Abstract
We would like to comment on a recent study where the redox-modulatory anti-protozoal drug chloroquine has shown to trigger a crosstalk between the antioxidant and cell wall integrity systems in the yeast Saccharomyces cerevisiae. This note discusses the redox-active potential of the natural compound 2-hydroxy-4-methoxybenzaldehyde (2H4M) which could also serve as a potent redox-cycler in fungal (yeast, molds) pathogens. Using S. cerevisiae as a molecular tool, we determined how 2H4M negatively affected both the antioxidant and cell wall integrity systems of fungi, thus indicating a similar crosstalk between the two systems under 2H4M-induced toxicity. The crosstalk functions as a fungal defense against redox-active drugs/compounds or environmental cues, and therefore, could be an effective target for antifungal treatment.
PUBLIC INTEREST STATEMENT
Food or crop contamination by fungi is a recurring food safety/security issue, with outbreaks of wheat, corn or tree nut contamination by mycotoxin-producing fungi directly affecting the public health. It has been estimated that fungal infection or contamination causes 60%–70% of world food/crop loss. Moreover, potentiation of mycotoxin production by conventional fungicides has been documented in fungal pathogens. Hence, there is a persistent demand to improve the efficacy of conventional antifungals or develop new intervention methods. Natural products have been studied as new, safe antifungal agents, which not only inhibit the growth of fungi but also disrupt the mycotoxin production. Redox-active natural compounds can function as potent antimicrobials that disrupt both the redox maintenance and redox-sensitive cell structures in fungi. Therefore, natural antifungals can enhance the efficacy of established microbe interventions, overcome fungal resistance, and alleviate environmental side effects associated with current intervention strategies.
In a recent paper (Baranwal et al., Citation2014), the redox-modulatory anti-protozoal drug chloroquine (CQ) has shown to trigger the crosstalk between the antioxidant and cell wall integrity systems in the model yeast Saccharomyces cerevisiae. The CQ-induced stress/toxicity was transmitted to both HOG1 (antioxidant) and SLT2 (cell wall integrity) signaling pathways, namely, Mitogen-Activated Protein Kinase (MAPK) pathways. HOG1 encodes the High Osmolarity Glycerol-responsive MAPK involved in osmo-/oxidative stress regulation pathway, while SLT2 encodes the Suppressor of the LyTic phenotype (namely, an alteration/deficiency in the biosynthesis of cell wall components or in the control of yeast autolysis), a serine/threonine MAPK involved in the control of cell wall integrity (Saccharomyces Genome Database, Citation2020). CQ inhibited S. cerevisiae growth in a dosage-dependent manner, where the phosphorylated HOG1 MAPK enzyme translocated (from the cytosol) to the nucleus to activate glycerol synthesis. The SLT2 MAPK system was then activated to regulate the cell wall damage-induced defense responses (Baranwal et al., Citation2014).
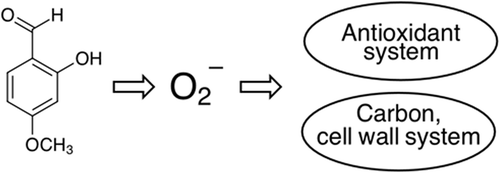
Meanwhile, we previously identified the natural product 2-hydroxy-4-methoxybenzaldehyde (2H4M) that targets cell wall integrity system of fungi (yeast, molds) (Kim et al., Citation2015). Fungi treated with 2H4M exhibited acute growth inhibition, while the sensitivity was alleviated by sorbitol. The 2H4M has been used as a flavoring agent, adjuvant or medicine (U.S. Food and Drug Administration, Citation2018) (Supplementary Table S1). Worthy of note, a recent investigation described the redox activity, namely possessing both antioxidant and prooxidant potential, of natural products and their interaction with cellular signaling pathways (Jacob, Citation2014). The redox-active 2H4M should also serve as a potent redox-cycler in fungal pathogens by disrupting redox homeostasis and/or redox-sensitive structures including fungal cell wall (Félix-Contreras et al., Citation2020; Guillen & Evans, Citation1994; Jacob, Citation2006).
Fungal cell wall integrity is regulated mainly by the sugar metabolism (Latgé et al., Citation2017). In S. cerevisiae, at least six systems contribute to the cell wall integrity, which include glycogen-, trehalose-, C6-, glucosamine- and chitin metabolism, as well as the upstream cell wall signaling pathway (Saccharomyces Genome Database, Citation2020). We selected 25 representative genes in these systems (Supplementary Table S2; Gene deletion mutants currently available) and found 13 out of the 25 genes are regulated further by stresses such as oxidative stress. For example, the expression of GPH1 encoding glycogen phosphorylase enzyme is regulated by stress-response elements and by the oxidative stress-driven MAPK signaling pathway, namely HOG1 MAPK, indicating the crosstalk between the “cell wall integrity” and “antioxidant” pathways (Table S2). We initially verified that five mutants (slt2Δ, bck1Δ, gph1Δ, tps1Δ, hxk2Δ) of the cell wall integrity system were relatively more susceptible to 2H4M or its structural derivatives (Sigma-Aldrich Co., St. Louis, MO, USA) compared to the parental strain (Table S2; Table S3; Figure S1), where 2H4M possessed the highest antifungal activity (Data not shown for the other mutants which did not exhibit increased sensitivity compared to the parental control).
The compound-induced yeast haploinsufficiency sensitivity bioassays (yeast gene deletion bioassays) use heterozygous gene deletion mutants of S. cerevisiae that are susceptible to a particular compound, thus identifies both the candidate compound and its possible gene targets (Giaever et al., Citation1999). Agar plate S. cerevisiae dilution bioassay was performed using the wild type (BY4741 MAT a his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and mutants (25 lacking each gene described in Table S2) to assess effects of the most potent benzaldehydes (six compounds) targeting the cell wall integrity system with a structure–activity relationship previously reported (Kim et al., Citation2015). Yeast strains were exposed to 0.5 to 7.0 mM of benzaldehydes.
Cells (1 x 106) of the wild type or mutants of S. cerevisiae, cultured on Yeast Peptone Dextrose (Bacto yeast extract 1%, Bacto peptone 2%, glucose 2%) plate, were serially diluted 10-fold in Synthetic Glucose (SG; Yeast nitrogen base without amino acids 0.67%, glucose 2% with appropriate supplements: uracil 0.02 mg/mL, amino acids 0.03 mg/mL) liquid medium five times to yield cell dilution cohorts of 106, 105, 104, 103, 102 and 101 cells. Yeast cells from each dilution of wild type or mutant strains were spotted on SG agar incorporated with individual benzaldehydes. Yeast cells were incubated at 30°C.
Results were monitored and the level of yeast growth was evaluated based on a designated value of the highest dilution where colonies became visible after 5 to 7 days of incubation, as follows: Score ‘0ʹ—no colonies were visible from any of the dilutions, Score ‘1ʹ—only a colony from the spot with the undiluted cells (106 cells), Score ‘2ʹ only colonies from the spots with the undiluted (106) and 105 cells were visible, etc., whereas Score ‘6ʹ—colonies were visible from all dilution spots. Therefore, each unit (1 to 6) of numerical difference as shown above was equivalent to a 10-fold difference in the sensitivity of the yeast strain to the benzaldehyde treatment (See also Figure S1). The sensitivity of S. cerevisiae presented in Table S3 reveals the differential susceptibility of test strains at the representative concentrations enough to detect the distinctive responses of the mutants.
To examine the possible crosstalk between the “cell wall integrity” and “antioxidant” pathways under the treatment of 2H4M, the susceptibility to 2H4M was investigated further in four antioxidant mutants of S. cerevisiae, which include: (a) yap1Δ, transcription factor mutant regulating four downstream genes of oxidative stress-response system, namely GLR1 (glutathione reductase), YCF1 (a glutathione S-conjugate pump), TRX2 (thioredoxin), and GSH1 (γ-glutamylcysteine synthetase) (Fernandes et al., Citation1997; Lee et al., Citation1999); (b) sod1Δ, cytosolic superoxide dismutase mutant; (c) sod2Δ, mitochondrial superoxide dismutase mutant; and (d) glr1Δ, glutathione reductase mutant (Figure S2). These representative groups are necessary for maintaining cellular redox homeostasis in both enzymatic (scavenging superoxide radicals) and non-enzymatic (glutathione homeostasis) aspects, where yap1Δ mutation negatively affects both enzymatic and non-enzymatic processes. In brief, among the four mutants tested, sod1Δ (cytosolic superoxide dismutase (SOD1)) mutant exhibited the highest susceptibility, strongly suggesting the crucial role of superoxide dismutase enzyme in the “cytoplasm” for fungal survival/defense against 2H4M-triggered toxicity (Figure S2).
We postulate that, while the redox-active 2H4M could directly disrupt the fungal cell wall components, the prooxidant characteristic of 2H4M also induces cellular oxidative stress, for which SOD1 plays an important role for fungal defense by scavenging the cytosolic superoxide radicals (O2−). Fungal mutants lacking key genes in these systems, such as SOD1 and SLT2 mutants, are highly susceptible to 2H4M (Table S3; Figure S2). Studies elucidating the dialogues between the antioxidant and cell wall integrity MAPK systems are currently emerging; the two MAPK systems are positively coordinated to counteract various stresses in S. cerevisiae (Rodríguez-Peña et al., Citation2010). For instance, the sequential activation of the HOG and cell wall integrity pathways has been determined during the yeast adaptation to zymolyase-treated cell wall stress. This cell wall-degrading enzyme activates both HOG1 and SLT2 MAPKs. The increased phosphorylation in SLT2 was via the SHO1 (Transmembrane osmo-sensor) branch of the HOG1 MAPK pathway, which also required the upstream essential components of the cell wall integrity pathway such as MAPK kinase (MAPKK), MAPKK kinase (MAPKKK), protein kinase C1, etc. (Rodríguez-Peña et al., Citation2010).
Whereas, the filamentous fungus Aspergillus fumigatus is a causative agent for the human invasive aspergillosis. A. fumigatus possesses two HOG MAPKs, namely SakA and MpkC which are Stress-Activated Protein Kinases (Mattos et al., Citation2020). Both SakA and MpkC play important roles countering the environmental stresses including oxidative-, osmotic- and cell wall stresses, where SakA physically associates with MpkC under the stresses (Manfiolli et al., Citation2019). A. fumigatus also possesses a separate cell wall integrity signaling system, for which the MpkA functions as the key MAPK enzyme (Mattos et al., Citation2020). Of note, both SakA and MpkC MAPKs are necessary for the phosphorylation of the MpkA during cell wall damage. Under the cell wall stress, SakA (oxidative stress MAPK) physically interacts with the MpkA (cell wall integrity MAPK) as well as the MAPK phosphatase, further indicating a close crosstalk between the HOG and cell wall integrity pathways also in the pathogenic A. fumigatus (Mattos et al., Citation2020).
The crosstalk between the HOG and cell wall integrity systems has been documented further in (a) Candida albicans where both HOG and Cek1 (cell wall construction) pathways were necessary to counteract the osmotic stress (Herrero-de-Dios et al., Citation2014), and (b) Pyrenophora graminea (a seed-borne barley disease pathogen) for which the pgpbs, an orthologous HOG pathway MAPKK gene, was required for both osmotic stress and cell wall integrity response (Liang et al., Citation2019).
In general, aldehydes are reactive molecules that inhibit the normal growth of fungi. Zhou et al. (Citation2015) identified in yeast assay that the transduction of the toxicity signals triggered by 5-hydroxymethyl-2-furaldehyde (5-Hydroxymethyl-2-furancarboxaldehyde), an aldehyde liberated from the treatment of lignocellulosic-biomass, was mediated via the cell wall integrity MAPK pathway in the industrial S. cerevisiae. Aldehydes are also the intermediates of many metabolic pathways in cells, which are reactive at high physiological concentrations thus causing the inhibition of cell growth or disruption of cellular integrity (Abdul et al., Citation2018). However, the antioxidant enzyme superoxide dismutase (SOD) plays a role in scavenging or controlling the level of cellular toxic aldehydes (Abdul et al., Citation2018). Altogether, studies indicate that fungal responses to the treatment of “aldehyde” derivatives (including 2H4M) involve the activity of SOD and cell wall integrity MAPK for fungal defense.
Comparing to the 2H4M presented here, certain natural flavonols such as quercetin exhibited cell-protective effects via the activation of cell wall synthesis/maintenance in yeast (Vilaça et al., Citation2012). For example, treatment of S. cerevisiae with quercetin upregulated genes involved in carbohydrate metabolism, which led to the accumulation of the disaccharide trehalose, a stress protectant (Vilaça et al., Citation2012). Quercetin also activated the cell wall integrity pathway by enhancing the cell wall proteins, SLT2 MAPK and the downstream transcription factor (RLM1) of the system, thus increased the oxidative stress tolerance of S. cerevisiae. Of note, quercetin treatment also increased yeast resistance to the lytic enzyme zymolyase that degrades the beta-1,3-glucans of cell wall structure (Vilaça et al., Citation2012).
Collectively, we propose that the cytosolic oxidative stress signals, such as superoxide radicals (O2−), triggered by 2H4M are transmitted further to activate the cell wall integrity pathway (via MAPKs as determined in CQ above), which then contributes to the maintenance of normal cell wall structure. The comprehensive characterization of the 2H4M-triggered signaling mechanism warrants future in-depth investigation including the measurement of SOD1 activity, genetic rescuing of sod1Δ (as performed by Kim et al., Citation2005) and/or MAPK phosphorylation.
The key is redox-active natural compounds, such as 2H4M, could serve as potent antifungal candidates where targeting cellular antioxidant and cell wall integrity systems is the proposed mechanism of action.
Supplemental Material
Download MS Word (414.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
Notes on contributors
Jong H. Kim
Jong H. Kim is Research Molecular Biologist. His research focuses on the development of intervention strategies for control of mycotoxigenic and phytopathogenic fungi. He provides chemo-biological expertise for resistance management.
Kathleen L. Chan
Kathleen L. Chan is research staff. Her work focuses on preventing mycotoxin production in edible crops. She provides antifungal bioassay and molecular biological expertise.
Christina C. Tam
Christina C. Tam is Research Microbiologist. Her research focuses on foodborne toxins including botulinum neurotoxin and abrin. She provides the detection, prevention and therapeutic methodologies for eliminating toxin-producing microorganisms.
Luisa W. Cheng
Luisa W. Cheng is Research Leader of the Unit. Her research focuses on the development of technologies for detecting toxins that impact food safety and food defense.
Kirkwood M. Land
Kirkwood M. Land is Associate Professor of Biological Sciences. He focuses on the control of microbial growth in humans, animals and food. He provides medicinal chemistry expertise for new drug discovery against pathogens.
References
- Abdul, W., Aliyu, S. R., Lin, L., Sekete, M., Chen, X., Otieno, F. J., Yang, T., Lin, Y., Norvienyeku, J., & Wang, Z. (2018). Family-four aldehyde dehydrogenases play an indispensable role in the pathogenesis of Magnaporthe oryzae. Frontiers in Plant Science, 9(2018), Article 980. https://doi.org/10.3389/fpls.2018.00980
- Baranwal, S., Azad, G. K., Singh, V., & Tomar, R. S. (2014). Signaling of chloroquine-induced stress in the yeast Saccharomyces cerevisiae requires the Hog1 and Slt2 mitogen-activated protein kinase pathways. Antimicrobial Agents and Chemotherapy, 58(9), 5552–7. https://doi.org/10.1128/aac.02393-13
- Félix-Contreras, C., Alba-Fierro, C. A., Ríos-Castro, E., Luna-Martínez, F., Cuéllar-Cruz, M., & Ruiz-Baca, E. (2020). Proteomic analysis of Sporothrix schenckii cell wall reveals proteins involved in oxidative stress response induced by menadione. Microbial Pathogenesis, 141(2020), Article 103987. https://doi.org/10.1016/j.micpath.2020.103987
- Fernandes, L., Rodrigues-Pousada, C., & Struhl, K. (1997). Yap, a novel family of eight bZIP proteins in Saccharomyces cerevisiae with distinct biological functions. Molecular and Cellular Biology, 17(12), 6982–6993. https://doi.org/10.1128/mcb.17.12.6982
- Giaever, G., Shoemaker, D. D., Jones, T. W., Liang, H., Winzeler, E. A., Astromoff, A., & Davis, R. W. (1999). Genomic profiling of drug sensitivities via induced haploinsufficiency. Nature Genetics, 21(3), 278–283. https://doi.org/10.1038/6791
- Guillen, F., & Evans, C. S. (1994). Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Applied and Environmental Microbiology, 60(8), 2811–2817. http://www.ncbi.nlm.nih.gov/pubmed/16349349
- Herrero-de-Dios, C., Alonso-Monge, R., & Pla, J. (2014). The lack of upstream elements of the Cek1 and Hog1 mediated pathways leads to a synthetic lethal phenotype upon osmotic stress in Candida albicans. Fungal Genetics and Biology, 69(2014), 31–42. https://doi.org/10.1016/j.fgb.2014.05.010
- Jacob, C. (2006). A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Natural Product Reports, 23(6), 851–863. https://doi.org/10.1039/b609523m
- Jacob, C. (2014). Special issue: Redox active natural products and their interaction with cellular signalling pathways. Molecules, 19(12), 19588–19593. https://doi.org/10.3390/molecules191219588
- Kim, J. H., Campbell, B. C., Yu, J., Mahoney, N., Chan, K. L., Molyneux, R. J., Bhatnagar, D., & Cleveland, T. E. (2005). Examination of fungal stress response genes using Saccharomyces cerevisiae as a model system: Targeting genes affecting aflatoxin biosynthesis by Aspergillus flavus Link. Applied Microbiology and Biotechnology, 67(6), 807–815. https://doi.org/10.1007/s00253-004-1821-1
- Kim, J. H., Chan, K. L., & Mahoney, N. (2015). Augmenting the activity of monoterpenoid phenols against fungal pathogens using 2-hydroxy-4-methoxybenzaldehyde that target cell wall integrity. International Journal of Molecular Sciences, 16(11), 26850–26870. https://doi.org/10.3390/ijms161125988
- Latgé, J. P., Beauvais, A., & Chamilos, G. (2017). The cell wall of the human fungal pathogen Aspergillus fumigatus: Biosynthesis, organization, immune response, and virulence. Annual Review of Microbiology, 71(1), 99–116. https://doi.org/10.1146/annurev-micro-030117-020406
- Lee, J., Godon, C., Lagniel, G., Spector, D., Garin, J., Labarre, J., & Toledano, M. B. (1999). Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. Journal Biological Chemistry, 274(23), 16040–16046. https://doi.org/10.1074/jbc.274.23.16040
- Liang, Q., Li, B., Wang, J., Ren, P., Yao, L., Meng, Y., Si, E., Shang, X., & Wang, H. (2019). PGPBS, a mitogen-activated protein kinase kinase, is required for vegetative differentiation, cell wall integrity, and pathogenicity of the barley leaf stripe fungus Pyrenophora graminea. Gene, 696(2019), 95–104. https://doi.org/10.1016/j.gene.2019.02.032
- Manfiolli, A. O., Mattos, E. C., de Assis, L. J., Silva, L. P., Ulaş, M., Brown, N. A., Silva-Rocha, R., Bayram, Ö., & Goldman, G. H. (2019). Aspergillus fumigatus high osmolarity glycerol mitogen activated protein kinases SakA and MpkC physically interact during osmotic and cell wall stresses. Frontiers in Microbiology, 10(2019), Article 918. https://doi.org/10.3389/fmicb.2019.00918
- Mattos, E. C., Silva, L. P., Valero, C., de Castro, P. A., Dos Reis, T. F., Ribeiro, L. F. C., Marten, M. R., Silva-Rocha, R., Westmann, C., da Silva, C. H. T. D., Taft, P., Al-Furaiji, C. A., Bromley, N., Mortensen, M., Benz, U. H., Brown, J. P., & Goldman, G. H. (2020). The Aspergillus fumigatus phosphoproteome reveals roles of high-osmolarity glycerol mitogen-activated protein kinases in promoting cell wall damage and caspofungin tolerance. mBio, 11(1), e02962–02919. https://doi.org/10.1128/mBio.02962-19
- Rodríguez-Peña, J. M., García, R., Nombela, C., & Arroyo, J. (2010). The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: A yeast dialogue between MAPK routes. Yeast (Chichester, England), 27(8), 495–502. https://doi.org/10.1002/yea.1792
- Saccharomyces Genome Database. Stanford University. Retrieved March 6, 2020, from https://www.yeastgenome.org
- U.S. Food and Drug Administration. (2018). Substances added to food. Retrieved March 6, 2020, from https://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm115326.htm
- Vilaça, R., Mendes, V., Mendes, M. V., Carreto, L., Amorim, M. A., de Freitas, V., Moradas-Ferreira, P., Mateus, N., & Costa, V. (2012). Quercetin protects Saccharomyces cerevisiae against oxidative stress by inducing trehalose biosynthesis and the cell wall integrity pathway. Plos One, 7(9), e45494. https://doi.org/10.1371/journal.pone.0045494
- Zhou, Q., Liu, Z. L., Ning, K., Wang, A., Zeng, X., & Xu, J. (2015). Genomic and transcriptome analyses reveal that MAPK- and phosphatidylinositol-signaling pathways mediate tolerance to 5-hydroxymethyl-2-furaldehyde for industrial yeast Saccharomyces cerevisiae. Scientific Reports, 4(1), 6556. https://doi.org/10.1038/srep06556