., Ko, S., Yeh, K. C., & Charng, Y. (2006). Isolation and characterization of tomato Hsa32 encoding a novel heat shock protein. Plant Science : An International Journal of Experimental Plant Biology, 5(170), 976–985. https://doi.org/10.1016/j.plantsci.2006.01.008
- Logan, B. A., Demmig-Adams, B., Adams, W. W., & Bilger, W. (2014). Context quantification and measurement guide for non-photochemical quenching of Chlorophyll fluorescence. In B. Demmig Adams, G. Garab, W. W. Adams, & Govindjee (Eds.), Non-photochemical quenching and energy dissipation in plants, algae and cynobacteria (pp. 187–201). Springer.
- Loka, D. A., & Oosterhuis, D. M. (2010). Effect of high night temperatures on cotton respiration. ATP levels, and carbohydrate content. Environmental and Experimental Botany, 3(68), 258–263. https://doi.org/10.1016/j.envexpbot.2010.01.006
- Lu, C., & Zhang, J. (1999). Effects of waters stress on Photosystem II photochemistry and its thermostability in wheat plants. Journal of Experimental Botany, 336(50), 1199–1206. https://doi.org/10.1093/jxb/50.336.1199
- Malnoë, M. (2018). Photoinhibtion or Photoprotection of photosynthesis? Update on the (newly termed) sustainable Quenching component tqH. Environmental and Experimental Botany, 1(154), 123–133. https://doi.org/10.1016/j.envexpbot.2018.05.005
- Malnoë, M., Schultink, A., Shahrasbi, S., Rumeau, D., Havaux, M., & Niyogi, K. K. (2017). The plastid Lipocalin LCN is required for sustained photo protective energy dissipation in Arabidopsis. The Plant Cell, 1(30), 96–208. https://doi.org/10.1105/tpc.17.00536
- Mathur, S., Agrawal, D., & Jajoo, A. (2014). Photosynthesis: Response to high temperature stress. Journal of Photochemistry and Photobiology B: Biology, 1(137), 116–126. https://doi.org/10.1016/j.jphotobiol.2014.01.010
- Mathur, S., Mehta, P., Jajoo, A., & Bharti, S. (2011). Analysis of elevated temperature induced inhibition of Photosystem II using Chlorophyll fluorescence induction kinetics. Plant Biology, 13(1), 1–6. https://doi.org/10.1111/j.1438-8677.2009.00319.x
- Maxwell, K., & Johnson, G. N. (2000). Chlorophyll fluorescence a practical guide. Journal of Experimental Botany, 345(51), 659–668. https://doi.org/10.1093/jexbot/51.345.659
- McKenzie, R. L., Björn, L. O., Bais, A., & Ilyasd, M. (2003). Changes in biologically active ultraviolet radiation reaching the Earth’s Surface. Photochemical & Photobiological Sciences, 1–3(46), 5–15. https://doi.org/10.1016/S1011-1344(98)00182-1
- Melis, A. (1999). Photosystem II damage and repair cycle in modulates the rate of photo damage in vivo? Trends in Plant Science, 4(4), 130–135. https://doi.org/10.1016/s1360-1385(99)01387-4
- Misson, L., Limousin, J. M., Rodriguez, R., & Letts, M. G. (2010). Leaf physiological responses to extreme droughts in Mediterranean Quercus ilex forest. Plant, Cell & Environment, 11(33), 1898–1910. https://doi.org/10.1111/j.1365-3040.2010.02193.x.
- Mohammed, A. R., & Tarpley, L. (2009). High night time temperatures affect rice productivity through altered pollen germination and spikelet fertility. Agricultural and Forest Meteorology, 6(149), 999–1008. https://doi.org/10.1016/j.agrformet.2008.12.003
- Murata, N., Allakhverdiev, S. I., & Nishiyama, Y. (2012). The mechanism of photoinhibition in vivo: Re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport. Biochimica et biophysica acta, 8(1817), 1127–1133. https://doi.org/10.1016/j.bbabio.2012.02.020
- Murata, N., Takahashi, S., Nishiyama, S., & Allakhverdiev, S. I. (2007). Photoinhibition of photosystem II under environmental stress. Biochimica et biophysica acta, 6(1767), 414–421. https://doi.org/10.1016/j.bbabio.2006.11.019
- Murchie, E. H., & Lawson, T. (2013). Chlorophyll fluorescence analysis: A guide to good practice and understandings new applications. Journal of Experimental Botany, 13(64), 3983–3998. https://doi.org/10.1093/jxb/ert208
- Mwanamwenge, J., Loss, S. P., Siddique, K. H. M., & Cocks, P. S. (1999). Effect of water stress during floral initiation, flowering and podding on the growth and yield of faba bean (Vicia faba L.). European Journal of Agronomy, 1(11), 1–11. https://doi.org/10.1016/S1161-0301(99)00003-9
- Nellaepalli, S., Zsiros, O., Toth, T., Yadavalli, V., Garab, G., Subramanyam, R., & Kovacs, L. (2014). Heat and light induced detachment of the light harvesting complex from isolated photosystemI supercomplexes. Journal of Photochemistry and Photobiology B, 1(137), 13–20. https://doi.org/10.1016/j.jphotobiol.2014.04.026 Epub 2014 May 6
- Nilkens, M., Kress, E., Lambrev, P., Miloslavina, Y., Muller, M., & Holzwarth, A. R. (2010). Identification of a slowly inducible Zeaxnthin component of non-photochemical quenching of chlorophyll fluorescence generated under steady state conditions. Arabidopsis. Biochem. Biophys. Acta, 4(1797), 466–475. https://doi.org/10.1016/j.bbabio.2010.01.001
- Nishiyama, Y., & Murata, N. (2014). Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Applied Microbiology and Biotechnology, 1(98), 8777–8796. https://doi.org/10.1007/s00253-014-6020-0
- Nishiyama, Y., Yamamoto, H., Allakhverdiev, S. I., Inaba, M., Yokota, A., & Murata, N. (2001). Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. The EMBO Journal, 2001(20), 5587–5594. https://doi.org/10.1093/emboj/20.20.5587
- Noctor, G., Ruban, A. V., & Horton, P. (1993). Modulation of 1PH dependent photochemical quenching of chlorophyll fluorescence in spinach chloroplasts. Biochimica et Biophysica Acta, 2-7(1183), 339–344. https://doi.org/10.1016/0005-2728(93)90237-A
- Nogués, S., & Baker, N. R. (2000). Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. Journal of Experimental Botany, 348(51), 1309–1317. https://doi.org/10.1093/jxb/51.348.1309
- Ohnishi, N., Allakhverdiev, S. I., Takahashi, S., Higashi, S., Watanabe, M., & Nishiyama, Y. (2005). Two-step mechanism of photodamage to Photosystem ǁ: Step 1 occurs at the oxygen evolving complex and step 2 occurs at the photochemical reaction center. Biochem, 23(44), 8494–8499. https://doi.org/10.1021/bi047518q
- Ohnishi, N., & Murata, N. (2006). Glycinebetaine counteracts the inhibitory effects of salt stress on the degradation and synthesis of D1 protein during photoinhibition in Synechococcus sp. PCC 7942. Plant Physiology, 1(141), 758–765. https://doi.org/10.1104/pp.106.076976
- Öquist, G., Chow, W. S., & Anderson, J. M. (1992). Photoinhibtion of photosynthesis represents mechanisms for the long term regulation of photosystem II. Planta, 1(186), 450–460. https://doi.org/10.1007/BF00195327
- Oukarroum, A., El Madidi, S., & Strasser, R. J. (2012). Exogenous glycine betaine and proline play a protective role in heat- stressed barley leaves (Hordeum vulgare L.): A chlorophyll a fluorescence study. Plant biosystems, 4(146), 1037–1043. https://doi.org/10.1080/11263504.2012.697493
- Oukarroum, A., Madidi, S. E., Schansker, G., & Strasser, R. J. (2007). Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environmental and Experimental Botany, 3(60), 438–446. https://doi.org/10.1016/j.envexpbot.2007.01.002
- Pal, M., Sengupta, U. K., Srivastava, A. C., Jain, V., & Meena, R. C. (1999). Changes in growth and photosynthesis of mung bean induced by UV-B radiation. Indian Journal of Plant Physiology, 2(4), 79–84.
- Papageorgiou, G. C., Tsimilli-Michael, M., & Stamatakis, K. (2007). The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: A viewpoint. Photosynthetic Research, 1(94), 275–290. https://doi.org/10.1007/s11120-007-9193-x
- Park, Y., Chow, W. S., Osmond, C. B., & Anderson, J. M. (1996). Electron transport to oxygen mitigates against the photoinactivation of photosystem II in vivo. Photosynthesis Research, 1(50), 23–32. https://doi.org/10.1007/BF00018218
- Peng, S., Huang, J., Sheehy, J. E., Laza, R. C., Visperas, R. M., Zhong, X., Centeno, G. S., Khush, G. S., & Cassman, K. G. (2004). Rice yields decline with higher night temperature from global warming. PNAS, 101(27), 9971–9975. https://doi.org/10.1073/pnas.0403720101
- Pradhan, G. P., Prasad, P. V., Fritz, A. K., Kirkham, M. B., & Gill, B. S. (2012). Effects of drought and high temperature stress on synthetic hexaploid wheat. Functional Plant Biology : FPB, 3(39), 190–198. https://doi.org/10.1071/FP11245
- Prasad, P. V., & Djanaguiraman, M. (2011). High night temperature decreases leaf photosynthesis and pollen function in grain sorghum. Functional Plant Biology, 12(38), 993–1003. https://doi.org/10.1071/FP11035
- Prasad, P. V., Pisipati, S. R., Ristic, Z., Bukovnik, U., & Fritz, A. K. (2008). Impact of night time temperature on physiology and growth of spring wheat. Crop Science, 23(48), 72–80. https://doi.org/10.2135/cropsci2007.12.0717
- Prochazkova, D. R., Sairam, K., Srivastava, G. C., & Singh, D. V. (2001). Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Science : An International Journal of Experimental Plant Biology, 4(161), 765–771. https://doi.org/10.1016/S0168-9452(01)00462-9
- Ranjan, S., Singh, R., Singh, M., Pathre, U. V., & Shirke, P. A. (2014). Characterizing photoinhibition and photosynthesis in juvenile-red versus mature-green leaves of Jatropha curcas L. Plant Physiology and Biochemistry, 1(79), 48–59. https://doi.org/10.1016/j.plaphy.2014.03.007 Epub 2014 Mar 13
- Reddy, K. R., Kakani, V. G., Zhao, D., Mohammed, A. R., & Gao, W. (2003). Cotton responses to ultraviolet -B radiation: Experimentation and algorithm development. Journal of Agricultural and Forest Meteorology, 120(1–4), 249–265. https://doi.org/10.1016/j.agrformet.2003.08.029
- Reicosky, D. C., Hatfield, J. L., & Sass, R. L. (2000). Agricultural contributions to greenhouse gas emissions. In K. R. Reddy & H. F. Hodges (Eds.), Climatic Change and Global Crop Productivity (pp. 37–50). CAB International.
- Ristic, Z. U. B., & Prasad, P. V. V. (2007). Correlation between heat stability of thylakoid membranes and loss of Loss of Chlorophyll in Winter Wheat under Heat Stress. Crop Science, 1(47), 2067–2073. https://doi.org/10.2135/cropsci2006.10.0674
- Rochaix, J. D. (2011). Regulation of photosynthetic electron transport. Biochimica et biophysica acta, 3(1807), 375–383. https://doi.org/10.1016/j.bbabio.2010.11.010 Epub 2010 Nov 29
- Ruban, A. V. (2016). Non-photochemical chlorophyll fluorescence quenching. Physiol, 1(170), 1903–1916. https://doi.org/10.1104/pp.15.01935
- Ruban, A. V., Johnson, M. P., & Duffy, C. D. P. (2012). The photoprotective molecular switch in the photosystem II antenna. Biochimica et Biophysica Acta, 1(1817), 167–181. https://doi.org/10.1016/j.bbabio.2011.04.007506285514732000
- Sairam, R. K., Srivastava, G. C., & Saxena, D. C. (2000). Increased antioxidant activity under elevated temperatures: A mechanism of heat stress tolerance in wheat genotypes. Plant Biology, 1(43), 245–251. https://doi.org/10.1023/A:1002756311146
- Sao, P. M. D. F., Ribeiro, R. V., da Silveira, J. A., Magalhaes Filho, J. R., & Machado, E. C. (2013). Rootstocks induce con trasting photosynthetic responses of orange plants to low night temperature without affecting the antioxidant metabolism. Theoretical and Experimental Plant Physiology, 1(25), 26–35. https://doi.org/10.1590/S2197-00252013000100004
- Savitch, L. V., Krol, P. T., Wilson, M., Greenberg, K. E., & Huner, N. P. A. (2001). Effects of growth under UV- A radiation on CO2 assimilation, carbon partitioning, PSII photochemistry and resistance to UV -B radiation in Brassica napus cv Topas. Australian Journal of Plant Physiology, 3(28), 203–212. https://doi.org/10.1071/PP00116
- Schansker, G., Toth, S. Z., Holzwarth, A. R., & Garab, G. (2014). Chlorophyll a fluorescence: Beyond the limits of the QA model. Photosynthetic Research, 1-2(120), 43–58. https://doi.org/10.1007/s11120-013-9806-5 Epub 2013 Mar 1
- Schrader, S. M., Wise, R. R., Wacholtz, W. F., Ort, D. R., & Sharkey, T. D. (2004). Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant, Cell & Environment, 27(2004), 725–735. https://doi.org/10.1111/j.1365-3040.2004.01172.x
- Schreiber, U., Hormann, H., Neubaur, C., & Klughammer. (1995). Assessment of photosystem quantum yield by chlorophyll fluorescence quenching analysis. Functional Plant Biology, 2(22), 209–220. https://doi.org/10.1071/PP9950209
- Sharkey, T. D., & Schrader, S. M. (2006). High temperature stress. In K. V. M. Rao, A. S. Raghavendra, & K. J. Reddy (Eds.), Physiology and Molecular Biology of Stress Tolerance in Plants (pp. 101–129). Springer.
- Simeneh, T. A., & Amsalu, G. R. (2020). Ultraviolet-B, end of day light and exclusion effect on photosynthetic efficiency of sweet potato (Ipomoea batatas L.) based on altitude. Journal of Horticulture and Postharvest Research, 3(SPECIAL ISSUE), 1–10. https://doi.10.22077/jhpr.2019.2882.1101
- Skotnica, J., Matouškova, M., Nauš, J., Lazár, D., & Dvořák, L. (2000). Thermoluminescence and fluorescence study of changes in Photosystem II photochemistry in desiccating barley leaves. Photosynthesis Research, 1(65), 29–40. https://doi.org/10.1023/A:1006472129684
- Sonoike, K. (2011). Photoinhibition of photosystem I. Physiologia Plantarum, 142(1), 56–64. https://doi.org/10.1111/j.1399-3054.2010.01437.x
- Srivastava, A., & Strasser, R. J. (1995). How do land plants respond to stress temperature and stress light? Arch Sci Geneve, 1(48), 135–146. https://doi.org/10.1186/1741-7007-9-79
- Stirbet, A., & Govindjee. (2011). On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. Journal of Photochemistry and Photobiology B, 1-2(104), 236–257. https://doi.org/10.1016/j.jphotobiol.2010.12.010 Epub 2011 Jan 4
- Stirbet, A., Lazár, D., Kromdijk, J., & Govindjee, G. (2018). Chlorophyll fluorescence induction: Can just a one second measurement be used to quantify a biotic stress response? Photosynthatica, 1(56), 86–104. https://doi.org/10.1007/s11099-018-0770-3
- Strasser, R. J., Srivastava, A., & Tsimilli-Michael., M. (2000). The fluorescence transient as a tool to characterize and screen photosynthetic samples. In P. Mohanty & P. Yunus (Eds.), Probing photosynthesis: Mechanism, regulation and adaptation (pp. 443–480). Taylor and Francis.
- Strasser, R. J., & Stirbet, A. D. (1998). Heterogeneity of photosystem II probed by the numerically simulated chlorophyll a fluorescence rise (O–J–I–P). Mathematics and Computers in Simulation, 48(1), 3–9. https://doi.org/10.1016/S0378-4754(98)00150-5
- Strasser, R. J., Tsimilli-Michael, M., & Srivastava, A. (2004). Analysis of the chlorophyll a fluorescence transient. In G. Papageorgiou & Govindjee (Eds.), Advances in photosynthesis and respiration. Chlorophyll a fluorescence: A signature of photosynthesis (pp. 321–362). Springer.
- Takahashi, S., & Badger, M. R. (2011). Photoprotection in plants: A new light on photosystem II damage. Trends in Plant Science, 1(16), 53–60. https://doi.org/10.1016/j.tplants.2010.10.001 Epub 2010 Nov 1
- Tevini, M. (2004). Plant responses to ultraviolet radiation stress. In G. C. Papageorgiou & Govindjee (Eds.), Chlorophyll fluorescence a signature of photosynthesis (pp. 605–621). Springer.
- Tezara, W., Mitchell, V. J., Driscoll, S. D., & Lawlor, D. W. (1999). Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature, 1(401), 914–917. https://doi.org/10.1038/44842
- Tian, J., & Yu, J. (2009). Changes in Ultrastructure and responses of antioxidant systems of algae (Dunaliella salina) during acclimation to enhanced Ultraviolet-B radiation. Journal of Photochemistry and Photobiology A, 3(97), 152–160. https://doi.org/10.1016/j.jphotobiol.2009.09.003
- Tikkanen, M., & Aro, E. M. (2014). Photosystem II photoinhibition repair cycle protects photosystem I from irreversible damage. Biochimica et Biophysica Acta (BBA), 1(1837), 210–215. https://doi.org/10.1016/j.bbabio.2013.10.001 Epub 2013 Oct 23
- Turnbull, M. H., Murthy, R., & Griffin, K. L. (2002). The relative impacts of daytime and nighttime warming on photosynthetic capacity in Populus detoides. Plant, Cell & Environment, 12(25), 1729–1737. https://doi.org/10.1046/j.1365-3040.2002.00947.x
- Valikhanov, K. M., Zakhidov, E. A., Zakhidova, M. A., Kasymdzhanov, M. A., Kurbanov, S. S., Nematov, S. K., & Khabibullaev, P. K. (2002). Kinetics of photoinhibition and delayed fluorescence in the plant photosynthetic system. Doklady Biochemistry and Biophysics, 1-6(387), 331–334. https://doi.org/10.1023/A:1021700432183
- Wang, W., Vinocur, B., & Altman, A. (2003). Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta, 1(218), 1–14. https://doi.org/10.1007/s00425-003-1105-5
- Yang, J., Sears, R. G., Gill, B. S., & Paulsen, G. M. (2002). Genotypic differences in utilization of assimilate sources during maturation of wheat under chronic heat and heat shock stresses. Euphytica, 1(125), 179–188. https://doi.org/10.1023/A:1015882825112
- Yang, J., Zhang, J., Wang, Z., Zhu, Q., & Liu, L. (2001). Water deficit induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agronomy Journal, 1(93), 196–206. https://doi.org/10.2134/agronj2001.931196x
- Yemane, Y., Kashino, Y., Koike, H., & Satoh, K. (1997). Increases in the fluorescence Fo level and reversible inhibition of Photosystem II reaction center by high-temperature treatments in higher plants. Photosynthesis Research, 1(52), 57–64. https://doi.org/10.1023/A:1005884717655
- Yordanov, I., Velikova, V., & Tsonev, T. (2000). Plant responses to drought, acclimation, and stress tolerance. Photosynthetica, 1(38), 171–186. https://doi.org/10.1023/A:1007201411474
- Yordanov, I., Velikova, V., & Tsonev, T. (2003). Plant responses to drought and stress tolerance. Bulgarian Journal of Plant Physiology. ( Special issue), 187-206.
- Zhang, L., Hao, X. M., Li, Y. G., & Jiang, G. M. (2010). Response of greenhouse tomato to varied low pre-night temperatures at the same daily integrated temperature. Hort Science, 11(45), 1654–1661. https://doi.org/10.21273/HORTSCI.45.11.1654
- Zhang, L. R., Xing, D., Wang, J. S., Zeng, L. Z., & Li, Q. (2007). Light induced delayed fluorescence as an indicator for UV –B radiation environment stress on plants. Journal of Optoelectronics·Laser, 7(18), 878–881.
- Zhao, D., Reddy, K. R., Kakani, V. G., Read, J., & Sullivan, J. (2003). Growth and physiological responses of cotton (Gossypium hirsutum L.) to elevated carbon dioxide and ultraviolet-B radiation under controlled environment conditions. Plant Cell & Environment, 5(26), 771–782. https://doi.org/10.1046/j.1365-3040.2003.01019.x
- Zivcak, M., Brestic, M., Balatova, Z., Drevenakova, P., Olsovska, K., Kalaji, M. H., & Allakhverdiev, S. I. (2013). Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynthesis Research, 1-3(117), 529–546. https://doi.org/10.1007/s11120-013-9885-3 Epub 2013 Jul 17
- Zivcak, M., Brestic, M., Olsovska, K., & Slamka, P. (2008). Performance index as a sensitive indicator of water stress in Triticum aestivum. Plant Soil & Environment, 4(54), 133–139. https://doi.org/10.17221/392-PSE
- Zlatev, Z. (2009). Drought-induced changes in chlorophyll fluorescence of young wheat plant. Biotechnol, 2009(23), 437–441. https://doi.org/10.1080/13102818.2009.10818458
- Zlatev, Z., & Yordanov, I. (2004). Effects of soil drought on photosynthesis and chlorophyll fluorescence in common bean plants. Bulgarian Journal of Plant Physiology, 3-4(30), 3–18.

