Abstract
The study evaluates the toxic effects of acute and sub-acute oral administration of methanol extracts of Geophila obvallata in rats. During acute study, a dose of 1600, 2900 and 5000 mg/kg bw of extract was orally administered to rats. Rats were observed for signs of toxicity for two weeks. During sub-acute study (28 days), the extract, at doses of 100, 500 and 1000 mg/kg bw were administered orally to rats while control rats were given only tap water. At the end of the study, samples were collected for analyses. In acute toxicity studies, the extract did not induce death after single dose administration. Hence, the LD50 was estimated above 5000 mg/kg. The results of sub-acute toxicity study show that no significant changes were observed in the body weights, organ weights, kidney function and organ histology. There were significant changes in hematology and biochemical indices investigated at elevated doses of 500 and 1000 mg/kg bw compared to the control. GOE may be considered non-toxic at a dose of 100 mg/kg with promising applications in drug therapy.
PUBLIC INTEREST STATEMENT
The study investigated the toxic effects of short and long-term consumption of Geophila obvallata methanol leaf extract on kidney and liver markers in rats. During the short term study, high doses ranging from 1600-5000 mg/kg bw of extract were orally administered to rats, following Lorkes method. Rats were observed for signs of toxicity for two weeks. During the long term study (28 days), the extract, at doses of 100, 500 and 1000 mg/kg bw were administered orally to rats while control rats were given only tap water. At the end of the study, samples were analyzed. The results of the study showed that short and long term consumption of the extract showed no pronounced toxicity with various histopathological and biochemical studies, albeit a mild biochemical response. It concludes the extract is safe for pharmacological use.
1. Introduction
Globally, there is an increase in the rate of herbal formulations consumption (Shri, Citation2003) because of the belief that they are organic, harmless and effective in the treatment of diseases (Arya et al., Citation2012). Developing countries in Africa mostly use herbal formulations as alternative treatment for various illnesses due to perceived disparities in conventional medicine (Pushpa et al., Citation2010; Zhu et al., Citation2002). According to Fabricant and Farnsworth (Citation2001), herbal formulations are safer and less damaging to biological systems with fewer side effects when compared with synthetic drugs. This is as a result of the presence of bioactive constituents translating into low animal and human toxicity. The World Health Organization recommends that complementary medicine should be adopted by member states in developing proactive policies that will strengthen the use of medicinal plants in keeping populations healthy (WHO, Citation2010).
One of the many medicinal plants used in Nigerian folkloric medicine is Geophila obvallata commonly known as“avbovbotor” and “ekoro” by the Edo and Yoruba tribes of Nigeria, respectively (Burkill, Citation1985). Its taxonomic classification includes; kingdom plantae, order Gentianales, family Rubiaceae and genus Geophila (Robbrecht & Manen, Citation2006). It is an edible rainforest plant that grows extensively in the tropical rain forest floors especially the Gelegele forests, Okomu oil palm reserves and Rubber Research Institute Iyanomo, located in Edo State, Nigeria (Obembe, Citation2015). This herb has been used by the rural natives of Edo state as a decoction in the treatment of abdominal troubles, headache, hypertension, tooth ache, jaundice, diabetes, stroke and cardiovascular diseases (Burkill, Citation1985). Aqueous and methanol leaf extracts of the plant were reported to possess antioxidant qualities as a result of its bioactive components (Iserhienrhien & Okolie, Citation2018). However, there has been no scientific evaluation of the toxicological implications on biological systems on short or long-term basis. This study therefore aims to assess the acute and sub-acute toxicity effects of GOE on some renal and hepatic indices in Wistar rats.
2. Materials and methods
2.1. Chemicals
The chemicals were purchased via a local vendor from Randox Ltd (USA) with a high quality.
2.2. Collection of plants and preparation of the extract
The fresh leaves of G. obvallata (GO) were collected by following leads supplied by a local healer at Ugbowo Quarters, Benin City, Nigeria. They were confirmed by Dr. Akinigboso (taxonomist), at the life science Department, University of Benin, Benin City and voucher number UBHa 0312 was assigned to it. GO was then deposited at the Plant Biology and Biotechnology herbarium, University of Benin, Benin City for future references.
A method modified by Agbai et al. (Citation2014) was adopted. Fresh leaves were washed and air-dried for seven days. Air-dried leaves were blended by a grinding machine (hammer type) (Meecan, CM/L-2264458, UK) until a smooth texture was obtained, and was later weighed and packaged. About 87.52 g of the blended leaves were extracted in the Soxhlet extractor using methanol (70%) (1:10 w/v) (Aiyelaagbe & Osamudiamen, Citation2009) prior to homogenization and continuous agitation for two days. Whatman’s paper (No. 1) was used to filter the homogenate and the filtrate was concentrated to aridness at 40°C (Edeoga et al., Citation2005) within 24 hrs to obtain about 46.20 g of methanol extract, and then, dried over anhydrous CuSO4 in a dessicator. The dried residues were stored in airtight containers at 4°C before laboratory experiments.
2.3. Experimental animals
Rats used in this experiment were obtained from the Department of Biochemistry animal house, University of Benin, Benin city. They were caged in a hygienic, conducive habitat with proper lighting. The rats weighed between 130 and 200 g. The rats were fed orally with rat pelleted feed (Agro feeds, Nigeria), they had access to dirt-free drinking water and they were housed in steel cages, in compliance with the National Research Council guidelines for the care and use of laboratory animals (National Research Council, Citation2011). Ethical principles regulating the use of living animals for research were strictly adhered to as adopted by Ward & Elsea (Citation1997). The research procedures for animal handling were endorsed by the ethical committee of the University of Benin, Benin City.
2.4. Acute toxicity study
A slight modification of Lorke’s method (Lorke, Citation1983) was employed in this study. Mixed genders of 20 Wistar rats were chosen and organized into four sets of five rats per set. The control rats were given tap water (10 ml/kg/body weight) while the other three sets were orally administered with a single dose of GOE at 1600, 2900 and 5000 mg/kg body weight. Observation for signs of toxicity was carried out 1, 2 and 4 hr after treatment and periodically during the first 24 hr, then, daily for two weeks following treatment. Changes in the skin, eyes and mucus membrane, body weight and behavioural patterns were noted during the test period (OECD 407, Citation2001).
2.5. Sub-acute toxicity
This investigation was completed in 28 days according to the OECD guidelines 407 (OECD 407, Citation2008). Experimental animals were divided into four sets of five rats per set of mixed sexes, both sexes were placed in separate cages to prevent mating. Set 1 served as control (i.e. the rats were fed without extract), while the other sets were daily fed by oral administration of GOE at different doses (100, 500, 1000 mg/kg) for 28 days.
On day 28, the rats were anaesthetized using ether after fasting for the night while blood samples were taken for biochemical and hematological analyses using both EDTA and plain vials while the kidney and liver were harvested for histological assessment.
2.6. Relative organ and body weights study
The changes in body weights were recorded on a weekly basis, while the organs (the liver, kidneys, brain and heart) were weighed using standard weighing balance to calculate relative organ weight for the different sets on the sacrifice day.
Relative organ weight (%) = [Absolute weight of organ (g)/weight of rat on sacrifice day (g)] x100
2.7. Hematological analysis
The indices analysed in the blood samples included haematocrit (HCT), corpuscular volume (CV), erythrocyte count, lymphocytes (LYM), neutrophils (NEU), monocytes (MONO), thrombocyte count, basophils (BASO), leucocyte count (WBC) were performed by means of an automated analyzer (BiopacBS-1100i, Shanghai, China).
2.8. Serum biochemistry
Dry tubes were used to collect blood samples which were spun at 3000 rpm for 10 min at 5°C to get the serum isolates used for the following experiments.
2.9. Liver function tests
The activities of liver enzymes (ALT, AST, and ALP) and the concentrations of total bilirubin, albumin and total proteins were analyzed using specific commercial kits according to the manufacturer’s protocol (Alpha Laboratories UK, London).
2.10. Kidney function tests
The kidney function tests investigated included: serum creatinine and urea as well as serum electrolytes (HCO3−, Na+, K+, Cl−). They were determined using specific commercial kits according to the manufacturer’s protocol (Alpha Laboratories UK, London).
2.11. Lipid profile
Total cholesterol (TC), serum triacylglycerol (TAG) and other lipid profile indices were evaluated according to the protocols outlined by Tiez (Tatefuji et al., Citation2014).
2.12. In vivo antioxidant study of GOE
2.12.1. Malondialdehyde (MDA) determination
Lipid peroxidation level was evaluated using spectrophotometry (Draper & Hadley, Citation1990) by measuring, malondialdehyde (MDA) which interacts with thiobarbituric acid (TBA) to produce a coloured complex at 532 nm in an acidic medium.
2.12.2. Determination of super oxide dismutase (SOD)
The SOD assay was carried out according to Xin et al. (Citation1991). Adrenaline solution was formed by dissolving (5 mg) adrenaline in 10 ml of distilled water. Then, 0.10 ml of serum was agitated in potassium buffer at pH 7.8. Buffer was mixed with 0.3 ml of adrenaline solution which was then added to 0.2 ml of the extract inside a cuvette, agitated and read at 450 nm.
2.12.3. Estimation of catalase
The method of Aebi (Citation1983) was employed in estimating catalase activity. This is based on the ultraviolet absorption and decomposition of hydrogen peroxide (H2O2) by catalase over time. Absorbance is easily measured at 240 nm.
2.12.4. Determination of reduced glutathione (GSH)
The technique of Xifan et al. (Citation2015) was employed in GSH determination. It is based on the principle that GSH interacts with alloxan and O2 in alkaline medium at a wavelength (320 nm).
2.13. Histopathology
On the 28th day, the liver and kidneys excised from the sets administered with the extracts and the control groups were collected and weighed and quickly set in 10% neutral buffered formalin at pH 7.4 and developed for histological studies. Following fixation, tissues were cleansed in graded series of alcohol, washed in xylene, inserted into paraffin, segmented by a microtome (5-μm thin) and tainted with dye in glass slides. Segments were viewed by a standard microscope (at X 100 and X 400) magnification (Pieme et al., Citation2006).
2.14. Analysis of data
ANOVA (One- way) was used to analyze data and data are presented as mean ± SEM. ANOVA was followed by Dunnett’s multiple comparison test. A P < 0.05 was considered statistically significant. Statistical analysis of data was done by using Minitab 16.
3. Results
3.1. Acute toxicity analysis
No signs of lethality or morbidity were detected in the rats given different doses up to 5000 mg/kg of GOE for two weeks. Therefore, the median lethal dose (LD50) of GOE was higher than 5000 mg/kg.
3.2. Sub-acute oral toxicity study
Administration of GOE for 28 days continuously did not induce morphological changes or general behavioural changes in treated rats compared to the control group. No deaths were observed during the period.
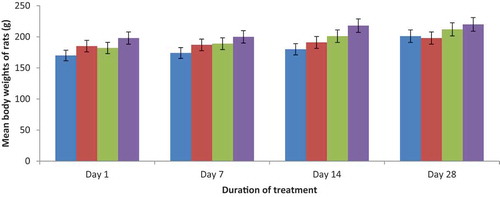
3.3. Body weights
The body weight alterations of rats given graded doses of GOE are indicated in Figure . Daily administration of GOE at different doses (100, 500 and 1000 mg/kg) did not result in significant changes in the body weight of GOE-fed rats when compared with the control.
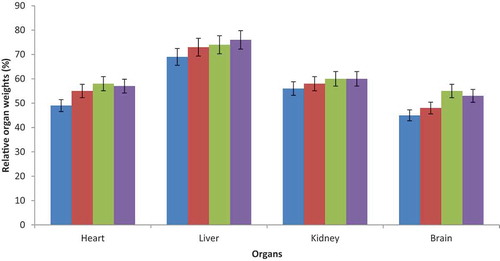
3.4. Relative organ weights
The weights of rats organs treated with GOE were non-significantly different from the control set (Figure ).
3.5. Biochemical analysis
3.5.1. MDA (mg/dl of wet tissue) activity
MDA (mg/dl of wet tissue) levels in both control and GOE-fed rats are indicated in Table . The results revealed significant increases (P < 0.05) in MDA levels in the liver, kidney and brain after sub-acute treatment with 500 and 1000 mg/kg bw doses of GOE for 28 days.
Table 1. Effect of sub-acute administration of Geophila obvallata extract on the activity of MDA (mg/dl of wet tissue)
3.5.2. Catalase activity
The catalase activity of both control and GOE-fed rats are indicated in Table . The results indicated no significant difference in catalase activity after sub-acute treatment with different doses of GOE for 28 days, when compared to control set.
Table 2. Effect of sub-acute administration of Geophila obvallata extract on the catalase activity (unit/mg of wet tissue)
3.5.3. Superoxide dismutase activity
Superoxide dismutase activities of GOE-treated and control rats are shown in Table . The results indicated significant increases (P < 0.05) in superoxide dismutase activity in the liver after sub-acute treatment with 500 and 1000 mg/kg bw of GOE for 28 days.
Table 3. Effect of sub-acute administration of Geophila obvallata extract on the superoxide dismutase activity (unit/mg of wet tissue)
3.6. GSH activity (mmol/GSH of wet tissue)
The reduced glutathione levels of GOE-treated rats and control rats are shown in Table . The results indicated no significant difference (p > 0.05) in reduced glutathione levels in the liver after sub-acute treatment with different doses of GOE for 28 days, when compared to control set.
Table 4. Effect of sub-acute administration of Geophila obvallata extract on the activity of GSH (mmol/GSH of wet tissue) of treated rats
3.7. Effects of GOE on hematological indices
The effects of sub-acute administration of GOE on haematological parameters are shown in Table . Daily administration of GOE for 28 days did not cause any significant difference in most of the hematological parameters when compared with the control group. However, there were significant decreases (p < 0.05) in hematocrit and haemoglobin (HB) concentration at 1000 mg/kg.
Table 5. Effect of sub-acute administration of Geophila obvallata extract on hematological profiles
3.8. Effects of GOE on liver indices
The effect of sub-acute administration of GOE on liver indices is presented in Table . A significant increase (P < 0.05) in ALP activity at 1000 mg/kg was observed while other liver markers showed normal levels.
Table 6. Effect of sub-acute administration of Geophila obvallata extract on liver indices
3.9. Effects of GOE on kidney function in rats
Sub-acute administration of GOE in the treated rats caused no significant difference (p > 0.05) in the kidney parameters (bicarbonates ion, creatinine, uric acid, sodium ion, potassium ion, urea and chloride ion levels) investigated (Table ).
Table 7. Effect of sub-acute administration of Geophila obvallata extract on kidney indices
3.10. Effects of GOE on lipid profiles in rats
Effects of sub-acute administration of GOE on the lipid profile of experimental rats are shown in Table . GOE treatment resulted in significant decreases (p˂0.05) in TC and TG concentrations at 100 mg/kg in treated rats. Alternately, GOE treatment at 100 and 500 mg/kg both resulted in elevated HDL levels in GOE-treated rats when compared to the control. LDL and VLDL levels in GOE treated rats were similar to the control set at 100 and 500 mg/kg bw.
Table 8. Effect of sub-acute administration of Geophila obvallata extract on the activity on lipid profiles in rats
3.11. Histopathology analysis
Histology results of assessment of GOE effects on the liver after 28 days of administration is shown in Figure -d). The microscopic examination revealed no significant pathological alterations in the liver for all experimental groups. It revealed unambiguous, observable rows of normal liver cells resulting from (A) central veins (B) hepatic sinusoids after 28 days of extract administration.
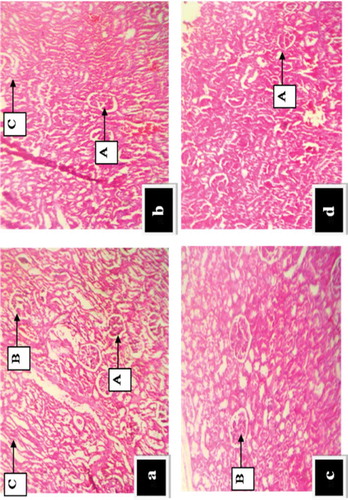
The histopathological effect of GOE on the kidney after 28 days of administration is revealed in Figure -d). The microscopic examination revealed no significant pathological alterations in the kidney for all experimental groups. It also revealed very clear and visible glomeruli as indicated by (A) renal tubules (B) renal corpuscles (C) medullary ray after 28 days of extract administration.
Figure 3. (a-d). Effect of Geophila obvallata on histology of the liver of rats after 28 days of extract administration (Haematoxylin and eosin staining; X 100). Key: a = Normal control showing normal hepatic histology; b = Administered with 100 mg/kg body weight of GOE, showing normal hepatic structure; c = Administered with 500 mg/kg body weight of GOE, showing normal hepatocytes; d = Administered with 1000 mg/kg body weight of GOE, showing abnormal hepatic structure (A) central veins (B) hepatic sinusoids.
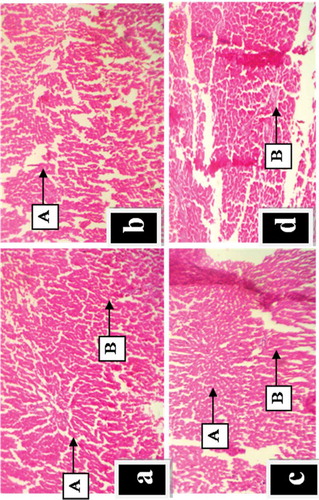
Figure 4. (a-d): Effect of G. obvallata on histology of the kidney of rats after 30 days of extract administration (Haematoxylin and eosin staining; X 100) Key: a = Normal control showing intact renal tubules, corpuscles and medullary rays; b = Administered with 100 mg/kg body weight of GOE, showing normal renal architecture and medullary rays; c = Administered with 500 mg/kg body weight of GOE, showing normal renal corpuscles; d = Administered with 1000 mg/kg body weight of GOE, showing abnormal renal architecture (A) Renal tubules (B) Renal corpuscles (C) Medullary rays.
4. Discussion
Information regarding the toxic effects of Geophila obvallata methanol extract in health care does not exist in previous research archives. To guarantee the quality of GOE for human consumption, a methodical toxicity assessment was needed to estimate the dangers of toxicity and to provide a basis for safe dose selection and scientific data in humans.
The acute toxicity study revealed that there were no signs of morbidity or death after two weeks of treatment. The rats were able to tolerate higher doses of GOE. Therefore, the LD50 of GOE is above 5000 mg/kg body weight when taken orally. Consequently, GOE should be considered a class 5 drug based on the OECD 423 guidelines (OECD 407, Citation2001) adopted worldwide synchronized categorization system (GSH) for chemical materials and concoctions.
In summary, it can be said that oral treatment with GOE caused no striking negative effects on the body weights and relative organ weights of the fed set in the sub-acute assessment. This is in agreement with Olorunnisola et al. (Citation2012) who reported that 28 day oral feeding of Wistar rats with graded doses of Tulbaghia violacea rhizomes had zero negative consequences to the organs of the extract-fed sets.
The bone marrow is a major location for novel blood cell manufacture and a vulnerable tissue targeted by toxic compounds in the hematopoietic system (Kifayatullah et al., Citation2015). In this study, there was a slight decrease in hematocrit and haemoglobin (HB) concentrations at 1000 mg/kg when compared with the control groups. However, these alterations were considered minor and toxicologically insignificant. This implies that GOE has no lethal implication on the hematopoietic system.
The liver biomarkers are specific tools in examining liver toxicity during drug biotransformation (Mukinda & Syce, Citation2007). The assessment of liver and kidney functions in this research revealed that GOE consumption at graded doses, had no effects on the ALT and AST liver indices, although, ALP levels increased significantly (P < 0.05) at 1000 mg/kg as an indication of biliary duct obstruction or cholestatic disease at higher doses (Burtis & Ashwood, Citation2001).
The notable potential of the liver to rejuvenate its cells makes it exceptional in overcoming various forms of necrosis and perturbations (Roberts et al., Citation2003). An increase in ALP was observed implying that hepato-biliary damage can be an effect of GOE consumption at higher doses thereby causing destruction of the liver cells. Illnesses like cholestasis of the liver and biliary cirrhosis are associated with elevated liver indices (Tietz, Citation1976).
Kidney disease can be detected by measurements of kidney indices like creatinine, uric acid, urea, bicarbonates, potassium, sodium, and chlorides and their normal levels reflect a reduced likelihood of renal problems (Dalle et al., Citation2006). In the present study, no significant alterations in plasma creatinine, uric acid, urea, bicarbonates, potassium, sodium, and chlorides levels in Geophila obvallata extract fed rats when compared to the control was observed. This indicates that the functional integrity of the kidney was not compromised after treatment with graded doses of the extract.
GOE effects on lipid peroxidation were evaluated by measuring malondialdehyde (MDA) levels, GSH levels, and SOD and catalase enzyme activities. Reduction in catalase, GSH, SOD activities and increases in MDA levels connotes an elevation in oxidative stress in biological entities thereby interfering with the system’s antioxidant defence mechanisms (Pajero et al., Citation2002). However, in this study, GOE administration at 500 and 1000 mg/kg bw significantly increased (p˂0.05) the MDA and SOD levels, especially in the liver of fed sets in comparison to the control. Also, GSH and catalase levels remained within the normal ranges. This suggests that G. obvallata methanol extract possesses beneficial properties due to its content of phytochemicals, in boosting the body’s defence.
Preliminary phytochemical analysis carried out on Geophila obvallata crude extract (GOE) revealed that, the methanol extract had the highest concentration of total flavonoid and phenolic compounds as well as the best antioxidant activity via DPPH, ABTS and hydroxyl scavenging assays compared to its aqueous solvent. (Iserhienrhien & Okolie, Citation2018).
GOE oral administration significantly increased (p˂0.05) total cholesterol (TC), serum triglyceride (TG) and LDL levels while HDL levels significantly decreased at elevated doses of 500 and 1000 mg/kg bw. This is in agreement with Moller’s (Citation2001) research which recommended that GOE administration may prove effective in the management of cardiovascular ailments, diabetes as well as deregulated blood pressure. This information is particularly relevant to the trado-medical practitioners who prepare the leaves of this plant in a decoction for its anti-hypertensive and cardiovascular potentials.
Histological observations of kidney and liver sections from the experimental animals demonstrated no significant pathological conditions in the test group as the liver and kidney tissues of the test groups were consistent with the normal histology of the control.
5. Conclusions
Oral doses of G. obvallata leaf extracts can be considered non-toxic especially at 100 mg/kg, as the extract did not elicit lethality in the acute and sub-acute toxicity studies in rats. The findings of this study give credence to the application of G. obvallata in folkloric traditional medicine. However, further pre-clinical assessments should be carried out to validate its effectiveness and long-term toxicological safety.
Competing interests
The authors declare that they have no competing interests.
Conflicts of interest
The authors have declared that there is no conflict of interest.
Acknowledgements
The author(s) hereby assert that no support was received from any sources for the publication or research of this article.
Additional information
Funding
Notes on contributors
Osafanme Lucky Iserhienrhien
Osafanme Lucky Iserhienrhien completed his First Degree in Biochemistry, Masters Degree in Environmental toxicology and Plant Biochemistry, and his Ph.D. in Environmental toxicology both from the University of Benin, Nigeria. He has been teaching at the Nigeria Maritime University, Okerenkoko for the last 3 years. His area of specialty is in Pharmacological studies, toxicology and Phytoremediation. The Author’s current project focuses on the identification, isolation and characterization of bio-compounds with potent antioxidant properties in the treatment of diseases related to oxidative damage.
References
- Aebi, H.E. (1983). Catalase. In Bergmeyer, H.U., Ed., Methods of Enzymatic Analysis, Verlag Chemie, Weinhem, 273-286..
- Agbai, E. O., Nwafor, A., & Ugwu, F. N. (2014). The hematological action of aqueous extracts of gongronema latifolium and ocimum gratissimum in alloxan induced diabetic rats. IJAPBC, 3(2), 235–13. https://doi.org/10.4103/2229-516X.179016
- Aiyelaagbe, O. O., & Osamudiamen, P. M. (2009). Phytochemical screening for active compounds in Mangifera indica leaves from Ibadan, Oyo State. Plant Science and Research, 2(1), 11–13. https://medwelljournals.com/abstract/?doi=psres.2009.11.13
- Arya, A., Mahmood, A. A., Batoul, S. H., & Mustafa, A. M. (2012). Screening for hypoglycemic activity on the leaf extracts of nine medicinal plants: In-vivo evaluation. E-Journal of Chemistry, 9(3), 1196–1205. https://doi.org/10.1155/2012/103760
- Burkill, H. M. (1985). The useful plants of West tropical Africa (2nd ed.). Royal Botanic Gardens, Kew.
- Burtis, C. A., & Ashwood, E. R. (2001). Enzymes: In tietz fundamentals of clinical chemistry (5th ed.). W.B. Saunders Company.
- Dalle, D. I., Rossi, R., Colombo, R., Giustarini, D., & Milzani, A. (2006). Biomarkers of oxidative damage in human disease. Clinical Chemistry, 52(4), 601–623. https://doi.org/10.1373/clinchem.2005.061408
- Draper, H. H., & Hadley, M. (1990). Malondiadehyde determination as index of lipid peroxidation. Methods in Enzymology, 86, 421–431. https://doi.org/10.1016/0076-6879(90)86135-I
- Edeoga, H. O., Okwu, D. E., & Mbaebie, B. O. (2005). Phytochemical constituents of some Nigerian medicinal plants. African Journal of Biotechnology, 4(7), 685–688. https://doi.org/10.5897/AJB2005.000-3127
- Fabricant, D. S., & Fansworth, N. R. (2001). The value of plants used in traditional medicine for drug discovery. Environmental Health Perspectives, 109(1), 69–76. https://doi.org/10.1016/0076-6879(90)86135-I
- Iserhienrhien, L. O., & Okolie, P. N. (2018). Phytochemical screening and in vitro antioxidant properties of methanol and aqueous leaf extracts of Geophila obvallata. AJRB, 3(2), 1–11. https://doi.org/10.9734/AJRB/2018/45052
- Kifayatullah, M., Mustafa, M. S., Senguptha, P., Sarker, M. M. R., Das, A., & Das, S. K. (2015). Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.)Merr inBALB/c mice. Journal of Acute Disease, 4(4), 309–315. https://doi.org/http://dx.doi.10.1016/j.joad.2015.06.010
- Lorke, D. (1983). A new approach to practical acute toxicity testing. Archives of Toxicology, 54(4), 275–289. https://doi.org/10.1007/BF01234480
- Moller, D. E. (2001). New drug targets for Type 2 diabetes and the metabolic syndrome: A review. Nature, 414(6865), 821–827. https://doi.org/10.1038/414821a
- Mukinda, J., & Syce, J. A. (2007). Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. Journal of Ethnopharmacology, 112(1), 138–144. https://doi.org/10.1016/j.jep.2007.02.011
- National Research Council. (2011). Guide for the care and use of laboratory animals. (8th. ed). The National Academies Press.
- Obembe, O. A. (2015). Studies on the stomata of some Rubiaceae. Academic Research International, 6(4), 2223–9553. https://doi.org/10.9734/arrb/2019/v32i530100
- OECD 407. (2001, December 17). Guidelines for the testing of chemicals. Acute oral toxicity -Fixed dose procedure. OECD/OCDE 407. Paris: OECD Publishing. https://doi.org/10.1787/9789264071001-en
- OECD 407. (2008, October 3). Guidelines for the testing of chemicals. Repeated dose 28-day oral toxicity study in rodents. OECD/OCDE 407. OECD Publishing.
- Olorunnisola, O. S., Bradley, G., & Afolayan, A. J. (2012). Acute and sub-chronic toxicity studies of methanol extract of Tulbaghia violacea rhizomes in Wistar rats. African Journal of Biotechnology, 11(83), 14934–14940. DOI: 10.5897/AJB12.1565
- Pajero, I., Viladomat, F., Bastida, J., Rosas-Romero, A., Fieriage, N., Burillo, J., & Codina, C. (2002). Between the free radical scavenging activity and anti- oxidant activity of six distilled and non distilled Mediterranean herbs and aromatic plants. Journal of Agricultural and Food Chemistry, 50(23), 6882–6890. https://doi.org/10.1021/jf020540a
- Pieme, C. A., Penlap, V. N., Nkegoum, B., Taziebou, C. L., Tekwu, E. M., & Etoa, F. X. (2006). Evaluation of acute and subacute toxicities of aqueous ethanolic extract of leaves of Senna alata (L.) Roxb (Ceasalpiniaceae). African Journal of Biotechnology, 5(3), 283–289. DOI: 10.5897/AJB
- Pushpa, L., Rama, M., Reddy, L., Mannur, I., & Vijaya, T. (2010). Medicinal plants and their derivatives as potential source in treatment of obesity. Asian Journal of Experimental Biological Sciences, 1(4), 719–727. http://refhub.elsevier.com/S2221-6189(15)00088-8/sref6
- Robbrecht, E., & Manen, J. F. (2006). The major evolutionary lineages of the coffee family (Rubiaceae, angiosperms). A new classification in two subfamilies, Cinchonoideae and Rubioideae. Systematics and Geography of Plants, 76(1), 85–146. https://www.jstor.org/stable/20649700
- Roberts, S., James, R. C., & Franklin, M. R. (2003). Principles of toxicology: Environmental and industrial applications (2nd ed.). John Wiley & Sons, Inc.
- Shri, J. N. M. (2003). Ginger: It‘s role in xenobiotic metabolism. ICMRBull, 33(6), 57–63. http://dx.doi.org/10.1016/j.jep.2013.12.034
- Tatefuji, T., Yanagihara, M., Fukushima, S., & Hashimoto, K. (2014). Safety assessment of melinjo (Gnetum gnemon L.) seed extract: Acute and subchronic toxicity studies. Food and Chemical Toxicology, 67(69), 230–235. https://doi.org/http://dx.doi.10.1016/j.fct.2014.02.030
- Tietz, N. W. (1976). Fundamentals of clinical chemistry. W.B. Saunders.
- Ward, J. W., & Elsea, J. R. (1997). Animal case and use in drug fate and metabolism. Methods and techniques. Markel Dekker.
- WHO. (2010). Monographs on selected medicinal plants commonly used in newly independent states. Geneva, Switzerland: WHO Press, World Health Organization. (ISBN97892 4 4597729).
- Xifan, Z., Chao, D., Jiangta, S., & Xuehui, D. (2015). Determination of reduced glutathione by spectrophotometry coupled with anti-interference compensation. Analytical Methods, 7(12), 5006. https://doi.org/10.1039/C5AY00825E
- Xin, Z., Waterman, D. F., Henken, R. M., & Harmon, R. J. (1991). Effect of copper status on neutrophil function, superoxide dismutase and copper distribution in steers. Journal of Dairy Science, 74(9), 3078. https://doi.org/10.3168/jds.S0022-0302(91)78493-2
- Zhu, M., Lew, K. T., & Leung, P. (2002). Protective effects of plants formula in ethanol-induced gastric lesions in rats. Phytotherapy Research, 16(3), 276–280. https://doi.org/10.1002/ptr.839