?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Leaves of Cadaba rotundifolia Forssk have widely been used by the community of Eastern Ethiopia as traditional phytomedicine against various diseases. The focus of this study was to screen major phytochemical classes and determine their contents, and evaluate in-vitro antioxidant activities of different solvent extracts of C. rotundifolia leaf. The total content of phenolic (TPC), flavonoid (TFC) and tannin (TTC), and in-vitro antioxidant activity (using DPPH and ferric reducing power assays) of four extracts (pure ethanol, pure methanol, 80% methanol and 80% ethanol) were determined using spectrophotometric method. The obtained data revealed that 80% ethanol extract scored the highest extraction yield (16.22%) followed by 80% aqueous methanol (12.90%), methanol (12.87%) and ethanol (12.86%); whereas the lowest crude extract percentage yield was recorded by petroleum ether (1.11%) followed by ethyl acetate (1.42%) and dichloromethane (1.65%). Flavonoids, phenols, alkaloids, saponins, carbohydrates and tannins were positively screened especially in the alcoholic and their corresponding aqueous extracts. The present finding also showed the presence of appreciable amounts of total phenolic (8.04 ± 1.04–10.46 ± 1.25) mgGAE/gDCE (gallic acid equivalents per gram of dried crude extracts), flavonoid (0.38 ± 0.05–0.51 ± 0.03) and tannin (0.75 ± 0.03–1.05 ± 0.03) mgCE/gDCE (catechin equivalent per gram of dried crude extracts). The 80% methanol extract exhibited the highest phenolic (10.46 ± 1.25 mgGA/gDCE) and flavonoid (0.51 ± 0.03 mgCE/gDCE) contents; whereas the highest total tannin content (0.92 ± 0.03 mgCE/gDCE) was recorded in aqueous (80%) ethanol extract. The aqueous ethanol and methanol extracts showed the highest scavenging activity against DPPH-free radical and ferric reducing antioxidant power (FRAP) than the non-aqueous parts; which is also correlated with the obtained total contents of phenolic, flavonoid and tannin expected to have antioxidant potency. In the immediate future, it is therefore very important to note that the hydro-alcoholic solvents should be prioritized while conducting any phyto-pharmacological studies on the C. rotundifolia leaves.
PUBLIC INTEREST STATEMENT
It is clearly known that the curing potential of medicinal plants is attributed to their harbored bioactive ingredients. In Ethiopia, the rural communities consider medicinal plants, including Cadaba rotundifolia, as the safeguards of their life to battle various lethal infectious and chronic diseases. In this study, an attempt has made to look at the effect of extraction solvents on the qualitative and quantitative profiles of Cadab arotundifolia leaf chemical ingredients by screening major phytochemicals, evaluating in-vitro antioxidants of flavonoids, phenols and tannins. The present finding revealed that the aqueous alcoholic extracts of Cadaba rotundifolia leaves are the promising sources of flavonoids, phenols and tannins with high contents and antioxidant activity. This is an assertion to the traditional preparations by a rural community in which water and/or traditional drinks are the solvent of choice. Therefore, our current study may support traditional medicine and contribute to modern medicines.
1. Introduction
This day, the chemical identity of reactive oxidative species and their devastative effect on the biological makeup of living cells have well been known. These restless and unstable reactive species destroy the major macromolecules of cells (such as proteins, lipids, enzymes and nucleic acids) thereby leading to the conversion of the functional cell to the malfunctioning one (Baba & Malik, Citation2015; Khatoon et al., Citation2013). Such malfunctioning cell causing oxidative stresses lead to various diseases like cancer, cardiovascular diseases, arteriosclerosis, neural disorders, Parkinson’s disease, Alzheimer’s disease, ageing, hypertension, inflammations, diabetes mellitus and chronic fatigue syndrome (Rebaya et al., Citation2015). Now, the question is how these reactive free radical species can be defended by the living cells? Thus far, researchers have spent their precious efforts and time on searching effective, safe and sustainable alternatives to tackle the causative effect of the reactive species. Of those alternatives, the natural sources originated antioxidants have been found as the champion one. Plants are in the front line as well-known homes of various natural antioxidants; which currently have been gained serious attentions (Khatoon et al., Citation2013; Lefahal et al., Citation2018; Truong et al., Citation2019). These medicinal plant based natural antioxidants have played tremendous roles on fighting free radicals via the chemistry of providing an electron and/or hydrogen and making the restless oxidative species stable (Rebaya et al., Citation2015). It is very apparent that the credit of biological activities, including antioxidants of medicinal plants has been given to the phytochemical classes like polyphenols, saponins, flavonoids, carotenoids, vitamin E and alkaloids they harbour in (Upadhya et al., Citation2015). Phenolic compounds and flavonoids are among the well-known phytochemical classes, which scavenge free radicals by acting as reducing agents, donating hydrogen-free radical, quenching singlet oxygen and chelating metals (Chigayo et al., Citation2016; Iloki-Assanga et al., Citation2015).
Cadaba rotundifolia Forssk, an evergreen shrub, is classified under the family Capparaceae and genus Cadaba. C. rotundifolia contains leaves, which are densely sparsely with swollen- based hairs (). The genus Cadaba also includs other 30 species which are distributed from Northeast Africa to South west Arabia (Karemy, Citation2001). In Ethiopia, C. rotundifolia is widely distributed in desert plain situated mainly within the Rift Valley lies in the Eastern and North Eastern part (Ahmed et al., Citation2017; Tikssa et al., Citation2009). It is also widely spread in Sudan, Kenya, Eritrea, Djibouti, Somalia, Saudi Arabia and Yemen (Karemy, Citation2001).
Different parts of C. rotundifolia have been reported for their different ethnomedicinal uses. For example, the leaves of the plant have been applied to treat ailments such as cough, stomach ulcer and cancer in Yemene (Al-Fatimi, Citation2019) and as antibiotics in Rwanda (Hassan-Abdallah et al., Citation2013). In Sudan also, the roots and leaves of C. rotundifolia are traditionally used to treat tumors and abscesses (Graham et al., Citation2000). In the Eastern part of Ethiopia, the leaves of C. rotundifolia in combination with Withania somnifera are traditionally used to treat extended flow of menstruation/Menometrorrhagia (Belayneh & Bussa, Citation2014; Hassan-Abdallah et al., Citation2013) external injury, wounds, skin infection and diarrhea (Teklehaymanot & Giday, Citation2010) and the aerial parts (leaves and stems) are known for treating arthritis, eye sickness, tonsillitis, flue, retained placenta, external parasite, brucellosis, bloating and bovine TB (Teklehaymanot, Citation2017). Moreover, ethanol extracts of the leaves also possess anti-malarial activity (Giday & Teklehaymanot, Citation2013). Regarding the nutritional value, C. rotundifolia has been reported for its high protein content (Belayneh et al., Citation2017). Thus far, Al Hamoud et al. (Citation2019), reported a chemistry study about the chemical constituents of C. rotundifolia, the ethanolic root extract of C. rotundifolia resulted a quaternary alkaloid (3-hydroxystachydrine); and the aerial parts provided flavonoid glycosides and other flavonoids constituents. However, no studies were reported so far on the qualitative and quantitative investigation of major phyto-compounds, and in-vitro antioxidant activity of C. rotundifolia leaf extracts. Conducting an experimental investigation on the major phytochemical families and anti-oxidative potency of various extracts of C. rotundifolia leaf is therefore urgently needed. The present study focused on the qualitative screening of major class of compounds, quantitative analysis of total phenolics, flavonoids and tannins contents; and in-vitro antioxidant activities of various extracts prepared from C. rotundifolia leaf
2. Materials and methods
2.1. Plant material collection
Leaves of Cadabarotundifolia were collected in January 2020from Harla and Dengego mountains (9°27′ and 9°39′N latitude and 41°38′ and 42°20′E longitude) found in the Eastern part of Ethiopia. The taxonomical name of the plant species was confirmed using the flora data/document of Haramaya University, Ethiopia and a voucher specimen was deposited at the University’s herbarium. The freshly collected leaves were then properly washed with tap water and rinsed with distilled water followed by air-drying under a shed at room temperature for a month. The dried leaves were ground into fine powder using an electrical grinder. The powdered leaf sample was then stored in a dark airtight glass container at 4°C refrigerator until extraction was commenced.
2.2. Chemicals and reagents
Ethanol (99.5%) and petroleum ether (40–60°C) were obtained from Carlo Erba reagents (France). Methanol (99%), dichloromethane (99%) and hydrochloric acid (37%) were purchased from Loba Chemie Pvt Ltd (India) while ethyl acetate from Sisco Research Laboratories (India) and DPPH (2, 2-Diphenyl picrylhydrazyl) from Alfa Aeser (Germany). Gallic acid and catechin reagents were bought from PS Park (Northampton, UK). Ascorbic acid- (L), potassium ferricyanide and sodium nitrite were purchased from AnalaRBDH (England). Aluminium chloride, trichloroacetic acid, sodium hydroxide and ferric chloride were procured from Blulux (India). Folin-Ciocalteureagent, sodium hydrogen carbonate, and vanillin were bought from Sigma-Aldrich (Sternheim, Germany). All the chemicals used, including the solvents were of analytical grade.
2.3. Preparation of plant extracts
Finely powdered leaves of Cadaba rotundifolia (40.00 g) was extracted with seven solvent systems (300 ml each), namely, petroleum ether, dichloromethane, ethyl acetate, 100% ethanol, 80% aqueous ethanol, 100% methanol and 80% aqueous ethanol, separately by placing on an orbital shaker at room temperature for 48 h. Each extracts were then filtrated using Whatman No.1 filter paper (Whatman International Ltd, England) and filtrates were concentrated using a rotary evaporator (Rotary vacuum, Jainsons, India) under reduced temperature and pressure; and the hydroalcoholic extracts were further dried over water bath at 90°C. Crude extracts were then stored in a refrigerator at 4°C for further analyses. The percentage of extraction yield (%) was determined using the following formula:
2.4. Phyto-chemical profiling test
Each crude extract was tested for the confrimation of the absence/or presence of some major classes of phytochemicals following the standard qualitative procedures described by Paterson (Citation1999) with a slight modification.
2.4.1. Tests for flavonoids
Alkaline reagent test: 2 ml of 2.0% NaOH was mixed with each plant crude extract. An intense yellow colour was formed which turned colourless on the addition of two drops of diluted acid which indicated the presence of flavonoids.
Lead acetate test: Extracts were treated with few drops of lead acetate solution. Formation of yellow colour precipitate also confirmed the positive test of flavonoids.
2.4.2. Test for alkaloids
The presence of alkaloids was tested using two reagents, Mayer’s and Wagner’s reagents. A few drops of the solution were added to the extract solution (0.5 ml). A reddish-brown turbidity or precipitate demonstrated the positive alkaloids.
2.4.3. Test for tannins (Ferric chloride test)
To the plant extracts solution (0.5 ml), few drops of 5% ferric chloride were added. Black or blue-green colouration or precipitate was taken as evidence for the presence of tannins.
2.4.4. Test for saponins
Few drops of NaHCO3 were added to the plant extract solution (0.5 ml) and shaken vigorously to froth and then allowed to stand for 15–20 min. A height of persistent foam greater than 1 cm indicated the presence of saponins.
2.4.5. Test for steroids and terpenoids
Salkowski test: 2 ml of each plant extract was mixed with 2 ml of chloroform followed by the addition of concentrated H2SO4 (2 ml) by shaking well. A red colour produced in the lower chloroform layer indicated the presence of steroids.
2.4.6. Test for carbohydrates
Molisch test: Plant extracts (2 ml) were treated with a few drops of alcoholic alpha-naphthol. Concentrated sulphuric acid (0.5 ml) was then poured slowly along the sides of the test tube. The appearance of purple to violet colour ring at the junction indicated the presence of carbohydrate.
2.4.7. Detection of phenols
Ferric chloride Test: Extracts were treated with 3–4 drops of ferric chloride solution. Formation of bluish-black colour indicated that the presence of phenols.
2.5. Quantitative determination
2.5.1. Total phenolic content (TPC)
The total phenolic content (TPC) in four plant extracts, namely, 100% ethanol, 100% methanol, 80% ethanol and 80% methanol extracts, was determined following Folin-Ciocalteu method described by Truong et al. (Citation2019) with minor modification. In brief, Folin-Ciocalteu reagent (5 ml, 10%) was added to each tested plant extract solution (1 ml, 1 mg/ml). After 5 min, Na2CO3 (4 ml, 7.5%) was added and the mixture was incubated at room temperature for 30 min. A set of standard solutions of gallic acid (10–100 µg/ml) were prepared in the same manner as described for the extracts. The absorbance of the extracts and standard solutions was measured against the reagent blank at 760 nm with a UV/Visible spectrophotometer (Cecil CE4001 UV/Vis, Cambridge, England). The total phenolic content in all tested extracts was determined from the calibration curve and expressed as milligram of gallic acid equivalent (mgGAE/gDCE) per gram of the dried crude extracts. The experiment was repeated thrice for all concentrations of the extracts and standards.
2.5.2. Total flavonoid content (TFC)
Total flavonoid content was determined using aluminium chloride colourimetric method (Anwar & Przybylski, Citation2012) with some modification. Briefly, each tested plant extract (1 ml,1 mg/ml) was taken into 10 ml volumetric flask and distilled water (5 ml) and NaNO2 (0.3 ml, 5%) were added. After 5 min, AlCl3 solution (0.5 ml, 5%) was added to the mixture followed by the addition of 1 M NaOH (2 ml) after another 5 min and diluted to the mark with distilled water. After the solution was vigorously mixed, its absorbance was measured at 415 nm using a UV/Visible spectrophotometer. Furthermore, serial standard solutions of catechin (70, 60, 50, 40 30, 20 and 10 µg/ml) were prepared from 0.1 mg/ml stock solution using the same technique described for the extracts above. The absorbance of the extracts and standard solutions was measured against the reagent blank at 415 nm with a UV/Visible spectrophotometer (Cecil CE4001 UV/Vis, Cambridge, England). The total flavonoid content was determined from the calibration curve and expressed as milligram of catechin equivalent per gram of dried crude extracts (mgCE/gDCE). The determinations of total flavonoid content in the tested extracts and standards were analyzed in triplicates.
2.5.3. Total condensed tannin content
Total condensed tannin (proanthocyanidin) contents in all extracts was analyzed using the modified procedure stated by Medini et al. (Citation2014) and Rebaya et al. (Citation2015) with the standard reference, catechin. In sum, vanillin (5 ml, 4% in methanol) and concentrated HCl (1.5 ml) were added to each tested plant extract solutions (1 ml, 1 mg/ml). In addition, serial standard solutions of catechin (60, 48, 36, 24, 30, 12 and 0 µg/ml) were prepared. After 20 min of incubation, the absorbance of both the extract and standard concentrations was measured at 500 nm against methanol as a blank and the total condensed tannin was expressed as mg equivalent of catechin per (mgCE/g DCE) dried crude extracts. All instrumental readings were conducted three times.
2.6. In-Vitro anti-oxidation activity evaluation of plant extracts
The in-vitro antioxidant activities of four extracts (100% ethanol, 100% methanol, 80% ethanol and 80% methanol) were investigated using two assays, DPPH free radical and Ferric Reducing Power (FRP).
2.6.1. DPPH assay
The free radical trapping activity of the extracts and the positive control, ascorbic acid was evaluated against the unstable radical DPPH by applying the method described by Khorasani Esmaeili et al. (Citation2015) with little arrangements. Five different concentrations, namely, 25, 50, 100, 150 and 200 μg/ml of tested plant extracts were prepared from a stock solution (1 mg/ml). Then, freshly prepared DPPH solution (2 ml, 0.004% in methanol) was added to each concentration and incubated at room temperature for 30 min. The absorbance of each concentration was measured in triplicate at 517 nm using UV-visible spectrophotometer (Cecil CE4001 UV/VIS, Cambridge, England). The methanolic solution of DPPH was used as a negative control. The free radical scavenging activity percentage of evaluated plant extracts against DPPH free radical was calculated using the following equation formula:
Where A0 and A are the absorbance of the negative control (0.004% w/v DPPH solution) and the DPPH containing plant extract solution.
2.6.2. Ferric reducing power assay
Ferric reducing antioxidant power (FRAP) of plant extracts was determined as per the procedure described by Do et al. (Citation2014). Briefly, from the stock solution (1 mg/ml), five serial concentrations of extracts (200, 150, 100, 50, 25 mg/ml) were prepared; mixed with phosphate buffer (2 ml, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 ml, 10%). After the solutions were incubated at 40°C for 30 min, trichloroacetic acid (2.5 ml, 10%) was added and the tubes were centrifuged at 3000 rpm for 10 min. Then, a supernatant (5 ml) of each solution was mixed with distilled water (2 ml) and ferric chloride (0.5 ml, 0.1%). Finally, the absorbance of the reaction mixtures was measured at 700 nm. Ascorbic acid was used as a positive control. Ferric reducing power was expressed as absorbance per specific amount of plant extract.
2.6.3. Statistical analysis
The obtained data were statistically analyzed using multivariate analysis of SPSS (version 20) (SPSS Inc., Chicago, USA) at 95% confidence level. All the experiments were done in duplicate and the results were expressed as Mean± SD. Mean comparisons were considered statistically significant when p ≤ 0.05.
3. Results and discussion
3.1. Percentage extraction yield
As the chemistry of various bioactive compounds in medicinal plants varied, different solvents of extraction may have different effects on the solubility, extraction yield and antioxidant activity of the phyto-compounds (Azabou et al., Citation2020). In the present study, the percentage extraction yield of seven different crude solvent extracts (ethanol, methanol, 80% ethanol, 80% methanol, ethyl acetate, dichloromethane and petroleum ether) prepared from C. rotundifolia leaf was calculated and presented in . From the statistical data obtained, the means value of percentage extract yield showed a significant difference (P ≤ 0.05) between each solvent of extractions. Among the solvent of extractions used in this study, 80% aqueous ethanol scored the highest extraction yield (16.22%) followed by 80% aqueous methanol (12.90%), methanol (12.87%) and ethanol (12.86%); whereas the lowest crude extract percentage yield was recorded by petroleum ether (1.11%) followed by ethyl acetate (1.42%) and dichloromethane (1.65%). The obtained results indicated that polar protic solvents gave better extraction yields and this may tell us that the plant is enriched with polar secondary metabolites which was alittle bit in agreement with previously reported results on the same plant species (Al-Fatimi et al., Citation2007). According to this author, the maximum extract yield was obtained from MeOH (14.5%) and water (11.2%) extracts; while the lowest yield was recorded by dichloromethane extract (3.2%). In the same way, aqueous methanol is one of the most promising potential in recovering the highest amounts of phenolic compounds (Sultana et al., Citation2009).This indicated that the extraction yield is not only affected by the extraction technique but also by the extraction solvent. Furthermore, using hydroalcoholic as solvent of extraction may facilitate the exploitation of chemical components as they may be soluble either in water and/or organic solvent parts (Do et al., Citation2014; Truong et al., Citation2019). In this study also, this rational may be the reason why the aqueous ethanol and methanol solvents scored the highest extract yields than the pure ethanol and methanol, and the rest solvent of extractions. Therefore, ethanol, methanol and their corresponding aqueous solvents could be preferred for further investigation on the biological activity and isolation of effective bioactive compounds of C. rotundifolia leaf.
Table 1. Percentage yields of seven different crude extracts of Cadaba rotundifolia leaf
3.2. Preliminary phytochemical profiling of crude extracts
In this study, the chemical profiles of all crude extracts prepared from C. rotundifolia leaf were screened using different reagents to confirm the absence/or presence of major phytochemical classes. Accordingly, eight phyto-compound families, namely, flavonoids, phenols, alkaloids, tannins, saponins, steroids, terpenoids and carbohydrates were screened as depicted in .
Table 2. Phytochemicals profile from different crude extracts of Cadaba rotundifolia leaf
As it can be seen from , the obtained preliminary qualitative analyses results revealed that flavonoids, phenols and saponins were strongly detected in EtOH, MeOH and their aqueous solvent crude leaf extracts of C. rotundifolia. Whereas tannin was moderately screened in all extracts, except in petroleum ether was negative. According to the previous study reported by Al Hamoud et al. (Citation2019), ethanolic extract of aerial parts of same plant species showed the presence of flavonoids which is in line with the present result. Also in this study, alkaloids and tannins were observed strongly and moderately, respectively, in dichloromethane extract. All extracts, except ethanol and methanol, showed a positive presence of carbohydrates (). On the contrary, steroids and terpenoids were completely absent in all tested crude plant extracts. From the present result, it is possible to say that the ethno-medicinal value of C. rotundifolia leaf such as treating cough, stomach ulcer and cancer (Al-Fatimi, Citation2019) might be attributed to the positively screened secondary metabolites such as alkaloids, flavonoids and tannins ().
3.3. Estimation of total phenolics, favonoids and tannins contents
The total phenolic content (TPC) in the ethanolic, methanolic and their aqueous (80%) crude extracts of C. rotundifolia leaf were presented in . The obtained results ranged from 10.46 to 8.04 mgGAE/gDCE which was determined from the linear regression equation of standard curve (y = 0.007x—0.009, R2 = 0.996). From the statistical data obtained, the difference in mean value between each extract was generally insignificance (p ≥ 0.05) except between extracts 1 and 4 (P ≤ 0.5). As shown from , the highest content of total phenolics (mgGAE/gDCE) was detected in 80% methanol extract (10.46) followed by 80% ethanol (9.63) and pure methanol (9.54), while the lowest total phenolic content was recorded in pure ethanol (8.04). The results generally confirmed that the hydroalcoholic crude extracts provide satisfactory phenolic content. According to Anwar and Przybylski (Citation2012), aqueous methanol and ethanol solvent extracts were found to be more effective for isolation and determination of phenolic compounds from different plant materials; and this statement is a confirmation for our findings reported in the present study.
Table 3. Total phenolic, flavonoid and tannin contents in four different crude leaf extracts of Cadaba rotundifolia
The total flavonoid content (TFC) (mgCE/gDCE) in the tested solvent extracts was obtained using the equation of standard curve (y = 0.028x +0.008; R2 = 0.998). The highest and lowest TFC values were ranged from 0.51 ± 0.04–0.38 ± 0.05 mgCE/Gdce (). The resulted statistical output indicated that the mean value between tested solvent extracts showed a significantly different (p ≤ 0.05), except between extracts 1 & 2 and 3 & 4 which was insignificance (p ≥ 0.05). The obtained results showed that the aqueous methanol and ethanol extracts exhibited the highest TFC value (0.49 ± 0.04 and 0.46 ± 0.02 mgCE/gDCE, respectively). As shown in also, the total content of tannin was calculated in the crude extracts from the standard calibration curve of catechin concentrations (y = 0.005x+0.011, R2 = 0.997). According to the obtained results, the highest total tannin content was recorded in 80% ethanol extract (1.05 mgCE/gDCE) followed by 80% methanol (0.92 mgCE/gDCE). Nevertheless, the obtained total tannin content in general did not show a significant difference between the tested crude extracts (p ≥ 0.05).
3.4. In-vitro anti-DPPH radical activity of Cadaba rotundifolia crude leaf extracts
In the present study, the anti-free radical scavenging potential of four crude extracts of C. rotundifolia leaf against DPPH free radical was evaluated. Ascorbic acid and plant extract free DPPH solution were used as positive and negative controls, respectively. The obtained DPPH free radical scavenging percentage of the tested crude extracts together with the ascorbic acid is presented in .
Figure 2. DPPH radical scavenging percentage (%) versus different concentrations of four crude extracts of Cadaba rotundifolia leaf and standard ascorbic acid
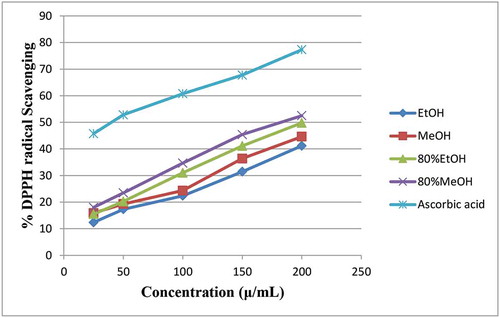
The obtained output of statistical analysis showed that the difference in anti-DPPH activity mean values between each tested extracts was significance (p ≤ 0.05) except between extracts 1 & 4 (p ≥ 0.05). As it can clearly be seen from , 80% methanol extract exhibited the greatest DPPH radical scavenging activity followed by 80% ethanol. The lowest scavenging percentage was recorded by the non-aqueous ethanol extract followed by methanol analogues. Overall, the methanol family was found to be the better extract than the ethanol family in the trapping of DPPH free radicals. According to many scholars, for example (Škerget et al., Citation2005), the anti-DPPH free radical trapping potential of plant extracts is mainly due to the presence of high contents of polyphenolic and flavonoid compounds which easily donate free hydrogen radical. This fact definitely supports our findings, in which the aqueous part of the methanol and ethanol crude extracts which scored the highest total phenolic, and flavonoid contents also exhibited the highest DPPH free radical scavenging percentage than the non-aqueous parts.
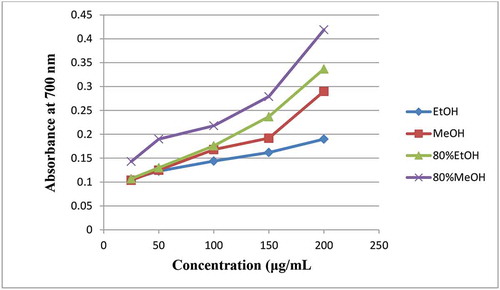
3.5. Ferric reducing antioxidant power (FRAP)
The reducing potential of chemical entities found in a given analyte such as plant extract could be evaluated by monitoring the reduction of ferric (Fe3+) into the ferrous (Fe2+) form. That is, ferric ions (Fe3+) originated from ferricyanide complex ([K3Fe(CN)6]) can be reduced to the ferrous ions (Fe2+) by observing the change in colour of the reaction solutions from yellow to green in the presence of antioxidants. The produced Fe2+ concentration is monitored by measuring its absorbance at 700 nm (Anwar & Przybylski, Citation2012; Sun et al., Citation2015). As shown from , the ferric reducing power of the tested plant extracts was positively correlated with their concentrations. The higher colour intensity of the mixtures revealed greater absorbance and higher reducing activity or antioxidants (the presence of more reductants) in the crude extracts. The hydroalcoholic (80% MeOH and 80% EtOH) crude extracts of C. rotundifolia leaf showed greater FRAP than the corresponding non-aqueous ones (). This result, like that of DPPH assay, showed a positive correlation between the ferric reducing antioxidant power (FRAP) and the total content of phenolics and flavonoids () the hydroalcoholic crude extracts recorded. The FRAP mean value between each evaluated extract was significantly different (p ≤ 0.05) according to the statistical data obtained.
4. Conclusion
As the chemistry of phyto-compounds varies, different solvents of extraction may have different effects on the solubility, extraction yield and antioxidant activity of the phyto-compounds. In the present study, among the solvent of extraction used, 80% aqueous ethanol scored the highest extraction yield followed by 80% aqueous methanol, methanol and ethanol. Therefore, ethanol, methanol and their corresponding aqueous solvents could be preferred for further investigation on the biological activity and isolation of effective bioactive compounds of C. rotundifolia leaf. Regarding the phytochemical screening test, major phytochemical classes such as flavonoids, phenolics, alkaloids, tannins, carbohydrates and saponins were positively observed in different crude extracts of C. rotundifolia leaf. It is, therefore, possible to say that the reported ethno-medicinal value of C. rotundifolia leaf might be attributed to such positively screened secondary metabolites. Quantitatively, the hydroalcoholic crude extracts (80% methanol and 80% ethanol) of C. rotundifolia leaf showed better total phenolics, flavonoids and tannins content; and such results were also correlated with the DPPH anti-free radical scavenging activity and ferric reducing antioxidant power (FRAP) of the mentioned extracts. In general, the overall findings obtained from the present study pave a way for the upcoming extensive research works which might be conducted on the pharmacological assay and phyto-compound investigations of C. rotundifolia leaf. From the present findings, it is also very important to note that the hydroalcoholic solvents should be prioritized while conducting any phyto-pharmacological studies on the C. rotundifolia leaf, in the near future.
Competing interests
The authors declare no competing interests.
Acknowledgements
The authors would like to acknowledge the Department of Chemistry, Central Laboratory and Department of Food Science and Postharvest Technology Haramaya University, for the chemicals and materials support.
Additional information
Funding
Notes on contributors

Teshome Gonfa
Teshome Gonfa obtained his M.Sc. degree in Organic Chemistry from Addis Ababa University in 2014. He is presently working as a lecturer and researcher in the Department of Chemistry, Haramaya University. His core areas of research interests are on natural product chemistry; isolation, structural elucidation by spectroscopic technique, and evaluation of bioactivity of crude extracts and isolated compounds.
Shewangizawe Teketle
Shewangizawe Teketle is a Senior Technical assistant in the Department of Food Science Haramaya University, Ethiopia. He has a B.Sc. degree in Food Science from Haramaya University and currently an M.Sc. student of Food Science and Technology at Haramaya University. His research interests are Food Quality Control, Food Chemistry and Analysis.
Tsegu Kiros
Tsegu Kiros obtained his M.Sc. degree in Organic Chemistry from Addis Ababa University in 2014. He is a researcher and lecturer at Haramaya University; and currently a Ph.D. candidate in Natural Product Chemistry at Adama Science and Technology University. His research interest focuses on the isolation and characterization of bioactive phyto-constituents.
References
- Ahmed, E., Ali, S., & Asefa, A. (2017). Structure and regeneration status of woody plants in the Hallideghie wildlife reserve, North East Ethiopia. International Journal of Biodiversity and Conservation, 9(6), 200–12. https://doi.org/10.5897/IJBC2017.1085
- Al Hamoud, G., Orfali, R., Sugimoto, S., Yamano, Y., Alothyqi, N., Alzahrani, A., & Matsunami, K. (2019). Four New Flavonoids Isolated from the Aerial Parts of Cadaba rotundifolia Forssk. (Qadab). Molecules, 24(11), 2167. https://doi.org/10.3390/molecules24112167
- Al-Fatimi, M. (2019). Ethnobotanical survey of medicinal plants in central Abyan governorate, Yemen. Journal of Ethnopharmacology, 241, 111973. https://doi.org/10.1016/j.jep.2019.111973
- Al-Fatimi, M., Wurster, M., Schröder, G., & Lindequist, U. (2007). Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. Journal of Ethnopharmacology, 111(3), 657–666. https://doi.org/10.1016/j.jep.2007.01.018
- Anwar, F., & Przybylski, R. (2012). Effect of solvent extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). Acta Scientiarum Polonorum. Technologia Alimentaria, 11(3), 293–301. PMID: 22744950.
- Azabou, S., Sebii, H., Taheur, F. B., Abid, Y., Jridi, M., & Nasri, M. (2020). Phytochemical profile and antioxidant properties of tomato by-products as affected by extraction solvents and potential application in refined olive oils. Food Bioscience, 36, 100664. https://doi.org/10.1016/j.fbio.2020.100664
- Baba, S. A., & Malik, S. A. (2015). Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. Journal of Taibah University for Science, 9(4), 449–454. https://doi.org/10.1016/j.jtusci.2014.11.001
- Belayneh, A., & Bussa, N. (2014). Ethnomedicinal plants used to treat human ailments in the prehistoric place of Harla and Dengego valleys, eastern Ethiopia. Journal of Ethnobiology and Ethnomedicine, 10(1), 18. https://doi.org/10.1186/1746-4269-10-18
- Belayneh, A., Bussa, N., & Deressa, M. (2017). The potential of camel milk and extracts of major plants browsed by the animal for diabetes treatment. East Africa Journal of Sciences, 11, (2), 129–138.
- Chigayo, K., Mojapelo, P. E. L., Mnyakeni-Moleele, S., & Misihairabgwi, J. M. (2016). Phytochemical and antioxidant properties of different solvent extracts of Kirkia wilmsii tubers. Asian Pacific Journal of Tropical Biomedicine, 6(12), 1037–1043. https://doi.org/10.1016/j.apjtb.2016.10.004
- Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E., Ismadji, S., & Ju, Y.-H. (2014). Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. Journal of Food and Drug Analysis, 22(3), 296–302. https://doi.org/10.1016/j.jfda.2013.11.001
- Giday, M., & Teklehaymanot, T. (2013). Ethnobotanical study of plants used in management of livestock health problems by Afar people of Ada’ar District, Afar Regional State, Ethiopia. Journal of Ethnobiology and Ethnomedicine, 9(1), 8. https://doi.org/10.1186/1746-4269-9-8
- Graham, J. G., Quinn, M. L., Fabricant, D. S., & Farnsworth, N. R. (2000). Plants used against cancer an extension of the work of Jonathan Hartwell. Journal of Ethnopharmacology, 73(3), 347–377. https://doi.org/10.1016/S0378-8741(00)00341-X
- Hassan-Abdallah, A., Merito, A., Hassan, S., Aboubaker, D., Djama, M., Asfaw, Z., & Kelbessa, E. (2013). Medicinal plants and their uses by the people in the region of Randa, Djibouti. Journal of Ethnopharmacology, 148(2), 701–713. https://doi.org/10.1016/j.jep.2013.05.033
- Iloki-Assanga, S. B., Lewis-Luján, L. M., Lara-Espinoza, C. L., Gil-Salido, A. A., Fernandez-Angulo, D., Rubio-Pino, J. L., & Haines, D. D. (2015). Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Research Notes, 8(1), 396-396. https://doi.org/10.1186/s13104-015-1388-1
- Karemy, Z. (2001). Capparaceae in the flora of Egypt. Taeckholmia, 21(2), 257–267. https://doi.org/10.21608/taec.2001.12468
- Khatoon, M., Islam, E., Islam, R., Rahman, A. A., Alam, A. K., Khondkar, P., Rashid, M., & Parvin, S. (2013). Estimation of total phenol and in vitro antioxidant activity of Albizia procera leaves. BMC Research Notes, 6(1), 121-121. https://doi.org/10.1186/1756-0500-6-121
- Khorasani Esmaeili, A., Mat Taha, R., Mohajer, S., & Banisalam, B. (2015). Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from In vivo and in vitro grown trifolium pratense L. (Red Clover). BioMed Research International, 643285. https://doi.org/10.1155/2015/643285
- Lefahal, M., Zaabat, N., Radia, A., Makhloufi, E., Lakhdar, D., Benahmed, M., & Akkal, S. (2018). In vitroassessment of total phenolic and flavonoid contents, antioxidant and photoprotective activities of crude methanolic extract of aerial parts of Capnophyllum peregrinum (L.) Lange (Apiaceae) growing in Algeria. Medicines, 5(2), 26. https://doi.org/10.3390/medicines5020026
- Medini, F., Fellah, H., Ksouri, R., & Abdelly, C. (2014). Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. Journal of Taibah University for Science, 8(3), 216–224. https://doi.org/10.1016/j.jtusci.2014.01.003
- Paterson. (1999). Phytochemical methods. A guide to modern techniques of plant analysis. Plant Pathology, 48(1), 146-146. https://doi.org/10.1046/j.1365-3059.1999.00318.x
- Rebaya, A., Belghith, S., Baghdikian, B., Leddet, V. M., Fathi, M., Olivier, E., & Trabelsi Ayadi, M. (2015). Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae). Journal of Applied Pharmaceutical Science, 5(01), 052–057. https://doi.org/10.7324/JAPS.2015.50110
- Škerget, M., Kotnik, P., Hadolin, M., Hraš, A. R., Simonič, M., & Knez, Ž. (2005). Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chemistry, 89(2), 191–198. https://doi.org/10.1016/j.foodchem.2004.02.025
- Sultana, B., Anwar, F., & Ashraf, M. (2009). Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules (Basel, Switzerland), 14(6), 2167–2180. https://doi.org/10.3390/molecules14062167
- Sun, C., Wu, Z., Wang, Z., & Zhang, H. (2015). Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evidence-based Complementary and Alternative Medicine: eCAM, (2015, 595393. https://doi.org/10.1155/2015/595393
- Teklehaymanot, T. (2017). An ethnobotanical survey of medicinal and edible plants of Yalo Woreda in Afar regional state, Ethiopia. Journal of Ethnobiology and Ethnomedicine, 13(1), 1-25. https://doi.org/10.1186/s13002-017-0166-7
- Teklehaymanot, T., & Giday, M. (2010). Quantitative ethnobotany of medicinal plants used by Kara and Kwego semi-pastoralist people in lower omo river valley, debub omo zone, southern nations, nationalities and peoples regional state, Ethiopia. Journal of Ethnopharmacology, 130(1), 76–84. https://doi.org/10.1016/j.jep.2010.04.013
- Tikssa, M., Bekele, T., & Kelbessa, E. (2009). Plant community distribution and variation along the Awash river corridor in the main Ethiopian rift. African Journal of Ecology, 48(1), 21–28. https://doi.org/10.1111/j.1365-2028.2009.01116.x
- Truong, D.-H., Nguyen, D. H., Ta, N. T. A., Bui, A. V., Do, T. H., & Nguyen, H. C. (2019). Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and invitro anti-inflammatory activities of Severinia buxifolia.. Journal of Food Quality, 2019, 8178294. https://doi.org/10.1155/2019/8178294
- Upadhya, V., Pai, S., & Hegde, H. (2015). Effect of method and time of extraction on total phenolic content in comparison with antioxidant activities in different parts of Achyranthes aspera. Journal of King Saud University - Science, 27, 204-208. https://doi.org/10.1016/j.jksus.2015.04.004